
Efficacy of concentrated growth factor combined with grafting materials vs. grafting materials alone for the treatment of periodontal intrabony defects: a systematic review and meta-analysis
Highlight box
Key findings
• Concentrated growth factor (CGF) in combination with grafting materials is more effective than the use of grafting materials alone in the treatment of periodontal intrabony defects.
What is known and what is new?
• CGF is known to promote tissue regeneration.
• Our new finding is that CGF in combination with grafting materials improves tissue regeneration compared to grafting materials alone.
What is the implication, and what should change now?
• For the treatment of periodontal intrabony defects, CGF has a significant additive effect when combined with grafting materials. In the future, we can prioritize CGF in combination with grafting materials for the treatment of periodontal intrabony defects to achieve optimal therapeutic outcomes.
Introduction
Periodontitis, with the common symptoms of gingival bleeding and chewing weakness, is a prevalent inflammatory condition (1). Its typical pathologic feature is the destruction of periodontal connective tissue and bone, with the main outcome of tooth loss in humans (2). The teeth defects lead to poor mastication, which not only reduces the living quality of patients with periodontitis, but also impairs their speech and aesthetic functions, which inhibits their social function (3). Subgingival scaling and root planing (SRP) have been employed as the main way to eliminate the local periodontal irritation, which can first stop inflammation from developing. However, restoring the periodontal tissues to their original state is an ideal outcome that SPR alone cannot fundamentally address (4,5). Given the large proportion of periodontitis patients worldwide, the exploration of preferable treatment has long represented a hot research topic. In the 1980s, guided tissue regeneration (GTR) involved the establishment of a barrier membrane around the intrabony defects to prevent the interlacing growth of epithelial cells and fibroblasts, providing suitable conditions for periodontal tissue regeneration (6). However, it is not easy to completely regenerate periodontal tissue due to inherent defects under the barrier membrane (7).
With the development of biomaterials, periodontal flap surgery combined with autologous bone, allograft bone, and xenografts has become a common clinical method. These tissues can support the growth and maturation of blood clots. However, the success of bone induction is still unpredictable (8,9). Exploring a valuable approach is critical to achieve the goal of optimal treatment of periodontal intrabony defects.
Endogenous regeneration technology has received increasing attention in recent studies focusing on functional regeneration of non-renewable tissues based on the autologous ‘regeneration agents’ (10,11). Autologous platelet concentrates (APCs) is an imperative candidate for endogenous regeneration technology. APCs, with their superior properties and convenient preparation, are widely used in all areas of oral therapy through the generation transition from platelet rich plasma (PRP) to platelet rich fibrin (PRF) (12,13).
Concentrated growth factor (CGF) is the third generation and is produced by the variable speed centrifugation. With higher fibrinogen matrix density and growth factor levels compared to the previous two generations (14), CGF makes it possible to promote true tissue regeneration. A previous study has proved that CGF have a role in promoting differentiation and growth of cells involved in the periodontal healing (15). However, it has also been claimed that adding CGF has no greater benefits in the treatment of periodontal intrabony defects than using grafting materials alone (16,17). A growing number of clinicians are choosing to use CGF in combination with grafting materials to promote tissue regeneration. To date, no evidence-based recommendations regarding CGF combined with grafting materials have more clinical advantages than grafting materials alone has been carried out. The purpose of this meta-analysis was to quantitatively assess all published randomized controlled trials (RCTs) and evaluate whether adding CGF could yield additional clinical benefits based on grafting materials, and determine CGF important for future treatment of periodontal intrabony defects. We present the following article in accordance with the PRISMA reporting checklist (18) (available at https://atm.amegroups.com/article/view/10.21037/atm-23-891/rc).
Methods
Protocol and registration
The study was performed according to the Cochrane Handbook for Systematic Reviews of Interventions (19). The registration number of literature in PROSPERO is CRD42022319325.
