
Healing mechanism of diabetic foot ulcers using single-cell RNA-sequencing
Highlight box
Key findings
• The CCL2-ACKR1 axis is closely associated with diabetic foot ulcer healing.
What is known and what is new?
• Both CCL2 and ACKR1 play a large role in angiogenesis during wound healing.
• We directly verified the role of the CCL2-ACKR1 signaling pathway in tissue stem cells in the healing process of diabetic wounds, and showed that the CCL2-ACKR1 signaling pathway activity of tissue stem cells in DFU wound healing tissues was closely related to the activity of endothelial cell subpopulation, which significantly accelerated wound healing.
What is the implication, and what should change now?
• A new pathway to promote the healing of diabetic foot ulcer has been found, but further clinical and animal studies are still needed on whether it can be used in clinical practice.
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disease of hyperglycemia caused by insulin resistance (1). It has been reported that by the end of 2021, there were 536.6 million diabetic patients worldwide, and that by 2045, the number of diabetes patients worldwide is expected to reach 693 million (2). Diabetes has become a major global epidemic, especially in China. At present, the number of diabetes patients in China has increased to 116 million (3), with a prevailing upward trend. T2DM is associated with a variety of complications, including cardiovascular disease, stroke, chronic renal failure, peripheral neuropathy, and diabetic skin wounds or ulcers, among others (4).
Diabetic foot (DF) is one of the most serious chronic complications of diabetes and the most common chronic wound. It refers to foot infections, ulcers, and/or deep tissue destruction in diabetic patients caused by distal nerve abnormalities in the lower extremities (5,6) and varying degrees of vasculopathy (6). A previous pathological study has indicated that the causes of difficult healing of DF wounds include microbial invasion, epithelial cell rupture, and impaired immune function (7). Among all possible complications of type II diabetes, DF is the main reason for hospitalization of patients. Studies have reported that 25% of diabetic patients may develop foot ulcers. Because of its long healing time and tendency to recur, it has become the primary cause of amputation (8,9). The current treatment of diabetic foot ulcer (DFU) mainly includes wound debridement, wound unloading, blood glucose regulation, and control of infection (10). In recent years, some emerging therapeutic modalities have been gradually incorporated into clinical practice, including hyperbaric oxygen therapy, application of dressings, negative pressure wound therapy (NPWT), growth factor therapy [nerve growth factor, platelet-rich plasma (PRP), growth factor], tissue stem cell therapy, and application of tissue-engineered skin, among others (9,11), all of which have achieved improved therapeutic results. A study in diabetic patients demonstrated that hyperbaric oxygen therapy can increase the expression and angiogenesis of vascular endothelial growth factor (VEGF), as well as reduce interferon-gamma (IFN-γ) and neutrophil aggregation (12) to promote wound healing; Hunter et al. found that local oxygen therapy can promote the formation of wound healing microbiota and inhibit the development of inflammation (13). Campitiello et al. found that NPWT can reduce leukocyte production and pain, promote fresh granulation tissue production on wounds, and greatly shorten the time to healing (14). A related study has reported that autologous PRP has become an important treatment for most chronic wounds due to its richness of various growth factors [epidermal growth factor (EGF), platelet-derived growth factor (PDGF), transforming growth factor-β1 (TGF-β1)], and ability to promote cell proliferation differentiation and inhibit inflammation (15).
Although various innovative technologies and drugs have been developed to treat DFUs, the therapeutic effect is still unsatisfactory. Due to its complex pathophysiological mechanisms, the annual amputation rate of patients with DF still reaches 5.1%, even with standardized care and treatment (16). In addition to the currently recognized pathophysiological mechanisms of lower limb vasculopathy and neuropathy (17), there are many pathogenic mechanisms that we have not recognized, such as the “invisible damage” of skin caused by hyperglycemia and the “edge effect” of chronic ulcer healing. Only by continuing to strengthen relevant basic research can we make great progress in clinical diagnosis and treatment.
