Pafolacianine, the magic wand of intraoperative imaging of folate-receptor positive ovarian cancer
Introduction
Pafolacianine was approved by the Food and Drug Administration on November 29, 2021, as an optical imaging agent for use as intraoperative adjunct for identification of cancerous lesions in adult ovarian cancer patients. More recently, on December 16, 2022, pafolacianine was also approved for intraoperative identification of lesions in lung cancer patients. Pafolacianine is a fluorescent imaging agent that consists of a folate analog conjugated with a near-infrared (NIR) cyanine dye, S0456, through an amino acid linker. It targets folate receptor alpha (FRα) that overexpresses in many tumors including, lung and ovarian cancers. With cancer being one of the leading causes of death worldwide, and the ever-increasing need for better cancer therapeutic and diagnostic tools, surgical removal of cancerous tumors remains one of the most important treatments. Real time identification and visualization of cancerous lesions during surgical resection of folate receptor positive tumors promise to improve the effectiveness of the surgery outcomes. Moreover, pafolacianine also seems to encourage the ongoing endeavors in developing folate receptor targeted cancer therapeutics and diagnostics.
Folate receptors (FR)
Folate or folic acid, also known as vitamin B9, is commonly taken as a vitamin supplement. An adequate intake of folic acid/folate is necessary for one carbon reactions that include folate cycles, which contribute to DNA synthesis and repair by providing one-carbon methyl units. Folate deficiency on the other hand, is associated with fetal nerve disorders and certain cardiovascular diseases (1). Folate absorption into the cells occurs partially through diffusion, given the anionic and hydrophilic nature of folate, and predominantly through the surface membrane transporters. There are three distinct transporters that are associated with folate intake: reduced folate carriers (RFC) that transport reduced form of folic acid; proton coupled high-affinity transporters that transport in acidic environments employing the proton gradient across the membrane, hence the major folate transporter in intestinal tract (2). Thirdly, and perhaps the most significant of all these transporters is the folate receptor, a high-affinity folate binding protein, that has attracted tremendous interest that far surpasses its role of being a folate transporter (1,2).
Folate receptor is the surface membrane bound, glycosylphosphatidylinositol (GPI)-linked protein that binds the folic acid and folate conjugates with high affinity and transports the folate or folate conjugates via receptor-mediated endocytosis (3). Once inside the cell, the acidic environment of the endosome allows the release of folate from the receptor to be subsequently transported into cytoplasm by proton coupled folate transporters (3,4). FR, with apparent molecular weight of 35–45 kDa have three different isoforms namely FRα, FRβ, and FRγ. The α and β variants of FR, predominantly expressed in human tissues are linked to surface GPI. While γ variant is only expressed in hematopoietic stem cells without the GPI component, which makes them soluble unlike the α and β variants (4). Generally, these FR are expressed in those adult human tissues that are associated with embryonic development i.e., placenta and neural tubes, and folate resorption as in kidneys to prevent the loss through urine, since most of the healthy cells uptake reduced form of folate via RFC (3,4). Among the different isoforms of FR, FRα is reported to be the most expressed form and is particularly overexpressed in malignant tumors. In normal adult human tissues, FRα is also expressed in pneumocytes of lungs and polarized epithelial cells of ovary, uterus, fallopian tubes, salivary and bronchial glands, and the retinal pigment epithelial cells (2,5,6). FRβ has also been reported to express in CD34+ cells but has considerably low affinity for folic acid and folate derivatives (5). FRα significantly overexpresses in certain cancers including breast, ovarian, cervical, lung, brain, kidney and endometrial cancers. Most of the cancers with overexpression of FRα tend to be those of epithelial origin (1). Since overexpression of FR is an important feature of many cancer tissues, several studies have been conducted regarding the possible role of FR in tumor cell proliferation and tumor development. One of the studies suggest that following the folate uptake by FRα, the receptor protein then translocates to the nucleus and binds with regulatory elements to act as transcription factor (1,7).