Search strategy
The databases of PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), China Biology Medicine Disc (CBM), Wanfang, and VIP were searched by 2 reviewers (MY and JH) for RCTs in English or Chinese that had been published by the end of June 2022. Search items were based on the combination of keywords and medical subject headings (MeSH), including ‘Periodontal disease’, ‘Periodontitis’, ‘intrabony defect*’, ‘infrabony defect*’, ‘bone defect*’, ‘osseous defect*’, ‘periodontal defect*’, ‘periodontal atrophy’, ‘Bone regeneration’, ‘concentrated growth factor*’, and ‘CGF’. Then, two authors (MY and JH) independently searched the electronic databases and retrieved the relevant studies. Article titles and abstracts were screened following removal of duplicates. After excluding the non-related literature, the remaining full texts were read for re-screening in order to determine the final included studies. Reference lists of included studies, conference materials, and unpublished studies were also searched for potential eligible studies.
Inclusion and exclusion criteria
The following people, intervention, comparison, outcomes, and study (PICOS) standards were applied: participants (P) were people who had periodontal intrabony defects confirmed by imaging and conformed to the indications of periodontal surgery; intervention (I) was the CGF combined with grafting materials as applied in the test group; comparison (C) was the single use of grafting materials in the control group; primary outcomes (O) included probing depth (PD) and clinical attachment loss (CAL), and secondary outcomes included gingival recession (REC) and bone filling (BF). The BF consists of the distance from the apex of the alveolar ridge defect to the bottom of the alveolar bone defect (A-B), the distance from the cementum-enamel junction to the apex of the alveolar bone defect (CEJ-A), and the distance from the cementum-enamel junction to the bottom of the alveolar bone defect (CEJ-B). The study (S) included only published RCTs.
The inclusion criteria were as follows: (I) RCTs include CGF combined with bone grafting materials compared to the single use of grafting materials; (II) periodontal intrabony defects were confirmed with clinical examination and imaging; (III) the outcomes included at least the primary outcome.
The exclusion criteria were as follows: (I) animal and in vitro studies; (II) case report, reviews, and conference summaries; (III) studies for which data were not available.
Data extraction
The 2 reviewers (MY and JH) conducted literature screening and data extraction independently, and any disagreements were resolved through discussion (LJ, RG, and XW). Data extraction included the following: (I) basic information about the included studies; (II) basic characteristics of participants; (III) interventions and follow-up; (IV) key elements of risk of bias assessment; and (V) primary clinical and imaging outcome measurement.
Risk of bias assessment
The Cochrane Collaboration’s risk of bias assessment tool (Cochrane RoB 2.0) (20) was used to assess the quality of 8 RCTs. The items of Cochrane tool included: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. The risk of bias in each item can be classified into three categories: low, high, and unclear risk of bias. If risk of bias in all items turns out to be “low” then the study is low risk of bias. If the risk of bias in some items is assessed as “unclear” and there is no “high”, then the study is unclear risk of bias. As long as one item of risk of bias assessment results “high” then the study is high risk.
Statistical analysis
The software Stata 15.0 (StataCorp., LLC, College Station, TX, USA) was employed for the meta-analysis. Weighted mean difference (WMD) with the 95% confidence interval (95% CI) was applied as pooled statistics for PD, CAL, REC, and BF. Heterogeneity testing was processed by chi-square test (α=0.1), and I2 statistics was verified to evaluate the heterogeneity. P value was two-sided and <0.05 was significant. A fixed-effect model was applied if I2<50%. When I2>50%, random-effect models were used and the source of heterogeneity was analyzed. Subgroup analysis was used to investigate the source of high heterogeneity. Sensitivity analysis was performed to determine the stability of the results. Publication bias was summarized if more than 10 articles were included in the study.
Results
Included studies
There were 570 articles identified, and 416 retrieved after removing duplicates. Through screening titles and abstracts, 57 articles remained, and after full-texts screening, 8 studies were finally included. There were 303 intrabony defect sites in 252 patients. Figure 1 depicts the literature screening process, and Table 1 depicts the basic characteristics of the articles included.