Single-cell sequencing technology is an ideal tool for single-cell research because of its high accuracy and specificity (18). It enables unbiased high-throughput studies with minimal sample starting volume, detects cell specificity and intercellular differences from the perspective of cell mapping, explores the synergistic operation of cells, and studies tissue heterogeneity (19). It helps researchers to understand the dynamic changes of genes, proteins, and so on (20). Although there have been many single-cell studies on DFU, its complex pathophysiological mechanisms remain incompletely elucidated. This study will focus on the interactions between cells in wound repair, aiming to provide a theoretical basis and experimental foundation for wound healing. We present the following article in accordance with the MDAR reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-23-240/rc).
Methods
Import of data
We searched, filtered, and downloaded the GSE165816 RNA-seq dataset from the Gene Expression Omnibus (GEO) official database, and screened foot and forearm skin samples and peripheral blood monocytes (PBMC) samples from 10 non-diabetic and 17 diabetic patients (11 with DFU and 6 without DFU). The Seurat package in R (The R Foundation for Statistical Computing, Vienna, Austria) was used to create Seurat objects separately for each sample and to filter out cells with less than 200 genes (min.features =200) and genes detected by less than 3 cells (min.cells =3). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
View of quality control (QC) indicators
We calculated the corresponding QC metrics and customized the parameters simultaneously. QC has two main parameters: (I) the number of the unique characteristics measured in each cell (the unique feature represents the number of genes detected in a cell, can be adjusted according to the quality of the data); (II) the proportion of mitochondrial genes detected in each cell, compared with the nuclear genome, theoretically mitochondrial genome only a small part. So cells with excessive expression of mitochondrial genes are filtered out. We then visualized the QC metrics by VlnPlot function and calculated QC correlation using the FeatureScatter function in R.
Cell selection and filtering
According to the QC metrics, cells with an excessive percentage of mitochondrial gene expression, and cells with extreme values of gene expression were removed. Parameter selection: nFeature_RNA represents the number of genes measured per cell, selection is greater than 200 and less than 7,500; nCount represents the sum of expression of all genes measured per cell, selects the proportion of mitochondrial genes measured by 100,000; percent.mt represents the proportion of the mitochondrial genes measured, less than the 20%; percent.HB represents the proportion of the red blood cell genes, selected for less than 5%.
Data normalization
Data normalization was performed by applying the sctransform function, which is a method for normalizing single-cell UMIcount data using variance-stabilized transformations. Its main functions are data normalization, identification of highly variable genes, and decentering of the data. Homogenization was performed to remove the influence of confounding factors such as mitochondria and red blood cells.
Reduced-dimensional clustering of cells
Linear dimensionality reduction was performed on single-cell objects. In identifying cell subtypes, a few principal components with the highest contribution were first picked out using principal component analysis (PCA), and then the already selected principal components were used for further cluster analysis. The FindNeighbors and FindClusters functions were used to find differentially expressed genes (DEGs) in each cell type from other classes and be used as biological marker genes for that cell type.
Cell type identification
DFU samples were automatically annotated using the SingleR package, which independently inferred possible cell types for each single cell from a reference transcriptome dataset of pure cell types. The annotated cell types were visualized with the UMP information contained therein by the DimPlot function.
Single-cell data integration and difference analysis
Tissue stem cell subpopulations were selected from the DFU group and Healed_DFU group respectively for integration, followed by reduction of dimensionality, clustering, and data normalization as described above. Tissue stem cell subpopulations were classified while searching for DEGs.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis
The obtained tissue stem cells subpopulation differential genes were analyzed for GO and KEGG enrichment analysis.
Analysis of intercellular communication in DFU
We performed intercellular communication analysis in DFU to further investigate the signaling connections and changes between immune cells and stromal cells, to analyze cell-cell communication of individual ligand receptors, and to explore the communication links between tissue cell stem cells and endothelial cells.
Statistical analysis
Statistical analysis was performed using the R software. Difference analysis was performed by Wilkerson and considered statistically significant if P value <0.05.
Results
Single-cell QC analysis
QC analysis was performed on single cells of healing DFU and DFU wounds, and Seurat objects were created for each sample. Filtering was conducted to detect cells with less than 200 genes (min.features =200) and genes with less than 3 cells (min.cells =3) (Figure 1).