Folate receptor targeted therapeutic and diagnostic agents
The significant overexpression of FR in cancerous tumors together with the ability of FR to retain their binding affinity for folate even if the folate is conjugated with other molecules, makes FR an attractive target for the cancer therapeutic and diagnostic tools. The Holy Grail of cancer therapeutics is the highly specific and targeted delivery of drugs to the cancer location such that it does not affect other healthy tissues along the way. In general, virtually all of the drug target proteins subject to cancer treatment drugs, are also expressed in critical body organs accessible by blood stream (2). In contrast, the expression of FRα is considerably low and inaccessible in normal tissues while it is considerably high and accessible in the tumor tissues. This feature suggests low toxicity for FRα targeted cancer therapeutics compared to conventional chemotherapy agents. Furthermore, targeting the FRα would not hinder the normal uptake of folate in healthy tissues given the other folate transporters responsible for folate uptake e.g., RFC (1,2).
In the area of folate conjugated FRα targeted drugs, one of the most prominent examples of folate conjugated anti-cancer drug is vintafolide, also known as EC145, which is a folate conjugated with microtubule destabilizing agent, vinblastine called desacetylvin-blastinemonohydrazide (DAVLBH). Vintafolide, a water-soluble conjugate selectively delivers the cytotoxic drug DAVLBH to the FRα that are overexpressed in tumors (1,4,8). A prerequisite of FR targeted drug application, for instance that of vintafolide in ovarian cancer patient, is the detection and more so the quantification of FR expression. In this regard, conventional tissue sampling methods e.g., biopsies may have limited applications as the sample is usually taken form single lesion, and the cancer may have heterogenous FRα in different lesions. Moreover, biopsy may not be the ideal diagnostic tool because of the invasive nature of the procedure. A solution to this challenge has been developed in the form of radioisotope labeled folate conjugates for whole body imaging as in a PET scan. One of the prominent FR targeting folate radioconjugate imaging agent is Etarfolatide also known as EC20 (9). Etarfolatide is composed of 99mtechnetium (Tc) complex linked to folic acid via a non-cleavable peptide linker. 99mTc, a well-known radionuclide used in diagnostic nuclear medicine has a half-life of 6 h and emit gamma radiations as result of radioactive decay. Phase II studies of 99mTc-etarfolatide has suggested that this FR targeted radio imaging can be used to determine the likelihood of ovarian or lung cancer to respond to the FRα targeted therapy, for instance, vintafolide (1,9). However, it is important to note that folate radioconjugates, e.g., 99mTc-etarfolatide cannot be used as intraoperative imaging tool because of the limitation of use of gamma camera and the hazards of gamma exposure.
Unmet clinical need for better intraoperative imaging tools
Among all the cancer treatment methods, surgical resection of the tumor remains one of the most important and effective method of treating cancer. The effectiveness of the tumor surgery, which is directly linked to the survival rate and life expectancy of cancer survivors, depends on the extent and accuracy of the identification of the cancerous lesions during the surgery. There are few conventional methods that are employed to achieve this target. First method is the reliance on preoperative imaging methods e.g., the use FR targeting radiographical imaging tools i.e., 99mTc-etarfolatide (9) which targets the FRα positive ovarian and lung cancers and allows the precise detection of the cancerous lesions by the detection of gamma emission (9). However there are obvious limitations to the translation of preoperative imaging results to that in the operation theatre (10). Secondly, most of the cancer tumors and nodes are visually distinguishable based on their distinct color, texture, morphology, elasticity and solidity form the surrounding healthy tissue. Very often surgeons rely on palpation in addition to visual observations. Another method is the use rapid intraoperative histopathological assessment (11). With a certain disconnect between preoperative imaging methods and significant limitations of visual or other intraoperative methods, there has been a huge need for the development of a sophisticated intraoperative tumor detection/visualization method that allows the compete resection of cancerous lesions without leaving positive margins. Positive margins left after the surgery are associated with recurrence and lacking prognosis (11).
The advent of near infrared fluorescence along with the advanced imaging tools has made fluorescence a desirable method to visualize the tumors tissues, which might otherwise be indistinguishable with conventional methods. Perhaps the most prominent examples of approved fluorophores for surgical purposes after the indocyanine green (ICG) are 5-aminolevulinic acid (5-ALA) for brain cancer and pafolacianine for ovarian cancer surgery (11).