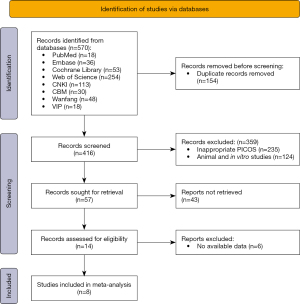
Table 1
| Study | Sample size (female/male) | Age (years; mean ± SD or range) | Intervention | Site (T/C) | Follow-up (months) | Outcome indicator | |
|---|---|---|---|---|---|---|---|
| T | C | ||||||
| Xu 2019 (21) | 58 (32/26) | 55.2±8.3 | CGF + Bio-Oss® | Bio-Oss® | 30/30 | 6, 12 | PD, CAL |
| Qiao 2016 (22) | 17 (8/9) | 47.7±13.9 | CGF + Bio-Oss® | Bio-Oss® | 15/16 | 12 | PD, CAL, REC, CEJ-A, CEJ-B, A-B |
| Zhao 2021 (23) | 30 (13/17) | T: 36.36±5.50 C: 37.31±4.24 |
CGF + DFDBA | DFDBA | 14/16 | 6 | PD, CAL, A-B, CEJ-A |
| Xu 2020 (24) | 57 (22/35) | 32.9±1.28 | CGF + Bio-Oss® | Bio-Oss® | 23/23 | 6, 12 | PD, CAL, A-B |
| Qiao 2017 (25) | 15 (ND) | 20–60 | CGF + Bio-Oss® | Bio-Oss® | 16/15 | 12 | PD, CAL, REC |
| Zhang 2021 (26) | 39 (ND) | 20–55 | CGF + Bio-Oss® | Bio-Oss® | 20/20 | 6, 12 | PD, CAL |
| Li 2020 (27) | 16 (ND) | 20–60 | CGF + Bio-Oss® | Bio-Oss® | 17/18 | 12 | PD, CAL, REC |
| Hu 2018 (28) | 20 (ND) | 25–60 | CGF + Bio-Oss® | Bio-Oss® | 15/15 | 12 | PD, CAL |
ND, no data; SD, standard deviation; T, test group; C, control group; CGF, concentrated growth factor; Bio-Oss®, deproteinated bovine-derived xenograft products; DFDBA, demineralized freezedried bone allograft; PD, probing depth; CAL, clinical attachment loss; REC, gingival recession; CEJ-A, distance from the CEJ to the most coronal extension of the alveolar bone crest; CEJ-B, distance from the CEJ to the base of the defect; A-B, defined as CEJ-A-CEJ-B.
Risk of bias
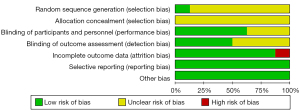
Based on the Cochrane Collaboration’s tool, a total of 8 RCTs (21-28) were evaluated. Only 1 of the studies (25) employed randomized digital table method. There was no mention of allocation hiding in any of the articles. A total of 5 studies (21,22,26-28) used the double-blind method, four reported blinding outcome assessment. One study (21) reported 4 cases lost to follow-up, and all studies had no selective reporting bias. Figure 2 depicts the results of the assessment. One article showed a high overall risk of bias due to loss of follow-up, while the rest showed an unclear overall risk of bias, as shown in Figure 3.
Results of meta-analysis
Among all the included RCTs, CGF combined with grafting materials was set as the test group, whereas single use of grafting materials was set as the control group. A further two subgroups were set based on follow-up duration (6 and 12 months after surgery). The short-term and long-term effects were evaluated respectively.
PD
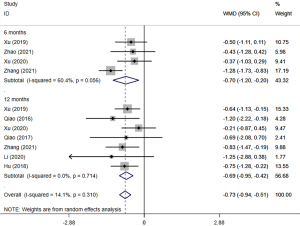
All 8 studies (21-28) evaluated changes of PD. The meta-analysis based on random-effect model (P=0.31, I2=14.1%) indicated that the test group was greater than the control group (WMD =−0.73, 95% CI: −0.94, −0.51, P=0.005). Meanwhile, the short-term effect (WMD =−0.70, 95% CI: −1.20, −0.20, P=0.006) and long-term effect (WMD =−0.69, 95% CI: −0.95, −0.42, P=0.001) were consistent with the results (Figure 4).
CAL
All 8 studies (21-28) evaluated improvements of CAL. The meta-analysis based on random-effect model (P=0.02, I2=52.7%) indicated that the CAL of the test group was greater than that of the control group (WMD =−0.56, 95% CI: −0.94, −0.19, P=0.003). In long-term effect, CAL was greater in test group (WMD =−0.68, 95% CI: −1.16, −0.19, P=0.007), but there was no statistically significant difference between the two groups in the short-term effect (WMD =−0.40, 95% CI: −1.05, 0.26, P=0.234) (Figure 5).