Introduction of cell subsets in wounds
The results showed that B cells, endothelial cells, epithelial cells, fibroblasts, keratinocytes, monocytes/macrophages, T cells, and tissue stem cells were distributed in both healing DFU and DFU wounds and accounted for a larger proportion. Among them, the increased infiltration of neutrophils, monocytes/macrophages and T lymphocytes into the wound site occurs in the inflammatory stage to remove bacteria and other microorganisms and prevent infection; fibroblasts are the key cells responsible for initiating angiogenesis, epithelialization and collagen production; keratinocytes, fibroblasts, endothelium, endothelial cells, epithelial cells and tissue stem cells are involved in the formation, epithelialization and tissue remodeling of granulation tissue (Figure 2).

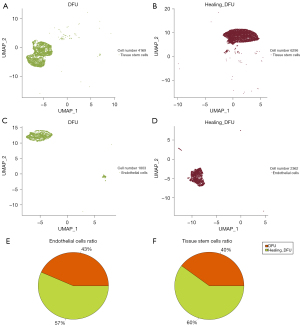
Comparison of tissue stem cell and endothelial cell numbers before and after wound healing
The results showed that the expression of tissue stem cells and endothelial cells in DFU wounds was 4,169 and 1,803, respectively, whereas the expression of both cells in healing DFU wounds was 6,296 and 2,362 (Figure 3).
Comparison of DEGs between tissue stem cells and endothelial cells before and after wound healing
DEG analysis showed that there were a total of 1,948 DEGs between tissue stem cells of healed and unhealed wounds, of which 1,198 genes were up-regulated and 685 genes were down-regulated; there were a total of 1,773 DEGs between endothelial cells of healed and unhealed wounds, of which a total of 984 genes were up-regulated and 789 genes were down-regulated (Figure 4).

GO and KEGG enrichment analysis
The results of GO enrichment functional analysis of tissue stem cells showed that extracellular matrix (ECM) organization, extracellular structural organization, external encapsulated structural organization, regulation of peptidase activity, and wound healing were the top 5 biological processes. Collagen-containing ECM, endoplasmic reticulum lumen, focal adhesion, cell-matrix junctions, and basement membrane were the top 5 cellular components. ECM structural components, ECM structural components that confer tensile strength, collagen binding, integrin binding, and proteoglycan binding were the top 5 molecular functions (Figure 5A).

Functional analysis of KEGG enrichment in tissue stem cells showed that local adhesion, ECM-receptor interaction, protein digestion and absorption, proteoglycans in cancer, regulation of the actin cytoskeleton, protein processing in the endoplasmic reticulum, the PI3K-Akt signaling pathway, human papillomavirus infection, vascular smooth muscle contraction, and platelet activation were the top 10 enriched signaling pathways (Figure 5B).
Functional analysis of endothelial GO enrichment showed that positive regulation of cytokine production, positive regulation of cell adhesion, regulation of cell-cell adhesion, extracellular structural organization, and ECM organization were the top 5 biological processes. Collagen-containing ECM, outer plasma membrane, cell-matrix junctions, focal adhesion, and endoplasmic reticulum lumen were the top 5 cellular components. Cellular matrix structural components, glycosaminoglycan binding, heparin binding, growth factor binding, and integrin binding were the top 5 ranked molecular functions (Figure 5C).
Endothelial cell KEGG enrichment function analysis showed that the PI3K-Akt signaling pathway, cytokine-cytokine receptor interaction, the MAPK signaling pathway, local adhesion, proteoglycans in cancer, human T-cell leukemia virus 1 infection, the Rap1 signaling pathway, cell adhesion molecules, fluid shear stress, and atherosclerosis and hematopoietic cell lines were the top 10 enriched signaling pathways (Figure 5D).
