
Mechanical comparison of application of locking intramedullary nail to locking compression plate in the ovine metatarsus
Highlight box
Key findings
• IMN constructs may provide superior mechanical properties for a critical-sized osteotomy model of the ovine metatarsus when compared to lateral LCP constructs.
What is known and what is new?
• The ovine metatarsal bone is commonly utilized in preclinical fracture models utilizing a locking compression plating (LCP) technique.
• More recently, intramedullary interlocking nails (IMN) have been utilized. The mechanical properties of this unique surgical technique utilizing an IMN has not yet been fully elucidated or compared to LCP technique.
What is the implication, and what should change now?
• Further in vivo investigation comparing characteristics of fracture healing between IMN and LCP is warranted.
Introduction
Ovine tibia has been used extensively for modelling fracture healing in a large variety of constructs including dynamic compression plates, locking compression plates (LCP), intramedullary pins, unlocked nails, and locking nails (1-8). Intramedullary nails (IMN) have shown to have the greatest stability in the tibia fracture model when compared to other constructs (4). While the ovine tibia is a well-established model for long bone fracture healing, the ovine metatarsus has been proposed as an alternative due to its vertical weight bearing orientation and more accessible distal anatomic location, both for orthopedic implantation and post-operative care and bandaging (9-12). A majority of studies to date have utilized plating systems or external fixation (10,11). Recently, Yang et al. described the first use of IMNs for stabilization of critical-sized defects of the metatarsus in a small cohort of animals (11). While this novel procedure was well-tolerated and provided appropriate mechanical stability throughout the study period, the mechanical properties of the IMN have not yet been fully elucidated or compared to the most commonly performed technique of LCP.
The lack of IMN use in metatarsal fracture studies is likely due to the complexity of the tarsus, which makes proximodistal insertion of an intramedullary nail less appealing. The ovine metatarsus articulates proximally with the second, third, and fourth tarsal bones at the tarsometatarsal joint, which is a low motion joint. The ovine metatarsus is made up of the fused third and fourth metatarsal bones, which becomes more apparent distally as the distal metatarsus forms a sagittal septum with both a medial and lateral metatarsophalangeal joint. Both medial and lateral joints are high motion and can be flexed and extended significantly in the dorsoplantar plane. Therefore, access to the medullary cavity of the metatarsus can be achieved in a distal to proximal direction through the sagittal septum of the distal metatarsus (13).
To provide additional information to aid in the appropriate selection of ovine preclinical fracture models, we aimed to compare the biomechanical properties of a metatarsal IMN construct with a critical-sized 3-cm mid-diaphyseal osteotomy to a laterally placed 3.5-mm, 9-hole LCP construct. We hypothesize that a mid-diaphysis metatarsal critical-sized osteotomy stabilized with an IMN will provide equivalent mechanical stability to LCP.
Methods
Specimens
Sixteen ovine hindlimbs were acquired from skeletally mature Columbia × Rambuoillet research purpose bred sheep (body weight estimated average 70±3 kg) with no known history of orthopedic disease, which had previously been enrolled in a separate study of the spine. The metatarsi were visually inspected prior to implant placement to verify that no macroscopic abnormalities were observed prior to inclusion. Eight hindlimbs were transected mid-femur with soft tissues intact prior to freezing for storage; eight of the hindlimbs were dissected for isolation of the metatarsus prior to freezing for storage. The limbs with intact soft tissues were used for placement of the IMNs in order to assess feasibility of the distal normograde approach. These were radiographed prior to IMN placement to assess size and confirm normal metatarsal structure. The metatarsi that were isolated from soft tissues were used for placement of LCP group and appeared grossly normal prior to implantation. All constructs were isolated from the soft tissues prior to servohydraulic testing.
Groups
For the IMN group, eight limbs (n=8) were implanted with an IMN through the sagittal septum of the metatarsophalangeal joint following creation of a mid-metatarsal 3-cm osteotomy. Detailed implantation methods are provided as follows:
The hindlimb was stabilized to simulate a dorsally recumbent animal. The limb was clipped free of wool. A 5-cm incision was made through the skin over the lateral aspect of the estimated midpoint of the metatarsus. The periosteum was elevated circumferentially and a 3-cm osteotomy was made first using an oscillating saw in the mid-diaphysis of the metatarsus. A custom cylindrical guide was then placed enabling consistency in the osteotomy gap creation without risking damage to the IMN after placement.
