
Association and predictive value of soluble thrombomodulin with mortality in patients with acute respiratory distress syndrome: systematic review and meta-analysis
Introduction
Acute respiratory distress syndrome (ARDS) is a life-threatening lung injury characterized by refractory hypoxemia and bilateral pulmonary infiltrates. This syndrome is different from cardiogenic pulmonary edema (1). ARDS accounts for 10.4% of intensive care unit (ICU) admissions, 23.4% of patients on mechanical ventilation, and 21% to 55.7% of hospital deaths (2,3). Early detection of the ARDS patients at a high risk of adverse outcomes is crucial for the risk-stratification and precise treatment of this heterogeneous syndrome (4).
The pathogenesis and progression of ARDS are attributed to pulmonary inflammation, damage to the alveolocapillary membrane, and dysregulation of the coagulation and fibrinolytic systems (5,6). Thrombomodulin (TM), which is highly expressed in pulmonary alveolar capillaries, is an endothelial membrane-bound protein that regulates inflammation and coagulation (7). When TM interacts with thrombin, a TM-thrombin complex is formed to activate protein C, thereby exerting anticoagulatory, anti-inflammatory, and profibrinolytic effects (8). As a major form of circulating TM, soluble TM (sTM) is produced when an intact protein is cleaved under pathologic conditions like cardiovascular diseases, inflammation, infection, and metabolic disorders (9). Hence, elevated sTM levels are associated with endothelial injury, impaired anticoagulation and fibrinolysis, and inflammatory status.
The correlation between sTM and ARDS has been investigated by some researchers, and the results are conflicting. Elevated levels of sTM were observed in nonsurvivors, and/or elevated levels of sTM were independently associated with ARDS mortality (10,11), but this finding was contradictory with other research (12,13). Furthermore, the predictive accuracy of sTM for ARDS hospital mortality was unclear, and mild to moderate predictive value was reported (14,15). The discrepancy may attribute to different measurement methods, study designs or limited sample sizes.
As a result, the present study aimed to probe into the relationship between sTM and hospital mortality in patients with ARDS and evaluate the role and predictive value of sTM in this unfavorable outcome. We present the following article in accordance with the PRISMA reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-23-432/rc) (16).
Methods
Search strategy
This study was registered on PROSPERO (International Prospective Register of Systematic Reviews; CRD42022368632). We searched PubMed, Web of Science, Cochrane Library, Embase, Chongqing VIP, WanFang, China National Knowledge Infrastructure (CNKI), and Chinese Biomedical Literature databases for relevant literature published before October 10, 2022. No restrictions were imposed on language or region. Two reviewers (LZ and LY) independently identified all potentially eligible studies. Any disagreements were mediated through a third reviewer (ZQ) for a final determination. Both free words and medical headings were used, including “thrombomodulin”, “ARDS”, “acute respiration distress syndrome”, “acute lung injury”, and “acute respiratory failure”. The detailed search procedure is depicted in Table S1.
Selection criteria
According to PECOS principle, the studies that met the criteria were eligible. Populations: patients were diagnosed with ARDS or had acute hypoxemic respiratory failure caused by ARDS, with no restrictions on age.ARDS was diagnosed based on the Berlin definition (1), American European Consensus Conference (17), or Pediatric Acute Lung Injury Consensus Conference (18); and international consensus criteria were used to define sepsis (19). Exposure: serum or plasma sTM levels were measured, and the high level of sTM was defined by the cutoff value in the original studies. Control: low level of sTM was defined according to the original studies. Outcome: (I) sTM level in survivors and nonsurvivors with ARDS; (II) the odds ratio (ORs) values of sTM associated with the ARDS mortality; (III) diagnostic four-grid table (true positive, false positive, false negative and true negative) for predicting the ARDS mortality. Study design: the types of included studies were cohort study, cross-sectional study, case control study, and propensity matching study.