Analysis of cell subpopulation communication within the wound surface
Healing DFU had 235 signaling pathways, whereas DFU had 184 signaling pathways, and the number of healing DFU signaling pathways was significantly higher (Figure 6A). We constructed a heat map showing the number and intensity of signaling pathways during wound healing among the cells (B cells, endothelial cells, fibroblasts, keratinocytes, T cells, tissue stem cells); among them, fibroblasts and endothelial cell subsets were closely related, and the number and intensity of communication were higher than those of other cells (Figure 6B). In both the wound tissues of healing DFU and DFU, the interaction between tissue stem cells and fibroblasts and endothelial cell subsets was relatively close (Figure 6C,6D). In the overall signaling pattern of the DFU versus healing DFU signaling pathways, CCL, VEGF, CALCR, and EDN were the most active in the functional activity of the endothelial cells (Figure 6E,6F). As can be seen from the data, the interleukin-1 (IL-1), IL-16, LIGHT, CHEMERIN, and IGF signaling pathways were only expressed within the DFU wound tissues; however, the CD70, CSF, BAG, ncWNT, and ANNEXIN signaling pathways were almost exclusively expressed in the healing DFU wound tissues. Of the signaling pathways that were expressed within both wound tissues, signaling pathways such as CCL, PROS, EDN, PERIOSTIN, and PARs were more highly expressed in healing DFU tissues than in DFU tissues; however, the signaling pathways such as FGF, SEMA 3, MK, PIN, and TGFb were more highly expressed in DFU wound tissues than in healing DFU wound tissues (Figure 6G).
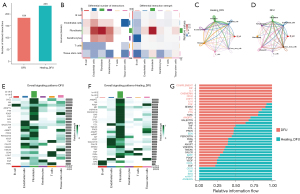
Analysis of each ligand and receptor in the CCL signaling pathway in tissue stem cells
As shown in the signal type diagram, the secretory signaling type accounts for 61.8%, the ECM-receptor type for 21.7%, and the cell-cell contact type for only 16.5%; 73% of these are from KEGG, and the remaining 27% are from literature (Figure 7A). Exploring the contribution of CCL in the functional activity of individual cells, it was most closely associated with endothelial cells, T cells, and fibroblasts, followed by tissue stem cells (Figure 7B). Based on the above signaling pathway studies, we adequately screened the highly expressed signaling pathways within healing DFU wounds and further determined the ligand-receptor relationship between the above signaling pathways. From the data, it can be seen that the CCL2-ACKR1 and ANGPTL2-(ITGA5 + ITGB1) signaling pathways in tissue stem cells had an impact on the endothelial cells of the healing DFU wound cell subpopulation, with the correlation of the CCL2-ACKR1 signaling pathway was significantly higher than that of the ANGPTL2-(ITGA5 + ITGB1) signaling pathway (Figure 7C). The results showed that the contribution of each ligand and receptor to the signaling pathway in the CCL signaling pathway in tissue stem cells within healing DFU wounds showed variability; the CCL2-ACKR1 signaling pathway made the greatest contribution to the signaling pathway. In the reciprocal relationship, it can be seen that there was a significant modulation of endothelial cells by tissue stem cells; thus we conclude that the CCL2-ACKR1 signaling pathway activity of tissue stem cells has an effect on the biological activity of endothelial cells subpopulation, which ultimately promotes the healing of DFU wounds (Figure 7D,7E).

Discussion
Wounds in diabetic patients are characterized by an impaired healing process, reduced neovascularization and matrix deposition, and delayed inflammatory regression (1,21). As one of the most serious complications of diabetes, DFU often lead to difficulties walking and even amputation or death (21). The healing process of DFU belongs to pathological healing, which is easy to form chronic refractory wound, and it is difficult to appear the step reaction of normal physiological healing. In addition to paying attention to the treatment of systemic factors, such as the control of blood sugar, blood pressure, blood lipid, anti-infection and the improvement of microcirculation, we must also pay attention to the standardized treatment of chronic wound itself. Only the comprehensive coordinated treatment of whole body and local can promote the healing of chronic difficult wound (22,23). DFU healing is a multi-step process that requires the coordination of 4 distinct but overlapping physiologically logical phases, including hemostasis, inflammation, proliferation, and remodeling (24). Tissue stem cells, an induced pluripotent stem cell, are well known for their remarkable regenerative properties and are now an important treatment for DF (25). El Hage et al. found that adipose-derived stem cells (ADSCs) can promote wound epithelialization and angiogenesis (26). It is well known that abnormal angiogenesis is an important cause of delayed healing of diabetic wounds, and that blood vessels can provide oxygen, nutrients, and repair cells to the injured site to maintain tissue regeneration (27). Experiments conducted by Komiyama et al. also showed that ADSCs secrete large amounts of angiogenic factor (28), which is consistent with our results.