REC
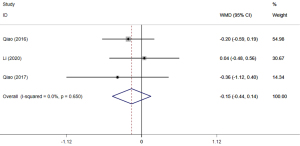
There were 3 studies (22,25,27) that reported the REC at 12 months after surgery. According to the meta-analysis, no statistically significant difference was found between the two groups (WMD =−0.15, 95% CI: −0.44, 0.14, P=0.312) (Figure 6).
BF
In addition to 1 study (23) that reported the BF at 6 months after surgery, 2 other studies (22,24) reported bone defect recovery at the 12th month post-surgery. The results were divided into three subgroups, depending on the content of evaluation. Meta-analysis based on the fixed-effect model (P=0.25, I2=23.9%) indicated that A-B, CEJ-A, and CEJ-B recovered in the test group more than the controls (WMD =−0.43, 95% CI: −0.65, −0.21, P=0.001) (Figure 7).

Sensitivity analysis and publication bias
Sensitivity analysis of the primary outcomes PD and CAL were performed. By eliminating 1 article at a time, we found little change in the merged results of the remaining papers, indicating that the results were stable (Figure 8). Since the number of included studies was 8 only, we did not conduct the publication bias.

Discussion
Summary of evidence
Periodontitis-induced intrabony defects remain a major challenge to periodontal treatment and the question of how to achieve predictable tissue regeneration is still unanswered (29). What seems certain is that the application of APCs provides a new direction for functional regeneration of periodontal tissues (30,31). CGF is not constant centrifugation, but centrifugation from the autologous venous blood at variable velocities (2,400 to 3,000 rpm) (32). A previous study has shown that CGF had greater tensile strength and induces the growth of osteoblasts and gingival fibroblasts than second generation PRF (33). Previous studies have found that CGF has advantages in expediting wound healing and promoting tissue formation when used to treat hollowing following tooth extraction (34,35). Moreover, the bone mineral density and trabecular architecture recovery of tissues after the addition of CGF were better than those without CGF (36,37).
Although most studies have shown that CGF can improve tissue regeneration efficiency, a study has indicated that CGF has no clinical advantage in promoting periodontal tissue recovery (38). After all, the use of CGF increases the financial burden on patients to some extent. The present meta-analysis evaluated the efficacy of CGF in combination with grafting materials and grafting materials alone for periodontal intrabony defects based on published RCTs. The results showed that the combination was more effective in PD, CAL, and BF improvement, providing a reference for the selection of a clinical treatment that achieves the desired outcome. However, due to the lack of clarity in the risk bias assessment of most RCTs, indicating the average quality of RCTs, caution is needed in interpreting the findings of this study.
In view of the superior structural properties of CGF, the addition of CGF not only saved the amount of grafting materials, but also provided a fiber scaffold for stem cell migration and new tissue growth (39). Fang et al. indicated that CGF induces durable osteogenesis in jaw defects (40). This may be a consequence of a large amount of growth factors, such as transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF), which contribute to the expansion of new tissue (41,42). These growth factors attach to a dense network of fibrinous scaffolds, not only protecting growth factors from early proteolysis, but also decelerating the release of growth factors, thus ensuring optimal outcomes for wound healing in the early- and long-term (43-45). However, based on the available research, the current findings are limited to Asian populations.
In PD, CAL, and BF, we found that the test group was more effective than the control group at the post-surgical 12th month, whereas there was no statistical difference in CAL between two groups at the 6th month. This outcome appears to influence the type of bone wall defects. It is well known that the repair of intrabony defects depends not only on the regenerative potential of residual tissue, but also on the local characteristics of the defect, such as the complexity of periodontal pocket and the number of bone walls (46,47). A previous study has shown that only 1-wall defects respond well to tissue regeneration surgery, whereas 2- and 3-wall defects do not have such high tissue regeneration due to excessive tissue collapse (48). The study included different types of wall defects, which may explain the uncertain clinical outcome.
Vaid et al. reported that fresh bone was detectable in the CGF group with or without grafting materials at the 6th month after surgery, and the formation of fresh bone was not significant between two groups (49). These results may suggest that the single use of CGF could achieve the same clinical benefit as combination of grafting materials in some cases, providing evidence for future use of CGF alone, thus saving the use of grafting materials. In our meta-analysis, we found no significant difference in the treatment of REC between CGF combination and individual grafting materials. The results are consistent with previous studies and indicate that CGF does not provide additional benefit in the treatment of REC (50). However, due to the limited number of included articles, the outcome of REC treatment should be interpreted with caution.