We excluded (I) studies with unclear or unreasonable diagnostic criteria for ARDS; (II) studies exploring the association between sTM and outcomes only based on histopathological and genetic levels; (III) the levels of sTM were not completely reported, or the correlation between sTM and the risk of ARDS mortality was investigated only by univariate analysis, or the diagnostic four-grid table could not be extracted directly or indirectly from the original studies; (IV) conference abstracts without full text, or animal-based studies.
Literature screening and data extraction
All searched studies were imported into the Endnote software (X9.2, Clarivate) for management. After removal of duplicates, we checked the titles and abstracts to eliminate unqualified studies. Finally, the full texts of the remaining articles were downloaded for eligibility assessment. A standard data extraction table was used to collect data from the included studies, including study design, patient characteristics, sTM levels, time-points of sTM measurement, and outcome. The results of data extraction were cross-checked.
Quality evaluation
The Newcastle-Ottawa Scale (NOS) (20) was adopted to evaluate the risk of bias in the included studies except the cross-sectional study by Benatti et al. (12), which was assessed by the modified NOS (21). For studies, a score of 7–9 indicated high quality, 4–6 moderate quality, and a 1–3 low quality. QUADAS-2 (Quality Assessment of Diagnostic Accuracy Studies), which was developed to evaluate the risk of bias in the studies on diagnostic accuracy, was unsuitable for the studies on prognostic predictive value. The risk of bias in the studies investigating the predictive value of sTM for ARDS mortality was evaluated by QUAPAS (Quality Assessment of Prognostic Accuracy Studies) tool, which is a modification of QUADAS-2 (22). Two reviewers (LZ, LY) independently performed the quality assessment, and a third reviewer (ZQ) was consulted to resolve the discrepancies in the assessment.
Statistical analysis
Due to the variability in detection methods and enzyme-linked immunosorbent assay (ELISA) kits, continuous variables are expressed as the standardized mean difference (SMD). The risk of mortality associated with increased sTM is presented as ORs with 95% confidence intervals (CIs) after adjustment for other confounding factors. Data were pooled by the inverse variance weighting method. The Cochrane Q and Higgins I2 tests were carried out to assess the heterogeneity. When P<0.05 and I2>50%, there was high heterogeneity. Thus, a random-effects model was employed for statistical analysis, and forest plots were drawn to present the analysis results. Due to the high degree of heterogeneity observed in this study, we carried out subgroup and meta-regression analyses to probe into the cause of heterogeneity. Sensitivity analysis was conducted by excluding noncohort studies and removing one study at a time. A funnel plot and Egger test or Deeks test were employed to determine publication bias. If publication bias was present, the trim-and-fill method was adopted. R version 4.0.3 (The R Foundation for Statistical Computing) was employed for statistical analysis. Stata 13.0 (StataCorp) was adopted for bivariate meta-analyses of pooled sensitivity, specificity, and the summary receiver operating characteristic curve (SROC). A 2-sided P<0.05 indicated a statistically significant difference.
Results
Study selection
We retrieved 891 records. After removing 504 duplicates, we examined the titles and abstracts to exclude unqualified studies. Based on a full-text review of 63 articles, we further ruled out 50 articles. Finally, 13 studies were included. The study selection process is shown in Figure 1.
Characteristics of the eligible studies
Thirteen studies were included in the study (10-15,23-29). The characteristics of the included studies are presented in Table 1. Among the 1,992 patients with ARDS, 538 patients died, and the median mortality was 37.1% (quartile interval 24.3–42.7%). The follow-up duration in the eligible studies was between 28 and 90 days. Children and adults were both included in our study. There were 12 trials, in which sTM was measured using ELISA kits produced by different manufacturers, such as R&D System, Abcam, Diagnostica Stago, and Shanghai Future. Gando et al. (28) employed the enzyme immunoassay (EIA) method for sTM detection. The included studies were of moderate to high quality, with NOS scores ranging from 5 to 8.