Our results showed that there were 1,948 DEGs between tissue stem cells of healing diabetic wounds and non-healing wounds, of which 1,198 genes were up-regulated and 685 genes were down-regulated. To understand the biological functions of these DEGs, we proceeded to perform GO and KEGG enrichment analysis. The results showed that tissue stem cells were closely associated with wound healing and promoted wound healing mainly through ECM-receptor interaction, the PI3K-AKT signaling pathway, and platelet activation. The ECM is an intricate network composed of macromolecules. It provides a suitable site for cell survival and activity and influences cell migration, proliferation, and differentiation, through signal transduction systems. Xie et al. concluded that impairment of the PI3K/AKT signaling pathway plays a decisive role in the development of ulcers and their subsequent complications. PI3K consists of a family of lipid kinases, and its normal activation promotes DNA repair, protein synthesis, cell migration, cell cycle, and more. AKT is a serine/threonine kinase, and its impairment can inhibit the activity of several downstream pathways that are commonly associated with cell proliferation, angiogenesis, and apoptosis. The PI3K/AKT signaling pathway can also regulate and control key downstream proteins, the most important of which is mammalian rapamycin (mTOR) (29). Ma et al. found that normal activation of the PI3K/AKT/mTOR pathway can promote epithelial mesenchymal transition (EMT), cell proliferation, and wound healing (30).
C chemokine receptor type 2 (CCL2) expression is significantly increased during the inflammatory phase of diabetic wound healing (31). CCL2 is produced in both autocrine and paracrine forms. In normal tissue cells, CCL2 can be produced by a variety of activated cells, such as fibroblasts, endothelial cells, lymphocytes, and monocytes/macrophages (32). CCL2 was first found to be used for targeted therapy in various malignancies (33) (breast, lung, nasopharyngeal, prostate, and colorectal cancers etc.), and two studies have reported that two non-classical pathways mTORC1-FOXK1 (34) and Notch1-NICD1 (35) are involved in the regulation of CCL2, thus promoting tumor-associated macrophages (TAMs) for the recruitment and infiltration of the population (36). Nakatsumi et al. found that mTORC1 promotes the expression of CCL2 by activating the response through downstream transcription factor FOXK1 dephosphorylation. Experiments in nude mouse animal models also demonstrated that the anti-tumor drug rapamycin (mTOR) attenuated the infiltration of TAMs (34). Lu et al. artificially inhibited the expression of CCL2 in mouse animal models and observed a prolonged wound healing time along with reduced neovascularization and epithelial tissue proliferation (37). Coincidentally, the experiments of Qian et al. also demonstrated that once the CCL2-CCR2 axis was inhibited in tumor cells, cellular VEGF expression also reduced, thus acting as an inhibitor of neovascularization in tumor cells (38). CCL2 normally binds preferentially to CCR2, and the overexpression of CCL2 and CCR2 can activate the ERK1/2 cellular pathway (39) and induce the expression of the matrix metalloproteinases MMP-2 and MMP-9, thus promoting metastasis in nasopharyngeal carcinoma. However, matrix metalloproteinases MMP-2 and MMP-9 have been shown by many investigators to be associated with angiogenesis, apoptosis, tissue repair, and wound healing. Recent genetic experiments by Nawaz et al. have confirmed that targeted inhibition of CCL2 expression inhibits the inflammatory response of retinal microvascular endothelial cells in a PDR model (40). Here, we downloaded the GSE165816 RNA-seq dataset from the GEO official website and observed that CCL2 expression was much higher in healing DFU than DFU while the number of vascular endothelial cells is also significantly increased, and these observations suggest that there is indeed a close relationship between CCL2 and angiogenesis in impaired skin wound healing.