With the metatarsophalangeal joint in full flexion, a 2-cm incision was made through the skin over the midline of the dorsal metatarsophalangeal joint, taking care to remain superficial to subcutaneous structures. Blunt dissection was then used to identify the extensor tendon and digital artery, which lie dorsal to the sagittal septum of the distal metatarsus. These structures, once identified, were retracted abaxial to the sagittal septum of the metatarsophalangeal joint (Figure 1A). The IMN was then applied using the I-Loc® IM Fixator (BioMedtrix) system1 according to the Manufacturer’s Instructions for Use (IFU) for 8 mm, 147-m hourglass-shaped interlocking nails. Specifically, a 2.4-mm Steinman pin was drilled through the central region of the intercondylar septum in alignment with the metatarsal bone until a majority of the pin was within the medullary canal of the metatarsus. An 8-mm cannulated drill bit was utilized to overdrill the pin tract until the medullary canal was accessed. The drill bit was removed and the medullary cavity was reamed using the 8-mm reamer included in the I-Loc system (Figure 1B). Following reaming, the IMN was placed in a distal normograde direction through the intercondylar septum and spanned evenly within the metatarsal bone from proximal to distal. The aiming guide was connected to the IMN ensuring accurate location for lateral to medial drilling of holes for the locking bolts. A 3.5-mm glide hole was created in the distolateral aspect of the cis cortex, beginning with the distal most bolt, through the drill guide attachment to the intramedullary nail. Complete drilling of the cis cortex to the nail was confirmed via tactile sensation of the nail threads via the feeler tool. The trans cortex was drilled using a 2.5-mm bit and guide attachment. An alignment post was inserted into each drilled hole through both cortices and nail to ensure no movement of the construct prior to placement of the bolts. These steps were repeated for each bolt consistent with the IFU. To maintain the 3cm osteotomy gap prior to placement of proximal transfixation bolts a custom-designed 3 cm spacer was placed within the osteotomy site. The aiming guide attachment was removed from the nail and the bolts were inserted from distal to proximal and tightened by hand. Appropriate application of the nail was confirmed radiographically (Figure 2). Following creation of the osteotomy and placement of the IMN, the metatarsi in this group were dissected from the soft tissues for mechanical testing (Figure 3).
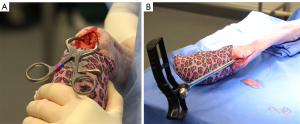


For the LCP group, eight (n=8) metatarsi were utilized. A 3.5-mm, 9-hole LCP (Depuy Sythes) was applied to the lateral aspect of the metatarsus using bicortically placed 3.5-mm locking head screws in the three most proximal and three most distal locking screw holes. Contouring of the plate was not necessary due to use of locking screws and relative straightness of the lateral metatarsus. Following plate application, the LCP was removed and a mid-metatarsal 3-cm osteotomy was created using an oscillating saw. The LCP was then replaced on the metatarsus to stabilize the ostectomy site.
Biomechanical testing
The metatarsal constructs were harvested and fitted directly to the bone with three multi-directional strain gauge rosettes (Micro Measurements, C2A-XX-062WW-350) on both the proximomedial and distomedial metatarsus approximately 3 cm from the proximal and distal most ends of the metatarsus stabilized with either the IMN or LCP. A third gauge was placed on the lateral aspect of the IMN or medial aspect of the LCP at the center of the defect site. The strain gauge rosettes were applied directly to the bone or metal of the construct, depending on location. Bone was prepared by removing all periosteal tissue then sanding and dehydrating the application area with ethanol. For application to the hardware, the metal surface was sanded with fine grit sandpaper and cleaned with a strain gauge manufacturer supplied solution. For the LCP, the screw hole at the center of the defect was filled with an elastomer, then sanded flush to the surface to ensure the strain gauge was uniformly attached to the surface. The stiffness of the elastomer was negligible compared to that of the plate contributing negligible resistance to the applied loads while experiencing similar strains. The rosettes were bonded to all surfaces using ethyl cyanoacrylate [Loctite Super Glue Precision Pen (Product# 2066118)].