Currently, the clinical CGF has two forms of adaptation: 1 is to mix the clot with grafting materials and put it into the defect, and the other is to press CGF into the film and then cover the defect surface (51). The membrane forms a barrier for rapid tissue repair in the intrabony defects area. Its barrier function in epithelial cells could influence the ultimate clinical outcome (52). Therefore, when CGF is applied, the format should also be considered.
Limitations
In the present study, the random sequence generation and allocation concealment in some of the original articles are not detailed enough, so the potential risk of bias persisted. In addition, only vertical tissue regeneration has been pooled due to the limitation of original studies. Actually, tissue reparation of intrabony defects should be evaluated from 3 dimensions. Further detailed and high-quality studies are needed to explore the stability of CGF after combining with grafting materials in the treatment of periodontal intrabony defects.
Conclusions
In our current meta-analysis, CGF combined with grafting materials is more effective than grafting materials alone in Asian patients. Its advantages are mainly in PD and CAL reduction, and BF gain.
Acknowledgments
Funding: This study was supported by the Scientific and Technological Foundation of Gansu (Nos. 20YF8FA073, 20JR10FA670) and the Lanzhou University Stomatology Fund (No. lzukqky-2019-t10).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-891/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-891/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-891/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Miani PK, do Nascimento C, Sato S, et al. In vivo evaluation of a metronidazole-containing gel for the adjuvant treatment of chronic periodontitis: preliminary results. Eur J Clin Microbiol Infect Dis 2012;31:1611-8. [Crossref] [PubMed]
- Baelum V, López R. Periodontal disease epidemiology - learned and unlearned? Periodontol 2000 2013;62:37-58. [Crossref] [PubMed]
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005;366:1809-20. [Crossref] [PubMed]
- Susin C, Fiorini T, Lee J, et al. Wound healing following surgical and regenerative periodontal therapy. Periodontol 2000 2015;68:83-98. [Crossref] [PubMed]
- Donos N, Dereka X, Calciolari E. The use of bioactive factors to enhance bone regeneration: A narrative review. J Clin Periodontol 2019;46:124-61. [Crossref] [PubMed]
- Villar CC, Cochran DL. Regeneration of periodontal tissues: guided tissue regeneration. Dent Clin North Am 2010;54:73-92. [Crossref] [PubMed]
- Bosshardt DD, Sculean A. Does periodontal tissue regeneration really work? Periodontol 2000 2009;51:208-19. [Crossref] [PubMed]
- Chen FM, Zhang J, Zhang M, et al. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials 2010;31:7892-927. [Crossref] [PubMed]
- Xu XY, Li X, Wang J, et al. Concise review: periodontal tissue regeneration using stem cells: strategies and translational considerations. Stem Cells Transl Med 2019;8:392-403. [Crossref] [PubMed]
- Anitua E, Sánchez M, Orive G. Potential of endogenous regenerative technology for in situ regenerative medicine. Adv Drug Deliv Rev 2010;62:741-52. [Crossref] [PubMed]
- Bartold PM, McCulloch CA, Narayanan AS, et al. Tissue engineering: a new paradigm for periodontal regeneration based on molecular and cell biology. Periodontol 2000 2000;24:253-69. [Crossref] [PubMed]
- Mijiritsky E, Assaf HD, Peleg O, et al. Use of PRP, PRF and CGF in Periodontal Regeneration and Facial Rejuvenation-A Narrative Review. Biology (Basel) 2021;10:317. [Crossref] [PubMed]
- Chou TM, Chang HP, Wang JC. Autologous platelet concentrates in maxillofacial regenerative therapy. Kaohsiung J Med Sci 2020;36:305-10. [Crossref] [PubMed]
- Masuki H, Okudera T, Watanebe T, et al. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int J Implant Dent 2016;2:19. [Crossref] [PubMed]