The atypical chemokine receptor 1 (ACKR1), which is a chemokine, has been shown to be enriched at endothelial cell junctions. Barkaway et al. demonstrated that ACKR1 can be expressed in the erythroid lines to regulate hematopoiesis and can also act as a plasma chemokine to trigger leukocyte adhesion and migration through the endothelium (41). CCL2, CCL5, CXCL8, and CCL11 have been shown to be inflammatory chemokines that bind strongly to ACKR1 on the surface of erythrocytes (42). A recent study has shown that erythrocyte infusion upregulates ACKR1 expression, affects the apoptosis of macrophages, and reduces inflammatory cytokine production (43) in a sepsis model.
Although both CCL2 and ACKR1 play a large role in angiogenesis during wound healing, to date, there is no evidence of synergistic effects between the 2 in promoting healing of DFU. The results of this study showed that there was variability in the contribution of each ligand and receptor to the CCL2 signaling pathway in tissue stem cells within healing DFU wound tissue, with the CCL2-ACKR1 signaling pathway making the greatest contribution to the signaling pathway. We also directly verified the role of the CCL2-ACKR1 signaling pathway in tissue stem cells in the healing process of diabetic wounds, and showed that the CCL2-ACKR1 signaling pathway activity of tissue stem cells in DFU wound healing tissues was closely related to the activity of endothelial cell subpopulation, which significantly accelerated wound healing.
This result provides us with a reliable basis for studying the biological functions and connections between tissue stem cells and endothelial cells in the complex context of DFU healing. But this study is based on bioinformatics analysis, lack of experimental validation is one of the limitations of this paper. Next, we will conduct animal modeling test for functional studies of key genes and signaling pathways, including immunofluorescence (IF) analysis, flow cytometry (FCM), western blot (WB) and qPCR, in order to provide a theoretical basis for clinical diagnosis and treatment through animal verification.
Conclusions
The CCL2-ACKR1 axis is closely associated with DFU healing.
Acknowledgments
Funding: The study was supported by Youjiang Medical University for Nationalities Research Project (No. yy2019bsky001); Baise Scientific Research and Technology Development Program (No. bsky 202123); Affiliated Hospital of Youjiang Medical University for Nationalities Research Project (No. yy2023bsky001); Guangxi Zhuang Autonomous Region Administration of Traditional Chinese Medicine Self-Funded Scientific Research Project (No. GXZYL20220302).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-240/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-240/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-240/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Burgess JL, Wyant WA, Abdo Abujamra B, et al. Diabetic Wound-Healing Science. Medicina (Kaunas) 2021;57:1072. [Crossref] [PubMed]
- Artasensi A, Pedretti A, Vistoli G, et al. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules 2020;25:1987. [Crossref] [PubMed]
- Chen XW, Ding G, Xu L, et al. A glimpse at the metabolic research in China. Cell Metab 2021;33:2122-5. [Crossref] [PubMed]
- Jaiswal V, Negi A, Pal T. A review on current advances in machine learning based diabetes prediction. Prim Care Diabetes 2021;15:435-43. [Crossref] [PubMed]
- Chatwin KE, Abbott CA, Boulton AJM, et al. The role of foot pressure measurement in the prediction and prevention of diabetic foot ulceration-A comprehensive review. Diabetes Metab Res Rev 2020;36:e3258. [Crossref] [PubMed]
