
Oncolytic adenovirus-mediated dual knockdown of survivin and OCT4 improves therapeutic efficacy in esophageal cancer
Highlight box
Key findings
• Dual-knockdown of survivin and OCT4 significantly inhibited the proliferative activity of ESCC cells and contributed to cell cycle arrest and apoptosis in vivo and in vitro.
• Dual-knockdown of survivin and OCT4 could reverse the process of EMT.
What is known and what is new?
• Survivin and octamer-binding transcription factor 4 (OCT4) are reportedly up-regulated in esophageal cancer (EC) and have been correlated with high tumor proliferative activity and poor prognosis.
• Oncolytic viruses containing shRNAs of survivin and OCT4 were found to perform a therapeutic effect in ESCC.
What is the implication, and what should change now?
• The dual target design strategy provided a novel and effective adjuvant target therapy for ESCC and should be profoundly explored.
Introduction
According to the International Agency for Research on Cancer (IARC), 19.3 million new cancer cases and nearly 10 million cancer deaths were reported worldwide in 2020, of which 604,100 were esophageal cancer (EC) cases and 544,000 were EC patient deaths (1). The incidence of EC in China ranks first in the world, accounting for 60% of new EC cases (2). Survivin has a full-length of 14.7 kb, is located on chromosome l7q25, and contains 4 exons and 3 introns. It is regarded as the smallest member of the inhibitor of apoptosis protein (IAP) family. The IAP family generally contains 2–3 tandem baculoviral IAP repeats (BIR), and the adjacent carboxyl terminus contains a ring-finger structure. Survivin has been found to be widely expressed in embryonic tissues and a few vigorously dividing mature tissues. Although rarely observed in well-differentiated tissues, it has been seen as highly expressed in tumor tissues which indicate its promotional role in tumorigenesis (3,4). Previous studies have revealed that survivin may act as an oncogene in lung cancer (5), colon cancer (6), pancreatic cancer (7), prostate cancer (8), breast cancer (9), lymphoma (10), and EC (3). Vallböhmer et al. demonstrated that survivin was significantly upregulated in esophageal adenocarcinoma compared to the adjacent normal tissues. Correlation analysis verified that survivin was not only related to the pathological stage and lymph node metastasis of EC, but was also implicitly related to 5-year overall survival (OS), with the OS of survivin-positive patients significantly lower than that of those who were survivin-negative (11). Our previous research results suggested that the expression of survivin protein in cancer cells was regulated by the p16 protein. In previous research, cancer cells with inactive p16 expressed higher levels of survivin, and upregulated p16 was shown to inhibit the expression of survivin and reduce the proliferation ability of cancer cells (12).
Meanwhile, octamer-binding transcription factor 4 (OCT4)-positive cancer cells with tumor stem cell characteristics have been observed in EC. OCT4 is known to positively upregulate survivin and maintain cell pluripotency. The possible mechanism involves directly upregulating the expression of the cyclin, CCND1, thereby promoting the proliferation of and indirectly activating the expression of survivin (13,14). As one of the members of the Pit-Oct-Unc (POU) transcription factor family, OCT4 activates its target genes by binding to its ATGCAAAT sequence. In stem cells, the promoter activity of 623 protein-coding genes and 5 messenger RNA (mRNA) genes is regulated by OCT4. An increasing number of evidence supports that survivin and OCT4 act as oncogenes in the occurrence and progression of EC, and they play an important role in maintaining the stemness of cancer cells. Therapeutic strategies targeting survivin and OCT4 are likely to be crucial in improving the clinical efficacy of EC treatments.
Immunotherapy has become an important part of a comprehensive tumor strategy. Oncolytic virus therapy, an immunotherapy method, is also receiving increasing attention (15,16). Oncolytic virus therapy not only bestows safety on treatment but also releases a variety of tumor-specific antigens and viral proteins when exerting an oncolytic effect and stimulating an immune response to produce specific or non-specific antibodies. Oncolytic viruses can also be used as carriers for gene therapy, with the specific replication of the virus in tumor cells and the copy number and expression of anti-cancer genes greatly increased (17,18). In this study, we used an oncolytic adenovirus carrying short hairpin RNA (shRNA) of survivin and OCT4 to inhibit the expression of OCT4 and survivin in the esophageal squamous cell carcinoma (ESCC) cell lines Eca109 and TE1, and then evaluated their roles in affecting cell proliferation, cell cycle, and xenograft growth. We aimed to explore whether dual-targeted therapy strategy targeting OCT4 and survivin expression could be an effective treatment method for ESCC. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4499/rc).