Significance of pafolacianine for ovarian cancer surgery
Ovarian cancer makes up to around 2.5% of all the cancers among female patients, and accounts for 5% of cancer related deaths in women (12). Compared to breast cancer, which is perhaps the single most prevalent cancer among women, ovarian cancer is less common, but it accounts for much greater number of deaths given the statistics that almost 75% of the patients with advanced stage cancer experience recurrence even after surgical resection and chemotherapy (12). The umbrella term ovarian cancer represents a wide heterogeneous array of ovarian carcinomas that differ in their origin site, epidemiology, pathology, genetic risk factors, response to chemotherapy, and prognosis (13). One of the biggest challenges in the treatment of ovarian cancer, of which 90% are of epithelial origin, is the poor early-stage diagnosis because of obscure clinical signs and symptoms (12). Another challenge is the lack of specific and targeted preoperative visualization methods, which leads to positive margin surgical resections where undetected lesions are left behind, often causing cancer recurrence (12,13).
Herein, pafolacianine comes in as targeted solution to enhance the effectiveness of cytoreductive surgery of ovarian cancer, which is the most effective treatment method along with chemotherapy. Pafolacianine combines three features that are perhaps most desirable for a successful negative margins ovarian cancer surgery (14). Firstly, it decreases the reliance on preoperative visualization methods by providing an intraoperative visualization method, which has obvious superiority over untargeted preoperative radiographic methods (15). Secondly, pafolacianine is a targeted imaging agent as it targets FRα that are overexpressed in most of epithelial ovarian cancers. And lastly, pafolacianine conjugates the FRα targeting analog, folate with a NIR cyanine dye, S0456, which exhibits fluorescence from 790 to 815 nm (12,15). Pafolacianine has competitive edge over other existing FRα targeting imaging agents e.g., EC17, a folate fluorescein conjugate, such that pafolacianine fluoresces in near infrared region, while EC17 fluoresces in the visible region with emission wavelength of 520 nm (16). NIR fluorescence is preferred over visible range fluorescence because NIR light causes less autofluorescence and light scattering, and penetrates much deeper into tissues compared to visible light, which allows highly sensitive detection during surgery (16,17).
Our group has been working on the development of the tissue specific NIR cyanine dyes given their excellent optical properties and the significance of NIR window for biomedical imaging (18-22). The tissue specificity of these flourophores is based on the structures, charge distribution and the functional moities of the cyanine dyes. For instance, cartilage specific NIR dyes include quateranry ammonium moities that target the negatively charged glycosaminoglycans (GAG) in the cartlilage (18,19); bone targeting NIR dyes are designed with phosphonate groups that inetercat with hydroxyapatite and calcium phosphate present in the bone (18,20). Moreover, the characteristic thyroid gland targeting with certain cyanine dyes has been observed to be associated with the presence of halogens, especially fluorine on the indole units of the structures (21). In addition to targeting moieties being employed in the NIR flourophores, the physicochemical properties of cyanine dyes modulated through funtional moities are also considered to play an important role in observed tissue specific targetting (22).
Design and synthesis of pafolacianine
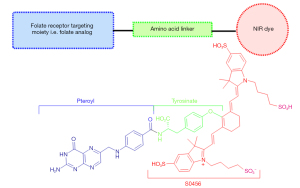
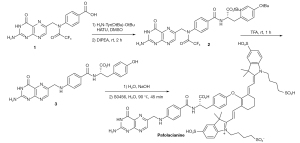
The endeavors to visualize malignant tumors intraoperatively via fluorescent dyes has two main routes that differ both in terms of design and mechanism of fluorescent targeting. The first approach involves the quenched-fluorescent activable fluorophores that are activated in the tumor in response to a particular enzyme, pH change or redox potential change (18). Hence the tumor specific activation of the otherwise “off” fluorescent dye allows the visualization of the tumor (18,23). The second approach involves the conjugation of the fluorescent dye with tumor specific ligand such that the conjugate selectively accumulates in the tumor because of the overexpression of that particular ligand’s receptor in the tumor. The active targeting fluorophores generally offer high signal to background ratio, rapid targeting and low toxicity (18). Design of Pafolacianine follows the second approach by incorporating the active targeting moiety i.e., the pteroyl unit of folic acid (23). This folate analog is linked with a tyrosine amino acid through amide linkage, which is then conjugated with NIR dye S0456 through a vinyl ether linkage as shown in Figure 1 (23,24). The direct linkage of the folic acid with the NIR dye has been reported to result in the formation of unwanted enamine byproduct, which was avoided by replacing the amine bearing glutamate moiety of the folic acid with tyrosinate moiety (25). This replacement not only increased the stability of the desired product upon conjugation with S0456 dye, but tyrosinate linker also exhibited optimum optical properties compared to other amino acid linkers (24,25). The synthesis is shown in Figure 2, with the commercially available triflouroacetylpteroic acid as the starting material (24,25).