Table 1
| Study | Patients | Country | Male [%] | Age, yearsa | Design | Causes of ARDS | Diagnostic criteria | Detection assay | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Benatti MN 2020 | Adults | Brazil | 17 [57] | 44±16 | Cross-section | Flu virus pneumonia | Berlin definition | ELISA | 5b |
| Sapru A 2015 | Adults | America | 242 [54] | 49.8±15.6 | Retrospective cohort | Mixed | Berlin definition | ELISA | 7 |
| Orwoll BE 2015 | Children | America | 136 [56] | 6.8±6.0 | Prospective cohort | Mixed | AECC | ELISA | 8 |
| McClintock D 2008 | Adults | America | 28 [56] | 55±16 | Prospective cohort | Mixed | AECC | ELISA | 7 |
| Gando S 2004 | Adults | Japan | 40 [70] | 50±5 | Prospective cohort | Mixed | AECC | EIA | 6 |
| Sun HZ 2022 | Adults | China | 61 [59] | 53±4 | Retrospective cohort | Nonpulmonary sepsis | Berlin definition | ELISA | 7 |
| Tan YH 2021 | Children | China | 20 [51] | 7.1±2.4 | Case-control | Mixed | PALICC | ELISA | 5 |
| He CL 2021 | Children | China | 39 [70] | 8.5±2 | Case-control | Mixed | PALICC | ELISA | 5 |
| Li CC 2020 | Children | China | 36 [62] | 0.5 (0.2–1.0) | Case-control | Pneumonia | Berlin definition | ELISA | 5 |
| Zhang Q 2020 | Adults | China | 106 [63] | 51.9±3.7 | Prospective cohort | Nonpulmonary sepsis | Berlin definition | ELISA | 8 |
| Song R 2021 | Adults | China | 56 [53] | 53±3 | Retrospective cohort | Mixed | Berlin definition | ELISA | 7 |
| Zheng YN 2022 | Adults | China | 93 [58] | 65.9±5.3 | Retrospective cohort | Nonpulmonary sepsis | Berlin definition | ELISA | 7 |
| Monteiro ACC 2021 | Children | America | 234 [54.2] | 4.1 (0.7–11) | Retrospective cohort | Mixed | PALICC | ELISA | 8 |
a, the data were expressed as mean ± standard deviation or median (25th percentile–75th percentile); b, risk of bias was evaluated by modified NOS. ARDS, acute respiratory distress syndrome; AECC, American European Consensus Conference; PALICC, Pediatric Acute Lung Injury Consensus Conference; ELISA, enzyme-linked immunosorbent assay; EIA, enzyme immunoassay; NOS, Newcastle-Ottawa Scale.
Difference in the sTM level between survivors and nonsurvivors
Twelve studies reported the sTM levels in nonsurvivors and survivors. Our meta-analysis demonstrated that nonsurvivors had more significantly increased sTM levels than did survivors (SMD =1.473; 95% CI: 0.874–2.072; P<0.001; I2=93%). The forest plot is illustrated in Figure 2. The difference in the sTM levels between nonsurvivors and survivors was significant in terms of patients with sepsis-related ARDS (SMD =1.057; 95% CI: 0.355 to 1.759; P<0.001; I2=91%) and nonpulmonary sepsis-induced ARDS (SMD =1.473; 95% CI: 0.675–2.270; P<0.001; I2=92%). In contrast, no significant difference was noted in the sTM levels in patients with direct ARDS (SMD =0.813; 95% CI: –0.673 to 2.229; P=0.253; I2=93%), as shown in Table 2.