Methods
Construction of shRNA vectors and survivin promoter-regulated adenoviruses
Plasmids expressing specific shRNA, including survivin-shRNA (shSRVN), OCT4-shRNA (shOCT4), dual-shRNA (shSRVN + shOCT4), and control shRNA (shNC), were synthesized by Wuhan Genesil Biotechnology Co., Ltd. (Wuhan, China). The corresponding interference sequences of genes are shown in Table 1. The shRNA structure was composed of U6 promoter, 19 nucleotides-sense DNA, loop (TTCAAGACG), antisense DNA, and tail (TTTTTT), and the dual-shRNA was arranged in a “foot-to-foot” structure.
Table 1
| Primer | Primer sequence |
|---|---|
| shSRVN | 5'-GAAAGTGCGCCGTGCCATC-3' |
| shOCT4 | 5'-CCCTCACTTCACTGCACTG-3' |
| shNC | 5'-GACTTCATAAGGCGCATGC-3' |
| Survivin upstream primer | 5'-CGGCTAGCCATAGAACCAGAG-3' |
| Survivin downstream primer | 5'-GAAGATCTGCCGCCGCCGCCACCT-3' |
shRNA, short hairpin RNA.
Based on the type 5-adenovirus (Ad5) backbone pBHGloxdeltaE13Cre (Microbix Biosystems; Ontario, Canada) with the deletion of the E1 and E3 regions, the aforementioned shRNAs were inserted into the E3 region to construct the pAd5-shRNA vector. Survivin upstream and downstream primers were used to amplify the survivin promoter sequence in the pSRVN-Luc plasmid and replace the mCMV promoter sequence in pDC315 (Microbix Biosystems) with the amplified product used to construct pDC315Spro. The E1a full-length complementary DNA (cDNA) sequence of Ad5 was synthesized, the Kozak consensus sequence was added, and the downstream section of the survivin promoter sequence in pDC315SPro was inserted to construct pDC315SPro-E1a. The luciferase plasmid, pSRVN-Luc, in which luciferase expression was under the control of the survivin promoter (nucleotides 1824-2800, GenBank U75285), was kindly provided by Himanshu Garg (Center of Excellence for Infectious Disease, Texas Tech University Health Sciences Center, TX, USA). The 293T cells were co-transfected with pDC315SPro-E1a and pAd5-shRNA. After intracellular recombination, the recombinant adenoviruses were purified and completed, and named AdSProE1a-shSRVN + shOCT4, AdSProE1a-shSRVN, AdSProE1a-shOCT4, and AdSProE1a-shNC, respectively. In addition, the replication control adenovirus, AdSProE1a-EGFP, and the replication-deficient control adenoviruses, Ad-shSRVN + shOCT4 and Ad-EGFP, were constructed with enhanced green fluorescent protein (EGFP) instead of the shRNA expression sequence (Figure 1A).
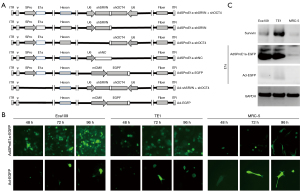
Cell culture
Human esophageal squamous cell cancer (ESCC) lines, Eca109, TE1, and human embryonic lung fibroblasts MRC-5, were purchased from the Chinese Academy of Sciences (Shanghai, China). All the cell lines were authenticated by short tandem repeat DNA fingerprinting. The Eca-109 cells were cultivated in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin solution.