The bones were fixed at the proximal and distal ends in custom-built potting boxes using epoxy (M325, Smooth-On, Inc., Macungie, PA). The constructs were tested in a servo-hydraulic material testing frame (MTS Landmark, MTS Systems Co., Eden Prairie, MN). For the compression and torsion tests, the metatarsal-hardware constructs were aligned vertically and centered so that the bone was within the circumference of the hydraulic piston of the servohydraulic testing frame. In 4-point bending, to account for the slightly different sizes of the osteotomy which could result in different moment arms, images were taken of each specimen with a scale and distances between fulcrum points were analyzed using the open-source image processing software ImageJ and computed with the known applied loads to calculate the maximum bending moment (14). The samples were preconditioned to 5 cycles for each testing condition. Data was collected for the following single loading conditions: 0 to 800 N in compression, −5 to 5 Nm in torsion, and 5.5 Nm maximum moment in four-point bending. The measured loads in this study were chosen and measured at estimated maximum values that the limb may experience under normal conditions. Due to lack of reported in vitro physiologic loading of the ovine metatarsus, compression values were estimated to approximate a 70-kg sheep bearing roughly 70–75% body weight on the limb. Torsion was estimated as a moderate turn with one foot planted, and bending was estimated as approximately 15 N lateral force applied to end of a fixed metatarsus. Loads were applied at a rate of approximately 50N/s in compression, ±1 Nm/s in torsion, and 1 Nm/s maximum moment in four-point bending. The load and strain were recorded at a rate of 100 Hz using a custom written LabView code (LabView, LabView 2018). Specimen hydration was maintained throughout the testing procedure with physiologic saline spray.
Statistical analysis
Maximum and minimum principal strains were compared at 500 N in compression and at the proximal (0.37 Nm), mid (5.3 Nm), and distal (0.075 Nm) aspects of the construct in four-point bending. Maximum shear strain was computed for torsional tests at +4.5 Nm of torque. Minimum and maximum principal strains were compared in compression and four-point bending, and maximum shear strain in positive and negative torsion.
Data normality was assessed using the Anderson-darling test. Statistical comparisons between fixation type (IMN vs. LCP) for each loading condition were performed using an unpaired Student’s t-test with an alpha (α) value of 0.05. A Wilcoxon rank-sum test was used for statistical comparisons when data were not normally distributed (GraphPad Prism).
Results
Placement of the IMN was possible through the sagittal septum while avoiding disruption of the dorsal soft tissues, including the extensor tendons and dorsal artery in all eight limbs. During drilling of the sagittal septum, minimal damage (estimated <5%) to the axial articulating surface of the metatarsophalangeal joint occurred (Figure 4).
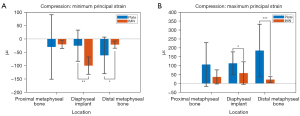
A summary of the mean strains across all groups is shown in Table 1. In compression at the implant diaphysis, the mean maximum principal strain was greater (P<0.05) in the LCP (112.9±64.1 µε) group than the IMN (57.7±63.5 µε) group, however, the magnitude of the mean minimum principal strain was greater (P<0.05) in the IMN (−99.8±32.4 µε) than the LCP (−25.2±68.4 µε) (Figure 5A,5B). The magnitudes of both maximum and minimum strains of the proximal and distal metaphyseal bones were higher in the LCP group than the IMN group, with the distal metaphyseal groups being statistically significant (P<0.05) (Table 1).