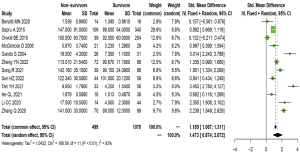
Table 2
| Subgroup | No. | Random-effects, SMD (95% CI) | I2 (%) | Meta-regression (P value)a |
|---|---|---|---|---|
| Detection timeb | ||||
| Early | 9 | 1.578 (0.858–2.298) | 95 | 0.135 |
| Late | 3 | 0.661 (0.320–1.004) | 24 | |
| Age group | ||||
| Adults | 8 | 1.399 (0.808–1.970) | 90 | 0.739 |
| Children | 4 | 1.638 (0.139–3.137) | 96 | |
| Typec | ||||
| Indirect | 4 | 1.330 (0.688–1.972) | 90 | 0.561 |
| Direct | 3 | 0.813 (−0.673–2.299) | 93 | 0.334 |
| Mixed | 7 | 1.543 (0.063–2.454) | 95 | |
| Cause | ||||
| Sepsis | 5 | 1.057 (0.355–1.759) | 91 | 0.434 |
| Mixed | 7 | 1.543 (0.631–2.454) | 95 | |
| Detection method | ||||
| ELISA | 11 | 1.186 (0.619–1.752) | 93 | 0.080 |
| EIA | 1 | 1.328 (0.735–1.921) | ||
| Follow-up daysd | ||||
| ≤30 days | 8 | 1.386 (0.657–2.115) | 92 | 0.801 |
| >30 days | 4 | 1.223 (0.051–2.395) | 94 | |
| Mortalitye | ||||
| High | 6 | 1.425 (0.633–2.217) | 91 | 0.762 |
| Low | 6 | 1.237 (0.286–2.187) | 94 | |
| Sample | ||||
| Plasma | 7 | 1.295 (0.302–2.288) | 94 | 0.877 |
| Serum | 5 | 1.394 (0.830–1.957) | 88 | |
| Definition | ||||
| Berlin | 6 | 1.464 (0.824–2.105) | 91 | 0.723 |
| PALICC | 2 | 1.859 (1.421–2.297) | 97 | 0.387 |
| AECC | 4 | 1.212 (0.029–2.395) | 94 |
a, the last categorical variate of the subgroup was used as the reference in the meta-regression analysis; b, an sTM level measured within 24 hours after the diagnosis of ARDS was define as early detection (baseline); otherwise, it was defined as late detection; c, the study by Orwoll separately reported sTM values in indirect, direct, and mixed types of ARDS; d, subgroup of follow-up >30 days comprised 60-day mortality and mortality in the intensive care unit or hospital; e, the mortality was divided into high and low using a 40% cutoff value. sTM, soluble thrombomodulin; ARDS, acute respiratory distress syndrome; ELISA, enzyme-linked immunosorbent assay; EIA, enzyme immunoassay; PALICC, Pediatric Acute Lung Injury Consensus Conference; AECC, American European Consensus Conference; SMD, standardized mean difference; CI, confidence interval.
According to the meta-regression analysis, detection time (early or late), patient age (adults or children), type of ARDS (indirect, direct, or mixed), cause of ARDS (sepsis or mixed), detection method (ELISA or EIA), follow-up time (short-term or long-term), mortality level (high or low), samples (plasma or serum), and definition of ARDS were not the source of heterogeneity (P>0.05). The analysis of the source of heterogeneity is presented in Table 2.
A funnel plot and Egger test were employed to determine publication bias, which indicated 6 studies were outside the funnel plot, and 4 studies were symmetrically distributed. The funnel plot of publication bias is depicted in Figure S1. Meanwhile, the Egger test revealed that the publication bias was not significant (P=0.297). The results of the Egger test are shown in Figure S2. Furthermore, when 2 studies were added with the trim-and-fill method, a similar main effect was observed (SMD =1.120; 95% CI: 0.414–1.827; P=0.002, I2=94%).
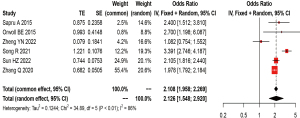
Elevated sTM and hospital mortality in patients with ARDS
Six studies analyzed the correlation between sTM and mortality in patients with ARDS by adjusted OR (10,13-15,24,26). Meta-analysis showed that elevated sTM levels had an independent correlation with higher ARDS mortality (pooled OR =2.126; 95% CI: 1.548–2.920; P<0.001) after adjustments were made for various confounding variables, including gender, age, illness severity score, and sepsis status. There was a large heterogeneity across these studies (I2=86%; P<0.01). The forest plot for the correlation between sTM and ARDS mortality is shown in Figure 3.