Phase III study of pafolacianine
The phase III study of pafolacianine as an adjunct has given positive results regarding its efficacy and safety for real-time intraoperative fluorescence imaging of folate receptor positive-ovarian cancer. The study consisted of subjects with confirmed or suspected ovarian cancer that were scheduled to undergo cytoreductive surgery to remove the tumor. Patients were given pafolacianine intravenously at least one hour prior to the intraoperative imaging to investigate if targeted fluorescence imaging would be able to detect lesions that otherwise remained undetected by the surgeon before and during the surgery. The primary objective of the study confirmed the efficacy of pafolacianine by exceeding the prescribed threshold of 10% (17). Thirty-six out of 109 patients [33.0%; 95% confidence interval (CI): 24.3% to 42.7%] in the full analysis set (FAS) who were randomly selected for NIR imaging had at least one additional cancer lesion detected by fluorescence and later confirmed by pathology to be malignant was found to be originally undetected and not planned for removal by conventional white light and palpation method. In the post-surgery questionnaire, surgeon reported that use of pafolacianine fluorescence allowed them to achieve enhanced complete debulking in 50.5% of the patients and complete resection in 62.4% of the patients. Moreover, the sensitivity of detecting the ovarian cancer was 83% with a false-positive rate of 24.8%. As for the safety profile of pafolacianine, 30% (45 of 150) of the patients reported drug-related adverse effects which mostly included abdominal pain, nausea, and vomiting (17).
Conclusions
Overall, the encouraging efficacy and safety results of pafolacianine and its approval as an intraoperative FRα targeting imaging agent for ovarian cancer is a valuable addition to the endeavors of enhancing the effectiveness of cancer surgeries and the very much associated survival rates for cancer patients. The relatively uncomplicated design and synthesis of pafolacianine also holds the potential for scaling up. It would encourage the development of other ligand-dye and drug-dye conjugates with NIR dyes for targeted therapeutic and diagnostic purposes. The ligand-dye design can potentially be applied to develop targeted imaging agents for other diseases and biological systems of interest. Perhaps the recent approval of pafolacianine for intraoperative imaging of FRα positive lung cancer in addition to prior approval for ovarian cancer is the first step in this direction.