Western blot and immunofluorescence staining
Western blotting was used to detect the expression levels of survivin, OCT4, E1a, and epithelial-mesenchymal transition (EMT) markers, including E-cadherin and vimentin. The cells were plated in a 24-well plate with 5×104 cells/well. The FBS medium was replaced with the serum-free medium after cells had attached, and the viruses AdSProE1a-shSRVN + shOCT4, AdSProE1a-shSRVN, AdSProE1a-shOCT4, and AdSProE1a, were diluted with serum-free medium. The short hair pin negative control (shNC) group and the Ad-shSRVN + shOCT4 group were used to infect cells at multiplicity of infection (MOI) =5 pfu/cell for 2 hours, and then culture medium containing 5% serum was used for 72 hours. A total of 30 µg protein was extracted from cells and separated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a polyvinylidene fluoride (PVDF) membrane via electroblotting. The membrane was blocked with 5% w/v nonfat, dry milk for 1 hour, and the antibodies were incubated at 4 ℃ with gentle shaking, overnight. Next, goat anti-mouse or anti-rabbit antibody was incubated for 1 hour at room temperature. Target proteins were then visualized with an enhanced chemiluminescent reagent.
Furthermore, the localization and relative expression of survivin and OCT4 in cells were detected by immunofluorescence staining. The cells were harvested 72 hours after transfection. Cells were transferred to a Lab-Tek chamber at a concentration of 1×104 cells/well, and fixed with 4% formaldehyde for 30 minutes. Survivin and OCT4 monoclonal antibodies were used and incubated overnight at 4 ℃. Fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG or tetramethylrhodamine (TRITC)-conjugated anti-mouse IgG were then added and incubated for another 1 hour at room temperature. The intensity of gene expression was observed under a laser confocal microscope.
Tumor-specific identification of oncolytic adenovirus replication
The cells were plated in a 24-well plate with 5×104 cells/well. After the cells had adhered, the serum-free medium was used, and the replication-type virus, AdSProE1a-EGFP, and the replication-defective virus, Ad-EGFP, were diluted with the serum-free medium, according to MOI =5 pfu/cell infected cells for 2 hours. They were then changed to medium containing 5% serum and continued in culture for 48, 72, and 96 hours. The expression of EGFP was observed by fluorescence microscope at 3 time points, and the proportion of positive cells was counted; the cells were collected at 96 hours, and the expression of E1a was detected by western blotting method.
Cells were plated in 96-well plates with 1×104 cells/well. After the cells had attached to the wall, a serum-free medium was used for transfection. Then, AdSProE1a-shSRVN + shOCT4, AdSProE1a-shSRVN, AdSProE1a-shOCT4, and AdSProE1a-shNC were diluted with serum-free medium. The Ad-shSRVN + shOCT4 was added to infect the cells at MOI =5 pfu/cell for 2 hours, and medium containing 5% FBS was applied and the culture continued for 0, 24, 48, 72, and 96 hours. Cells were collected at 5 time points and virus titers were determined by the 50% Tissue Culture Infectious Dose (TCID50) method.
Cell Counting Kit-8 (CCK-8) assay and flow-cytometry
Cells were plated in 96-well plates with 1×104 cells/well, and serum-free medium was used after cells had attached to the wall. Then, AdSProE1a-shSRVN + shOCT4, AdSProE1a-shSRVN, AdSProE1a-shOCT4, and AdSProE1a-shNC were diluted with serum-free medium. The Ad-shSRVN + shOCT4, with concentrations of MOI =0, 0.5, 1.0, 5.0, 10.0, 50.0, and 100.0 pfu/cell, was added to infect the cells for 2 hours, and then cells were cultured in medium containing 5% FBS for 7 days. A total of 8 replicate wells were set corresponding to each MOI value. The CCK-8 (Dojindo Laboratories, Kumamoto, Japan) was used according to the manufacturer’s instructions to detect cell viability, and the IC50 value of each group was calculated and compared.
Cells were plated in 6-well plates with 1×105 cells/well. Then, AdSProE1a-shSRVN + shOCT4, AdSProE1a-shSRVN, AdSProE1a-shOCT4, and AdSProE1a-shNC were diluted with serum-free medium and were added to infected cells for 2 hours at a concentration of MOI =5 pfu/cell. The medium was replaced and culturing continued for 72 hours. Cells were collected, washed twice with PBS, and then fixed with 75% ethanol overnight at 4 ℃. A total of 5 µL propidium iodide (PI) was added and incubated for 30 minutes in the dark, and the cell cycle was detected by flow cytometry.