Table 1
| Variables | Implant | Location, mean ± standard deviation | ||
|---|---|---|---|---|
| Proximal metaphyseal bone | Diaphyseal implant | Distal metaphyseal bone | ||
| Compression | ||||
| Max principal (με) | LCP | 106.2±122.8 | 112.9±64.1* | 184.5±147.8* |
| IMN | 36.2±39.7 | 57.7±63.5* | 21.3±16.3* | |
| Min principal (με) | LCP | −29.9±120.6 | −25.2±68.4* | −61.4±68.5* |
| IMN | −20.2±15.6 | −99.8±32.4* | −21.1±13.4* | |
| Torsion | ||||
| Positive torsion max shear (με) | LCP | −196.8±97.1* | −1,237.6±487.4* | 283.7±111.0* |
| IMN | 55.6±33* | −641.6±54.8* | 52.2±40.0* | |
| Negative torsion max shear (με) | LCP | −181.6±81.3* | 1,222.0±482.0* | 225.0±74.9* |
| IMN | 43.8±22.1* | 658.8±48.8* | 49.1±32.3* | |
| 4-point bending | ||||
| Max principal (με) | LCP | −9.5±23.9 | 461.4±127.9* | −1.0±7.9* |
| IMN | 5.3±4.3 | 790.7±182.2* | 16.5±15.4* | |
| Min principal (με) | LCP | −32.4±27.6* | −26.9±94.0* | −19.4±12.1 |
| IMN | −9.6±7.3* | −274.7±77.4* | −15.4±14.2 | |
*, statistically significant (P<0.05). LCP, locking compression plating; IMN, intramedullary nails.
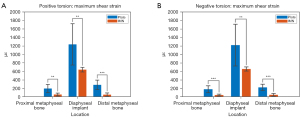
The mean maximum shear strains at the diaphyseal implants were similar in magnitude in positive and negative torsion, with the LCP (−1,237.6±487.4, 1,222.0±482.0 µε) having higher strain magnitudes (P<0.05) than the IMN (−641.6±54.8, 658.8±48.8 µε) (Figure 6A,6B). The magnitude of strains on the metaphyseal bone of both proximal and distal constructs were greater (P<0.05) in the LCP group than the IMN group (Table 1).
In 4-point bending, the maximum principal strains at the diaphyseal implants were greater (P<0.05) in the IMN (790.7±182.2 µε) than the LCP (461.4±127.9 µε). The magnitude of the minimum principal strains at the diaphyseal implants were greater (P<0.05) in the IMN (−274.7±77.4 µε) than in the LCP (−26.9±94.0 µε) (Figure 7A,7B).

Discussion
Yang et al. recently showed that this IMN technique can be safely performed in the ovine metatarsus with appropriate stability in a large critical-sized (5 cm) osteotomy over a 24-week study period (11). This cadaver trial described herein proved distal normograde application of the IMN to be simple in application without disruption of the dorsal soft tissue structures, specifically the extensor tendon and dorsal artery of the metatarsus which runs axially in the sagittal septum and only minimal disruption of the cartilage surface of the metatarsophalangeal joint. The IMN constructs showed overall comparable stiffness and less variance between constructs than the LCP constructs. The stiffness of constructs in this study was defined as the measured strain experienced by the material components of the constructs, including the bone and implant. Greater strain implies greater deformation and therefore reduced stiffness of the construct. It is therefore possible to conclude that an IMN is an acceptable alternative in a metatarsal osteotomy model when compared to the laterally placed LCP. This is evidenced most clearly via testing in compression, as the magnitudes maximum and minimum principal strains are more similar with less variance in the IMN group, as compared to the LCP group in which the strains infer both a tension and compression surface of the plate. This is likely due to the lateralized location of the plate and infers that there are asymmetric strains across the construct in the LCP group. We therefore accept our hypothesis that the IMN construct has comparable mechanical properties for stabilizing the ovine metatarsus following a mid-diaphyseal osteotomy. As such, the IMN construct can be expected to perform well in a critical-sized osteotomy in vivo model. This is recently proven further by the in vivo study performed by Yang et al. (11). A direct comparison of IMN to LCP in vivo performance will need to be performed to fully understand the novel IMN technique’s clinical/preclinical performance.
The metatarsus was originally described as a model for fracture healing in 2004 by Viateau et al. using dynamic compression plates (10). According to a recent review of 11 in vivo metatarsal defect models in sheep, the models were all able to mimic human conditions due to the vertical weight-bearing cortical bone region and were more easily accessible to surgery in comparison to tibia and femur models (15). LCP have also been described for use in fracture healing models of the metatarsus in combination with external fixator, which would be impossible in more proximal bones (9,16). Our team recently described the first use of this novel IMN technique in the ovine metatarsus and this mechanical study described herein characterizes the mechanical properties and potential mechanical advantages of utilizing this IMN construct for preclinical fracture models.