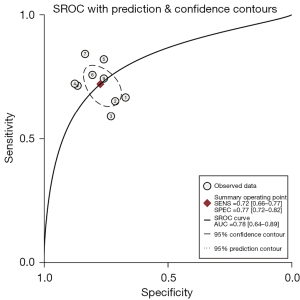
Predictive value of sTM for ARDS hospital mortality
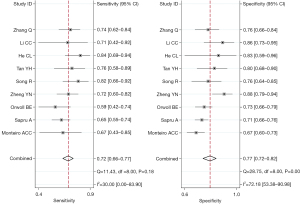
Nine studies investigated the performance of sTM in predicting ARDS mortality (11,13-15,23-27). The risk of bias mainly centered on participant and analysis domains (Table 3). The sTM showed satisfactory performance in predicting ARDS hospital mortality (SROC =0.78; 95% CI: 0.64–0.89). The SROC of sTM in predicting ARDS mortality is shown in Figure 4. The pooled sensitivity was 72% (95% CI: 66–77%; I2=30%), and the pooled specificity was 77% (95% CI: 72–82%; I2=72.18%). The pooled sensitivity and specificity of sTM in predicting ARDS mortality are shown in Figure 5. No publication bias was found (Figure S3).
Table 3
| Study | Participants | Index test | Outcome | Flow and timing | Analysis |
|---|---|---|---|---|---|
| Zhang Q 2020 | Low | Low | Low | Low | Low |
| Li CC 2020 | High | High | Low | Low | High |
| He CL 2021 | High | High | Low | Low | Low |
| Tan YH 2021 | High | Low | Low | Low | High |
| Song R 2021 | Low | Low | Low | Low | High |
| Zheng YN 2022 | Low | Low | Low | Low | High |
| Orwoll BE 2015 | Low | Low | Low | Low | Low |
| Sapru A 2015 | Low | Low | Low | Low | Low |
| Monteiro ACC 2021 | Low | Low | Low | Low | Low |
sTM, soluble thrombomodulin; QUAPAS, Quality Assessment of Prognostic Accuracy Studies.
Sensitivity analysis
Cohort studies have less potential bias than do case-control and cross-sectional studies. Hence, this sensitivity analysis only included cohort studies. The results showed that patients who died of ARDS had elevated sTM levels (SMD =1.377; 95% CI: 0.768–1.986; P<0.001; I2=93%). The results of sensitivity analysis without noncohort studies is shown in Figure S4. The OR values were extracted from 6 cohort studies. Removing 1 study at a time in the sensitivity analysis indicated that our analysis results were stable (Figure S5).
Discussion
The mortality of ARDS is still very high (nearly 40%) due to the complex, diversified, and poorly known molecular mechanism, which impedes precise prognosis and treatments (2). For the diagnosis of ARDS, risk stratification, and outcome prediction, biomarkers may be useful. Previous meta-analyses (30,31) have reported that soluble receptor for advanced glycation end-products (sRAGE), a marker for lung epithelial injury, and N-terminal probrain natriuretic-peptide (NT-ProBNP), a cardiac stretch marker, have a close correlation with the mortality of ARDS. Nonetheless, no comprehensive meta-analysis has evaluated the association between sTM and the mortality of ARDS.
Our meta-analysis demonstrated that the nonsurvivor group showed more significantly increased sTM levels than did the survivor group, suggesting that patients who died from ARDS had aggravated vascular injury, severely impaired anticoagulation and fibrinolysis, and serious disruption of the alveolar-capillary barrier. Moreover, elevated sTM levels had an independent correlation with increased ARDS mortality.