Acknowledgments
The authors would like to thank the Department of Chemistry at Georgia State University for the support for Zaryab Gul.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Translational Medicine. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-467/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cheung A, Bax HJ, Josephs DH, et al. Targeting folate receptor alpha for cancer treatment. Oncotarget 2016;7:52553-74. [Crossref] [PubMed]
- Salazar MD, Ratnam M. The folate receptor: what does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev 2007;26:141-52. [Crossref] [PubMed]
- Chen C, Ke J, Zhou XE, et al. Structural basis for molecular recognition of folic acid by folate receptors. Nature 2013;500:486-9. [Crossref] [PubMed]
- Fernández M, Javaid F, Chudasama V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem Sci 2018;9:790-810. [Crossref] [PubMed]
- Sudimack J, Lee RJ. Targeted drug delivery via the folate receptor. Adv Drug Deliv Rev 2000;41:147-62. [Crossref] [PubMed]
- Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev 2004;56:1067-84. [Crossref] [PubMed]
- Boshnjaku V, Shim KW, Tsurubuchi T, et al. Nuclear localization of folate receptor alpha: a new role as a transcription factor. Sci Rep 2012;2:980. [Crossref] [PubMed]
- Vergote I, Leamon CP. Vintafolide: a novel targeted therapy for the treatment of folate receptor expressing tumors. Ther Adv Med Oncol 2015;7:206-18. [Crossref] [PubMed]
- Maurer AH, Elsinga P, Fanti S, et al. Imaging the folate receptor on cancer cells with 99mTc-etarfolatide: properties, clinical use, and future potential of folate receptor imaging. J Nucl Med 2014;55:701-4. [Crossref] [PubMed]
- Tummers QR, Hoogstins CE, Gaarenstroom KN, et al. Intraoperative imaging of folate receptor alpha positive ovarian and breast cancer using the tumor specific agent EC17. Oncotarget 2016;7:32144-55. [Crossref] [PubMed]
- Van Keulen S, Hom M, White H, et al. The Evolution of Fluorescence-Guided Surgery. Mol Imaging Biol 2023;25:36-45. [Crossref] [PubMed]
- Dindere ME, Bucur O. Cancer detection during surgery: FDA-approved use of pafolacianine. Discoveries Reports 2022;5:e30.
- Prat J. New insights into ovarian cancer pathology. Ann Oncol 2012;23:x111-7. [Crossref] [PubMed]
- Dindere ME, Tanca A, Rusu M, et al. Intraoperative Tumor Detection Using Pafolacianine. Int J Mol Sci 2022;23:12842. [Crossref] [PubMed]
- Pafolacianine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Pafolacianine (accessed Decemeber 22, 2022).
- De Jesus E, Keating JJ, Kularatne SA, et al. Comparison of Folate Receptor Targeted Optical Contrast Agents for Intraoperative Molecular Imaging. Int J Mol Imaging 2015;2015:469047. [Crossref] [PubMed]
- Tanyi JL, Randall LM, Chambers SK, et al. A Phase III Study of Pafolacianine Injection (OTL38) for Intraoperative Imaging of Folate Receptor-Positive Ovarian Cancer (Study 006). J Clin Oncol 2023;41:276-84. [Crossref] [PubMed]
- Owens EA, Henary M, El Fakhri G, et al. Tissue-Specific Near-Infrared Fluorescence Imaging. Acc Chem Res 2016;49:1731-40. [Crossref] [PubMed]
- Hyun H, Owens EA, Wada H, et al. Cartilage-Specific Near-Infrared Fluorophores for Biomedical Imaging. Angew Chem Int Ed Engl 2015;54:8648-52. [Crossref] [PubMed]
- Hyun H, Wada H, Bao K, et al. Phosphonated near-infrared fluorophores for biomedical imaging of bone. Angew Chem Int Ed Engl 2014;53:10668-72. [Crossref] [PubMed]
- Hyun H, Park MH, Owens EA, et al. Structure-inherent targeting of near-infrared fluorophores for parathyroid and thyroid gland imaging. Nat Med 2015;21:192-7. [Crossref] [PubMed]
- Wada H, Hyun H, Vargas C, et al. Pancreas-targeted NIR fluorophores for dual-channel image-guided abdominal surgery. Theranostics 2015;5:1-11. [Crossref] [PubMed]
- Winum JY. Synthesis and composition of amino acid linking groups conjugated to compounds used for the targeted imaging of tumors: a patent evaluation of US20160011199A1. Expert Opin Ther Pat 2016;26:1223-6. [Crossref] [PubMed]
- Wang Q, Han J, Sorochinsky A, et al. The Latest FDA-Approved Pharmaceuticals Containing Fragments of Tailor-Made Amino Acids and Fluorine. Pharmaceuticals (Basel) 2022;15:999. [Crossref] [PubMed]
- Mahalingam SM, Kularatne SA, Myers CH, et al. Evaluation of Novel Tumor-Targeted Near-Infrared Probe for Fluorescence-Guided Surgery of Cancer. J Med Chem 2018;61:9637-46. [Crossref] [PubMed]