Relevant to repeatability and consistency of gap osteotomy models, the variance of strain between specimens in the IMN group was less than the variance among specimens in the LCP group. In addition, the strains measured in the metaphyseal bone were consistently low in the IMN groups, across all testing modalities. This implies stress shielding of the bone, which is not present in the LCP group, where there is variable and increased strain measured in the metaphyseal bone across testing modalities. Increased measured strain associated the metaphyseal bone in the LCP groups could indicate an increased morbidity at the bone implant interface, such as implant loosening or pull out which may be less likely in the IMN groups due to stress shielding through the IMN construct.
While previous osteotomy models have utilized LCP constructs of the ovine metatarsus, no biomechanical ex vivo data is available categorizing the stability of the constructs. In a study comparing union and non-union osteotomy groups using an LCP construct, the reported calcified tissue within the non-union and union osteotomies respectively were 10.4%±20.1% and 44.7%±27.7% (17). While these data are not directly comparable to the direct LCP microstrains, the variability in the percent fracture healing within groups could reflect the wide variability in LCP stability in the current study as compared to relatively smaller variability within the IMN construct group. In a previous LCP model study in vivo, sleeve-casting of the limb was required to ensure stability of the construct (10). It has been established, however that the use of a cast reduces mechanical stresses, which contribute to bone healing (18). With in vivo application of the IMN, post-operative sleeve-casting was not necessary and therefore eliminates this potential for added variance in a gap osteotomy model due to casting between implants. Further investigation is needed for comparison of in vivo fracture healing using an IMN construct to that of LCP construct. To this point, the magnitude of strains of the metaphyseal bones were higher in the LCP groups when tested in compression and torsion, compared to the IMN group. This implies a stress shielding of the metaphyseal bone associated with the IMN constructs.
In clinical application, the IMN may provide an alternative to plating in cloven hooved species with a fused metatarsus and a sagittal septum, such as sheep, goats, and cattle. This could provide benefit in cases of mid diaphyseal fractures of the metatarsus of small ruminants, which have a reported prevalence of 20% in one retrospective study (19). In vivo testing for joint tolerance of distal normograde application should be pursued prior to clinical application. The authors have applied a distal normograde IMN in an in vivo setting without complication, however long-term tolerance beyond 6 months has yet to be assessed (11). Additionally, the IMN system may prove to be cost prohibitive for livestock species in hospitals that do not already have access to the IMN surgical system.
The ex vivo nature of this study limits the ability to conclude definitively the reduction in variability of results in a fracture healing model. Further live animal studies will be needed to verify this extrapolation from the ex vivo data. This is also true for assessing the tolerance of the metatarsophalangeal joint to the distal normograde application in a live animal, both short term for fracture models and long term for clinical application. Only a single size of LCP and IMN were analyzed for this study, different size/thickness of hardware would almost surely change the measured strains. Due to a lack of standardization of fracture healing models in the literature, further examination of these differences may provide a more complete understanding of the ideal methodology for repeatability and consistency between constructs.
Conclusions
Use of IMNs in the ovine metatarsus may prove to be a more consistent preclinical fracture model than previously used LCP constructs, providing less variability in hardware strains between specimens. This implies a more reliable response of the IMN construct mechanical loading, leading to more precision of the experiment when analyzing multiple specimen defects. This reduction in variability of strain may decrease confounding factors in osteotomy gap models, thus improving the ability to interpret comparisons between groups. Additionally, the distal normograde application of IMN in the ovine metatarsus is no more technically difficult to apply than the LCP, does little to disrupt the normal anatomy of the metatarsophalangeal joint, and does not require post-operative casting, which can contribute to variability in forces on the construct.
Acknowledgments
The authors also thank Kelsea Ericksen, MS for providing the illustration for Figure 1.
Funding: None.
Footnote
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2746/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2746/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
1IM Nails applied based on the BioMedtrix I-Loc® IM Fixator system instructions for use.