The mechanism for TM shedding has been reported on. The sTM is formed due to the proteolytic cleavage of proteases, including neutrophil-derived proteases, rhomboids, and metalloproteinases, which are released during vascular damage-related diseases, including inflammation, infection, and sepsis (9). Furthermore, oxygen radicals can rapidly induce endothelial cells to chemically release sTM. Thus, elevated circulating sTM is an indicator of the severity of endotheliopathy, mirroring worse lung intravascular thrombosis, increased vascular permeability and extravascular leakage, impaired microcirculation, and organ dysfunction. The multifactorial mechanism leads to the progressive deterioration of ARDS.
Our subgroup analysis showed no significant difference in the sTM levels in patients with direct ARDS (primary or pulmonary ARDS) between nonsurvivors and survivors. Direct and indirect lung injuries are two distinct subphenotypes of ARDS. The former features alveolar epithelial injury and local alveolar inflammation, whereas the latter is characterized by inflammatory mediator-induced systemic vascular endothelial damage (32). Existing evidence has demonstrated that patients with indirect ARDS have more significantly elevated sTM levels than do those with direct ARDS (13), suggesting that circulating sTM cannot reflect the severity of lung injury in direct ARDS. Therefore, caution is needed in using sTM to predict adverse outcomes in patients with direct ARDS. Detection time and the cause of ARDS are important considerations. The study by Sapru et al. (14) found an increased level of sTM on day 3 compared to day 1 and indicated that sTM had comparable performance in predicting ARDS mortality (AUC =0.72 for both baseline and day 3 sTM). Our meta-analysis revealed that the sTM levels, regardless of early or late measurement, are correlated with the mortality of ARDS and may be helpful for the early detection of ARDS in patients at a high risk of experiencing adverse outcomes from the perspective of clinical application. The major contributors to ARDS are pneumonia and nonpulmonary sepsis (3). Sepsis-related ARDS is characterized by a strong inflammation response to infection, substantial immune cell infiltration, and high mortality (33). Sepsis-related ARDS exhibits more significantly increased levels of sTM than those of ARDS induced by trauma or other causes (14). Our meta-analysis demonstrated that elevated sTM levels were correlated with higher mortality in sepsis-related and nonpulmonary sepsis-related ARDS. Furthermore, the multivariate logistic regression revealed that the association was independent of sepsis.
The bivariate analysis and SROC suggested that sTM had a moderate predictive performance for in-hospital mortality in ARDS. This finding highlights the fact that sTM alone cannot accurately predict the high risk of death in the mixed ARDS population. However, sTM, when combined with multidimensional variates such as clinical and multiomics data, is a potential candidate biomarker for predicting the prognosis of ARDS. Additionally, sTM may be a valuable indicator for subphenotyping ARDS because it significantly contributes to the pathogenesis of ARDS.
This study still has some limitations which should be noted. First, the heterogeneity among the studies was high, and this might be attributable to the variability in patient symptoms, lung injury types, measurement kits, and causes and severity of ARDS. However, except for the injury type, the subgroup analysis yielded consistent results across all groups and was corroborated by the sensitivity analysis. High intrasubgroup heterogeneity may imply a flawed grouping method based on clinical phenotype. Second, some included studies did not perform multivariate analysis or report the effect value of OR, so there was potential publication bias. Third, despite the fact that mortality rates often reflect the severity of diseases, the predictive value of sTM for mortality in patients with mild, moderate, or severe ARDS could not be fully elucidated due to a lack of available data. Finally, our meta-analysis did not include patients with COVID-19. COVID-19-related ARDS has longer-lasting hyperinflammation and a greater incidence of thrombosis than does traditional ARDS (34). Elevated sTM levels were reported in patients with COVID-19 (35-37), but these studies did not focus on patients with ARDS. Therefore, they were deemed ineligible for this meta-analysis.