- Chen X, Wang J, Yu L, et al. Effect of Concentrated Growth Factor (CGF) on the Promotion of Osteogenesis in Bone Marrow Stromal Cells (BMSC) in vivo. Sci Rep 2018;8:5876. [Crossref] [PubMed]
- Döri F, Huszár T, Nikolidakis D, et al. Effect of platelet-rich plasma on the healing of intra-bony defects treated with a natural bone mineral and a collagen membrane. J Clin Periodontol 2007;3:254-61. [Crossref] [PubMed]
- Döri F, Arweiler N, Húszár T, et al. Five-year results evaluating the effects of platelet-rich plasma on the healing of intrabony defects treated with enamel matrix derivative and natural bone mineral. J Periodontol 2013;11:1546-55. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [Crossref] [PubMed]
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3. Cochrane, 2022. Available online: www.training.cochrane.org/handbook
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Xu Y, Qiu J, Sun Q, et al. One-Year Results Evaluating the Effects of Concentrated Growth Factors on the Healing of Intrabony Defects Treated with or without Bone Substitute in Chronic Periodontitis. Med Sci Monit 2019;25:4384-9. [Crossref] [PubMed]
- Qiao J, Duan J, Zhang Y, et al. The effect of concentrated growth factors in the treatment of periodontal intrabony defects. Future Sci OA 2016;2:FS136. [Crossref] [PubMed]
- Zhao J, Wang Y, Han S. Clinical evaluation of demineralized freeze dried bone allografts in combination with concentrated growth factor in the treatment of periodontal intrabony defects. Chinese Journal of Practical Stomatology 2021;14:166-70.
- Xu H, Zhang G, He F, et al. Application of concentrated growth factor combined with guided tissue regeneration in the treatment of periodontal infra-bony defects. Journal of Practical Hospital Clinic 2020;14:8-11.
- Qiao J, Duan J, Chu Y, et al. Effect of concentrated growth factors on the treatment of degree II furcation involvements of mandibular molars. Journal of Peking University 2017;49:36-42. (Health Sciences).
- Zhang M, Zhou G, Chang L, et al. Clinical study of the comprehensive treatment of severely damaged mandibular molars with type II furcation involvement. Beijing Journal of Stomatology 2021;29:27-31.
- Li F, Qiao J, Duan J, et al. Effect of concentrated growth factors combined with guided tissue regeneration in treatment of class II furcation involvements of mandibular molars. Journal of Peking University 2020;52:346-52. (Health Sciences). [Crossref] [PubMed]
- Hu Y, Yu X, Han X, et al. Effect of Guided bone regeneration using concentrated growth factors on the treatment of degree II furcation involvements of mandibular molars. General Journal of Stomatology 2018;5:60-2.
- Cortellini P, Tonetti MS. Clinical concepts for regenerative therapy in intrabony defects. Periodontol 2000 2015;68:282-307. [Crossref] [PubMed]
- Talaat WM, Ghoneim MM, Salah O, et al. Autologous Bone Marrow Concentrates and Concentrated Growth Factors Accelerate Bone Regeneration After Enucleation of Mandibular Pathologic Lesions. J Craniofac Surg 2018;29:992-7. [Crossref] [PubMed]
- Xu J, Gou L, Zhang P, et al. Platelet-rich plasma and regenerative dentistry. Aust Dent J 2020;65:131-42. [Crossref] [PubMed]
- Marchetti E, Mancini L, Bernardi S, et al. Evaluation of Different Autologous Platelet Concentrate Biomaterials: Morphological and Biological Comparisons and Considerations. Materials (Basel) 2020;13:2282. [Crossref] [PubMed]
- Lee HM, Shen EC, Shen JT, et al. Tensile strength, growth factor content and proliferation activities for two platelet concentrates of platelet-rich fibrin and concentrated growth factor. J Dent Sci 2020;15:141-6. [Crossref] [PubMed]
- Kamal A, Salman B, Abdul Razak NH, et al. The Efficacy of Concentrated Growth Factor in the Healing of Alveolar Osteitis: A Clinical Study. Int J Dent 2020;2020:9038629. [Crossref] [PubMed]