- Liu Y, Liu Y, Deng J, et al. Fibroblast Growth Factor in Diabetic Foot Ulcer: Progress and Therapeutic Prospects. Front Endocrinol (Lausanne) 2021;12:744868. [Crossref] [PubMed]
- Patel BK, Patel KH, Huang RY, et al. The Gut-Skin Microbiota Axis and Its Role in Diabetic Wound Healing-A Review Based on Current Literature. Int J Mol Sci 2022;23:2375. [Crossref] [PubMed]
- Pereira MG, Vilaça M, Carvalho E. Effectiveness of Two Stress Reduction Interventions in Patients with Chronic Diabetic Foot Ulcers (PSY-DFU): Protocol for a Longitudinal RCT with a Nested Qualitative Study Involving Family Caregivers. Int J Environ Res Public Health 2022;19:8556. [Crossref] [PubMed]
- Baltzis D, Eleftheriadou I, Veves A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv Ther 2014;31:817-36. [Crossref] [PubMed]
- Bardill JR, Laughter MR, Stager M, et al. Topical gel-based biomaterials for the treatment of diabetic foot ulcers. Acta Biomater 2022;138:73-91. [Crossref] [PubMed]
- Huang F, Lu X, Yang Y, et al. Microenvironment-Based Diabetic Foot Ulcer Nanomedicine. Adv Sci (Weinh) 2023;10:e2203308. [Crossref] [PubMed]
- Anguiano-Hernandez YM, Contreras-Mendez L, de Los Angeles Hernandez-Cueto M, et al. Modification of HIF-1α, NF-aκB, IGFBP-3, VEGF and adiponectin in diabetic foot ulcers treated with hyperbaric oxygen. Undersea Hyperb Med 2019;46:35-44. [Crossref] [PubMed]
- Hunter P, Greco E, Cross K, et al. Topical Oxygen Therapy Shifts Microbiome Dynamics in Chronic Diabetic Foot Ulcers. Wounds 2020;32:81-5. [PubMed]
- Campitiello F, Mancone M, Corte AD, et al. Expanded negative pressure wound therapy in healing diabetic foot ulcers: a prospective randomised study. J Wound Care 2021;30:121-9. [Crossref] [PubMed]
- Oneto P, Etulain J. PRP in wound healing applications. Platelets 2021;32:189-99. [Crossref] [PubMed]
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 2017;377:644-57. [Crossref] [PubMed]
- Hicks CW, Selvin E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr Diab Rep 2019;19:86. [Crossref] [PubMed]
- Kolodziejczyk AA, Kim JK, Svensson V, et al. The technology and biology of single-cell RNA sequencing. Mol Cell 2015;58:610-20. [Crossref] [PubMed]
- Armand EJ, Li J, Xie F, et al. Single-Cell Sequencing of Brain Cell Transcriptomes and Epigenomes. Neuron 2021;109:11-26. [Crossref] [PubMed]
- Chambers DC, Carew AM, Lukowski SW, et al. Transcriptomics and single-cell RNA-sequencing. Respirology 2019;24:29-36. [Crossref] [PubMed]
- Bolton L. Managing Patients With Diabetic Foot Ulcers. Wounds 2018;30:380-1. [PubMed]
- Wang S, Pan LF, Gao L, et al. Randomized research on the mechanism of local oxygen therapy promoting wound healing of diabetic foot based on RNA-seq technology. Ann Palliat Med 2021;10:973-83. [Crossref] [PubMed]
- Gan MS, Yang B, Fang DL, et al. IL-1B can serve as a healing process and is a critical regulator of diabetic foot ulcer. Ann Transl Med 2022;10:179. [Crossref] [PubMed]
- Rousselle P, Montmasson M, Garnier C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol 2019;75-76:12-26. [Crossref] [PubMed]
- Si Z, Wang X, Sun C, et al. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed Pharmacother 2019;114:108765. [Crossref] [PubMed]
- El Hage R, Knippschild U, Arnold T, et al. Stem Cell-Based Therapy: A Promising Treatment for Diabetic Foot Ulcer. Biomedicines 2022;10:1507. [Crossref] [PubMed]
- Velazquez OC. Angiogenesis and vasculogenesis: inducing the growth of new blood vessels and wound healing by stimulation of bone marrow-derived progenitor cell mobilization and homing. J Vasc Surg 2007;45 Suppl A:A39-47.