Conclusions
sTM is associated with in-hospital mortality in ARDS and shows moderate predictive performance. Hence, it is a potential candidate for predicting the mortality of ARDS. However, caution is needed when sTM is used to predict adverse outcomes in patients with direct ARDS. Future investigations targeted toward the subphenotype of ARDS or COVID-19-related ARDS may benefit this population.
Acknowledgments
Funding: The study was funded by the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZYJC18006).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-432/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-432/prf
Conflicts of interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-432/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Pham T, Rubenfeld GD. Fifty Years of Research in ARDS. The Epidemiology of Acute Respiratory Distress Syndrome. A 50th Birthday Review. Am J Respir Crit Care Med 2017;195:860-70. [Crossref] [PubMed]
- Alipanah N, Calfee CS. Phenotyping in acute respiratory distress syndrome: state of the art and clinical implications. Curr Opin Crit Care 2022;28:1-8. [Crossref] [PubMed]
- Sivapalan P, Bonnesen B, Jensen JU. Novel Perspectives Regarding the Pathology, Inflammation, and Biomarkers of Acute Respiratory Distress Syndrome. Int J Mol Sci 2020;22:205. [Crossref] [PubMed]
- Whyte CS, Morrow GB, Mitchell JL, et al. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J Thromb Haemost 2020;18:1548-55. [Crossref] [PubMed]
- Kawanami O, Jin E, Ghazizadeh M, et al. Heterogeneous distribution of thrombomodulin and von Willebrand factor in endothelial cells in the human pulmonary microvessels. J Nippon Med Sch 2000;67:118-25. [Crossref] [PubMed]
- Watanabe-Kusunoki K, Nakazawa D, Ishizu A, et al. Thrombomodulin as a Physiological Modulator of Intravascular Injury. Front Immunol 2020;11:575890. [Crossref] [PubMed]
- Boron M, Hauzer-Martin T, Keil J, et al. Circulating Thrombomodulin: Release Mechanisms, Measurements, and Levels in Diseases and Medical Procedures. TH Open 2022;6:e194-212. [Crossref] [PubMed]
- Sun H, Sun H, Li Y. Relationship between serum levels of sTM,suPAR and Ang-2 and inflammatory factors and prognosis in patients with sepsis complicated with ARDS. Laboratory Medicine and Clinic (China) 2022;19.
- Monteiro ACC, Flori H, Dahmer MK, et al. Thrombomodulin is associated with increased mortality and organ failure in mechanically ventilated children with acute respiratory failure: biomarker analysis from a multicenter randomized controlled trial. Crit Care 2021;25:271. [Crossref] [PubMed]
- Benatti MN, Fabro AT, Miranda CH. Endothelial glycocalyx shedding in the acute respiratory distress syndrome after flu syndrome. J Intensive Care 2020;8:72. [Crossref] [PubMed]
- Orwoll BE, Spicer AC, Zinter MS, et al. Elevated soluble thrombomodulin is associated with organ failure and mortality in children with acute respiratory distress syndrome (ARDS): a prospective observational cohort study. Crit Care 2015;19:435. [Crossref] [PubMed]
- Sapru A, Calfee CS, Liu KD, et al. Plasma soluble thrombomodulin levels are associated with mortality in the acute respiratory distress syndrome. Intensive Care Med 2015;41:470-8. [Crossref] [PubMed]
- Zheng Y, Sha H, Jiang W. Value of E/Em combined with serum soluble thrombomodulin to the prediction of prognosis of sepsis patients complicated with acute respiratory distress syndrome. Journal of Chinese Practical Diagnosis and Therapy (China) 2022;36:283-7.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1-34. [Crossref] [PubMed]
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-24. [Crossref] [PubMed]
- Khemani RG, Smith LS, Zimmerman JJ, et al. Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015;16:S23-40. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Modesti PA, Reboldi G, Cappuccio FP, et al. Panethnic Differences in Blood Pressure in Europe: A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0147601. [Crossref] [PubMed]
- Lee J, Mulder F, Leeflang M, et al. QUAPAS: An Adaptation of the QUADAS-2 Tool to Assess Prognostic Accuracy Studies. Ann Intern Med 2022;175:1010-8. [Crossref] [PubMed]
- Tan Y, Yu B, Yu Q, et al. Changes of plasma soluble thrombomodulin and interleukin-22 in children with acute respiratory distress syndrome and their significances. Journal of Chinese Practical Diagnosis and Therapy (China) 2021;35:255-8.