- Ma F, Lin Y, Sun F, et al. The impact of autologous concentrated growth factors on the alveolar ridge preservation after posterior tooth extraction: A prospective, randomized controlled clinical trial. Clin Implant Dent Relat Res 2021;23:579-92. [Crossref] [PubMed]
- Pirpir C, Yilmaz O, Candirli C, et al. Evaluation of effectiveness of concentrated growth factor on osseointegration. Int J Implant Dent 2017;3:7. [Crossref] [PubMed]
- Durmuşlar MC, Balli U, Dede FÖ, et al. Histological Evaluation of the Effect of Concentrated Growth Factor on Bone Healing. J Craniofac Surg 2016;27:1494-7. [Crossref] [PubMed]
- Isler SC, Soysal F, Ceyhanlı T, et al. Regenerative surgical treatment of peri-implantitis using either a collagen membrane or concentrated growth factor: A 12-month randomized clinical trial. Clin Implant Dent Relat Res 2018;20:703-12. [Crossref] [PubMed]
- Rochira A, Siculella L, Damiano F, et al. Concentrated Growth Factors (CGF) Induce Osteogenic Differentiation in Human Bone Marrow Stem Cells. Biology (Basel) 2020;9:370. [Crossref] [PubMed]
- Fang D, Long Z, Hou J. Clinical Application of Concentrated Growth Factor Fibrin Combined With Bone Repair Materials in Jaw Defects. J Oral Maxillofac Surg 2020;78:882-92. [Crossref] [PubMed]
- Yu M, Wang X, Liu Y, et al. Cytokine release kinetics of concentrated growth factors in different scaffolds. Clin Oral Investig 2019;23:1663-71. [Crossref] [PubMed]
- Rodella LF, Favero G, Boninsegna R, et al. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc Res Tech 2011;74:772-7. [Crossref] [PubMed]
- Park HC, Kim SG, Oh JS, et al. Early Bone Formation at a Femur Defect Using CGF and PRF Grafts in Adult Dogs: A Comparative Study. Implant Dent 2016;25:387-93. [Crossref] [PubMed]
- Isobe K, Watanebe T, Kawabata H, et al. Mechanical and degradation properties of advanced platelet-rich fibrin (A-PRF), concentrated growth factors (CGF), and platelet-poor plasma-derived fibrin (PPTF). Int J Implant Dent 2017;3:17. [Crossref] [PubMed]
- Christgau M, Moder D, Hiller KA, et al. Growth factors and cytokines in autologous platelet concentrate and their correlation to periodontal regeneration outcomes. J Clin Periodontol 2006;33:837-45. [Crossref] [PubMed]
- Isler SC, Soysal F, Ceyhanlı T, et al. Efficacy of concentrated growth factor versus collagen membrane in reconstructive surgical therapy of peri-implantitis: 3-year results of a randomized clinical trial. Clin Oral Investig 2022;26:5247-60. [Crossref] [PubMed]
- Zhang Y, Tangl S, Huber CD, et al. Effects of Choukroun's platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: a histological and histomorphometric study. J Craniomaxillofac Surg 2012;40:321-8. [Crossref] [PubMed]
- Lei L, Yu Y, Han J, et al. Quantification of growth factors in advanced platelet-rich fibrin and concentrated growth factors and their clinical efficacy as adjunctive to the GTR procedure in periodontal intrabony defects. J Periodontol 2020;91:462-72. [Crossref] [PubMed]
- Vaid T, Kumar S, Mehta R, et al. Clinical and radiographic evaluation of demineralized freeze-dried bone allograft with concentrated growth factor versus concentrated growth factor alone in the treatment of intrabony defects. Med Pharm Rep 2021;94:220-8. [Crossref] [PubMed]
- Bozkurt Doğan Ş, Öngöz Dede F, Ballı U, et al. Concentrated growth factor in the treatment of adjacent multiple gingival recessions: a split-mouth randomized clinical trial. J Clin Periodontol 2015;42:868-75. [Crossref] [PubMed]
- Honda H, Tamai N, Naka N, et al. Bone tissue engineering with bone marrow-derived stromal cells integrated with concentrated growth factor in Rattus norvegicus calvaria defect model. J Artif Organs 2013;16:305-15. [Crossref] [PubMed]
- Tayşi M, Atalay B, Çankaya B, et al. Effects of single- and double-layered resorbable membranes and platelet-rich fibrin on bone healing. Clin Oral Investig 2018;22:1689-95. [Crossref] [PubMed]
(English Language Editor: J. Jones)