- Komiyama S, Sakakura C, Murayama Y, et al. Adipose-derived stem cells enhance tissue regeneration of gastrotomy closure. J Surg Res 2013;185:945-52. [Crossref] [PubMed]
- Xie Y, Shi X, Sheng K, et al. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia Mol Med Rep 2019;19:783-91. (Review). [PubMed]
- Ma Z, Lou S, Jiang Z. PHLDA2 regulates EMT and autophagy in colorectal cancer via the PI3K/AKT signaling pathway. Aging (Albany NY) 2020;12:7985-8000. [Crossref] [PubMed]
- Ridiandries A, Tan JTM, Bursill CA. The Role of Chemokines in Wound Healing. Int J Mol Sci 2018;19:3217. [Crossref] [PubMed]
- Sha K, Yeh S, Chang C, et al. TNF signaling mediates an enzalutamide-induced metastatic phenotype of prostate cancer and microenvironment cell co-cultures. Oncotarget 2015;6:25726-40. [Crossref] [PubMed]
- Wang Y, Tiruthani K, Li S, et al. mRNA Delivery of a Bispecific Single-Domain Antibody to Polarize Tumor-Associated Macrophages and Synergize Immunotherapy against Liver Malignancies. Adv Mater 2021;33:e2007603. [Crossref] [PubMed]
- Nakatsumi H, Matsumoto M, Nakayama KI. Noncanonical Pathway for Regulation of CCL2 Expression by an mTORC1-FOXK1 Axis Promotes Recruitment of Tumor-Associated Macrophages. Cell Rep 2017;21:2471-86. [Crossref] [PubMed]
- Tsuyada A, Chow A, Wu J, et al. CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res 2012;72:2768-79. [Crossref] [PubMed]
- Yang H, Zhang Q, Xu M, et al. CCL2-CCR2 axis recruits tumor associated macrophages to induce immune evasion through PD-1 signaling in esophageal carcinogenesis. Mol Cancer 2020;19:41. [Crossref] [PubMed]
- Lu J, Zhong H, Chu T, et al. Role of anlotinib-induced CCL2 decrease in anti-angiogenesis and response prediction for nonsmall cell lung cancer therapy. Eur Respir J 2019;53:1801562. [Crossref] [PubMed]
- Qian J, Wang C, Wang B, et al. The IFN-γ/PD-L1 axis between T cells and tumor microenvironment: hints for glioma anti-PD-1/PD-L1 therapy. J Neuroinflammation 2018;15:290. [Crossref] [PubMed]
- Mohamed HT, El-Ghonaimy EA, El-Shinawi M, et al. IL-8 and MCP-1/CCL2 regulate proteolytic activity in triple negative inflammatory breast cancer a mechanism that might be modulated by Src and Erk1/2. Toxicol Appl Pharmacol 2020;401:115092. [Crossref] [PubMed]
- Nawaz MI, Van Raemdonck K, Mohammad G, et al. Autocrine CCL2, CXCL4, CXCL9 and CXCL10 signal in retinal endothelial cells and are enhanced in diabetic retinopathy. Exp Eye Res 2013;109:67-76. [Crossref] [PubMed]
- Barkaway A, Rolas L, Joulia R, et al. Age-related changes in the local milieu of inflamed tissues cause aberrant neutrophil trafficking and subsequent remote organ damage. Immunity 2021;54:1494-1510.e7. [Crossref] [PubMed]
- Girbl T, Lenn T, Perez L, et al. Distinct Compartmentalization of the Chemokines CXCL1 and CXCL2 and the Atypical Receptor ACKR1 Determine Discrete Stages of Neutrophil Diapedesis. Immunity 2018;49:1062-1076.e6. [Crossref] [PubMed]
- Jenkins BD, Martini RN, Hire R, et al. Atypical Chemokine Receptor 1 (DARC/ACKR1) in Breast Tumors Is Associated with Survival, Circulating Chemokines, Tumor-Infiltrating Immune Cells, and African Ancestry. Cancer Epidemiol Biomarkers Prev 2019;28:690-700. [Crossref] [PubMed]
(English Language Editor: J. Jones)