- Song R, Qi H, Li X, et al. Study on the relationship between serum KL-6 and sTM levels and the condition and prognosis of patients with acute respiratory distress syndrome. Chinese Journal of Difficult and Complicated Cases (China) 2021;20.
- He C, Wang X, Liu R, et al. Application of serum vWF:Ag,Claudin-5 and sTM combined detection in early diagnosis and prognostic evaluation of children patients with ARDS. International Jounal of Lab Medicine (China) 2021;42.
- Zhang Q, Hu X. The predictive value of serum KL-6, sTM and CC-16 in patients with sepsis complicated with acute respiratory distress syndrome. Journal of Clinical Emergency (China) 2020;21:711-6.
- Li C, Wu L, Hu L. Prognostic value of plasma soluble thrombomodulin in children with pneumonia and acute respiratory distress syndrome. Chinese Journal of Clinical Research (China) 2020;33:638-41.
- Gando S, Kameue T, Matsuda N, et al. Systemic inflammation and disseminated intravascular coagulation in early stage of ALI and ARDS: role of neutrophil and endothelial activation. Inflammation 2004;28:237-44. [Crossref] [PubMed]
- McClintock D, Zhuo H, Wickersham N, et al. Biomarkers of inflammation, coagulation and fibrinolysis predict mortality in acute lung injury. Crit Care 2008;12:R41. [Crossref] [PubMed]
- Jabaudon M, Blondonnet R, Pereira B, et al. Plasma sRAGE is independently associated with increased mortality in ARDS: a meta-analysis of individual patient data. Intensive Care Med 2018;44:1388-99. [Crossref] [PubMed]
- Jayasimhan D, Foster S, Chang CL, et al. Cardiac biomarkers in acute respiratory distress syndrome: a systematic review and meta-analysis. J Intensive Care 2021;9:36. [Crossref] [PubMed]
- Chiu LC, Chuang LP, Lin SW, et al. Comparisons of Outcomes between Patients with Direct and Indirect Acute Respiratory Distress Syndrome Receiving Extracorporeal Membrane Oxygenation. Membranes (Basel) 2021;11:644. [Crossref] [PubMed]
- Ming T, Dong M, Song X, et al. Integrated Analysis of Gene Co-Expression Network and Prediction Model Indicates Immune-Related Roles of the Identified Biomarkers in Sepsis and Sepsis-Induced Acute Respiratory Distress Syndrome. Front Immunol 2022;13:897390. [Crossref] [PubMed]
- Batra R, Whalen W, Alvarez-Mulett S, et al. Multi-omic comparative analysis of COVID-19 and bacterial sepsis-induced ARDS. PLoS Pathog 2022;18:e1010819. [Crossref] [PubMed]
- Maldonado F, Morales D, Díaz-Papapietro C, et al. Relationship Between Endothelial and Angiogenesis Biomarkers Envisage Mortality in a Prospective Cohort of COVID-19 Patients Requiring Respiratory Support. Front Med (Lausanne) 2022;9:826218. [Crossref] [PubMed]
- Francischetti IMB, Toomer K, Zhang Y, et al. Upregulation of pulmonary tissue factor, loss of thrombomodulin and immunothrombosis in SARS-CoV-2 infection. EClinicalMedicine 2021;39:101069. [Crossref] [PubMed]
- Marchetti M, Gomez-Rosas P, Sanga E, et al. Endothelium Activation Markers in Severe Hospitalized COVID-19 Patients: Role in Mortality Risk Prediction. TH Open 2021;5:e253-63. [Crossref] [PubMed]