References
- Lu Y, Nemke B, Lorang DM, et al. Comparison of a new braid fixation system to an interlocking intramedullary nail for tibial osteotomy repair in an ovine model. Vet Surg 2009;38:467-76. [Crossref] [PubMed]
- Berger L, Fischerauer S, Weiß B, et al. Unlocked and locked elastic stable intramedullary nailing in an ovine tibia fracture model: a biomechanical study. Mater Sci Eng C Mater Biol Appl 2014;40:267-74. [Crossref] [PubMed]
- Wolynski JG, Sutherland CJ, Demir HV, et al. Utilizing Multiple BioMEMS Sensors to Monitor Orthopaedic Strain and Predict Bone Fracture Healing. J Orthop Res 2019;37:1873-80. [Crossref] [PubMed]
- Heitemeyer U, Claes L, Hierholzer G, et al. Significance of postoperative stability for bony reparation of comminuted fractures. An experimental study. Arch Orthop Trauma Surg 1990;109:144-9. [Crossref] [PubMed]
- Letechipia J, Alessi A, Rodríguez G, et al. Design and preliminary testing of an active intramedullary nail. Rev Invest Clin 2014;66:S70-8. [PubMed]
- Andrews JL, Sutherland J, Ghosh P. Distribution and binding of glycosaminoglycan polysulfate to intervertebral disc, knee joint articular cartilage and meniscus. Arzneimittelforschung 1985;35:144-8. [PubMed]
- Dozza B, Salamanna F, Baleani M, et al. Nonunion fracture healing: Evaluation of effectiveness of demineralized bone matrix and mesenchymal stem cells in a novel sheep bone nonunion model. J Tissue Eng Regen Med 2018;12:1972-85. [Crossref] [PubMed]
- Rössig C, Angrisani N, Helmecke P, et al. In vivo evaluation of a magnesium-based degradable intramedullary nailing system in a sheep model. Acta Biomater 2015;25:369-83. [Crossref] [PubMed]
- Gadomski BC, McGilvray KC, Easley JT, et al. An in vivo ovine model of bone tissue alterations in simulated microgravity conditions. J Biomech Eng 2014;136:021020. [Crossref] [PubMed]
- Viateau V, Guillemin G, Yang YC, et al. A technique for creating critical-size defects in the metatarsus of sheep for use in investigation of healing of long-bone defects. Am J Vet Res 2004;65:1653-7. [Crossref] [PubMed]
- Yang YP, Labus KM, Gadomski BC, et al. Osteoinductive 3D printed scaffold healed 5 cm segmental bone defects in the ovine metatarsus. Sci Rep 2021;11:6704. [Crossref] [PubMed]
- Vidal L, Kampleitner C, Krissian S, et al. Regeneration of segmental defects in metatarsus of sheep with vascularized and customized 3D-printed calcium phosphate scaffolds. Sci Rep 2020;10:7068. [Crossref] [PubMed]
- Duncan JS, Singer ER, Devaney J, et al. The radiographic anatomy of the normal ovine digit, the metacarpophalangeal and metatarsophalangeal joints. Vet Res Commun 2013;37:51-7. [Crossref] [PubMed]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671-5. [Crossref] [PubMed]
- Veronesi F, Martini L, Giavaresi G, et al. Bone regenerative medicine: metatarsus defects in sheep to evaluate new therapeutic strategies for human long bone defect. A systematic review. Injury 2020;51:1457-67. [Crossref] [PubMed]
- Gadomski BC, Lerner ZF, Browning RC, et al. Computational characterization of fracture healing under reduced gravity loading conditions. J Orthop Res 2016;34:1206-15. [Crossref] [PubMed]
- McGilvray KC, Unal E, Troyer KL, et al. Implantable microelectromechanical sensors for diagnostic monitoring and post-surgical prediction of bone fracture healing. J Orthop Res 2015;33:1439-46. [Crossref] [PubMed]
- Parente EJ, Nunamaker DM. Stress protection afforded by a cast on plate fixation of the distal forelimb in the horse in vitro. Vet Surg 1995;24:49-54. [Crossref] [PubMed]
- Kofler J, Hochschwarzer D, Schieder K, et al. Frakturen von Gliedmaßenknochen bei 32 kleinen Wiederkäuern – Methoden und Ergebnisse der Behandlung. Tierarztl Prax Ausg G Grosstiere Nutztiere 2017;45:201-12. [Crossref] [PubMed]