Xenograft experiment
Animal experiments were approved by the Ethics Committee of Shanghai Chest Hospital, in compliance with guidelines for the care and use of animals of Shanghai Jiao Tong University School of Medicine. A total of 50 5-week-old male BALB/c nude mice were used to conduct a xenograft experiment. We subcutaneously injected 5×106 cells under the forelimb in each mouse. After tumors had formed in all mice after 14 days, the 4 larger and the 4 smaller tumors were excluded and the remaining 42 animals were randomly divided into 7 groups (AdSProE1a-shSRVN + shOCT4, AdSProE1a-shSRVN, AdSProE1a-shOCT4, AdSProE1a-shNC, Ad-shSRVN + shOCT4, Ad-EGFP, and negative control). Mice in each group were given an intra-tumoral injection of the therapeutic virus, 2×108 pfu each time, once every other day, 5 consecutive times. The tumor size was measured routinely. The animal welfare guidelines for the care and use of laboratory animals were approved. The experiment was terminated when the average tumor volume in any group exceeded 2,500 mm3.
At the end of the observation period, the mice were sacrificed, and tumor specimens were removed, fixed in 10% formalin overnight, and then paraffin-embedded for sectioning. The expressions of adenovirus protein E1a, OCT4, survivin, E-cadherin, and vimentin were measured by immunohistochemical (IHC) staining. The apoptosis ratio was evaluated by the terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) method.
Statistical analysis
Differences between the treated and control groups were analyzed using the Student’s t-test or one-way analysis of variance (ANOVA) if they followed a normal distribution, otherwise, the Mann-Whitney test was adopted. All statistical analyses were performed using the software SPSS 24.0 (IBM Corp., Armonk, NY, USA). A value of P<0.05 was considered statistically significant.
Results
Oncolytic adenovirus replicated stably in tumor cells
In order to achieve dual knockdown of survivin and OCT4, an oncolytic adenovirus carrying survivin-shRNA (shSRVN) and/or OCT4-shRNA (shOCT4) was constructed and transferred to ESCC cells (Figure 1A). We first detected the intracellular replication viability of different viruses, and the results revealed that the oncolytic adenovirus, AdSProE1a-EGFP, effectively infected cancer cells, and the number of copies of virus increased over time according to epidermal growth factor receptor (EGFR) expression, whereas EGFP did not increase over time in MRC-5 cells. After the replication-deficient adenovirus Ad-EGFP infected cancer cells, there was no significant up-regulation of EGFP at 48, 72, and 96 hours (Figure 1B). By detecting the expression of survivin and E1a in cells after virus infection, we found E1a was strongly expressed in cancer cells after AdSProE1a-EGFP infection, while it was barely expressed in MRC-5 cells. The expression of survivin was consistent with E1a, which was up-regulated after transfection in cancer cells and with no significant change in MRC-5 cells. Neither E1a nor survivin was expressed in cells infected with Ad-EGFP (Figure 1C).
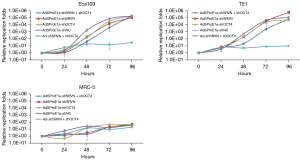
The virus titer was determined by TCID50 assay to evaluate the replication viability of the virus in different cells. The results showed that the oncolytic adenoviruses, AdSProE1a-shSRVN + shOCT4, AdSProE1a-shSRVN, AdSProE1a-shOCT4, and AdSProE1a-shNC, could all replicate in cancer cells, and replication activity gradually increased over time and started to drop at 96 hours after transfection. The oncolytic adenovirus containing AdSProE1a-shSRVN + shOCT4 had the highest replication of 192,085 times in Eca109 cells, and the oncolytic adenovirus carrying AdSProE1a-shSRVN had the highest replication of 620,055 times in TE1 cells; however, none of the oncolytic viruses had significant replication in MRC-5 cells. The replication-deficient adenovirus, Ad-shSRVN + shOCT4, had no obvious replication activity in both cancer cells and normal cells (Figure 2).
Effects of oncolytic adenovirus on gene expression in ESCC cells
Western blotting detected the expression of survivin, OCT4, E-cadherin, and vimentin. The results indicated that AdSProE1a-shSRVN + shOCT4, AdSProE1a-shSRVN, AdSProE1a-shOCT4, and Ad-shSRVN + shOCT4 could reduce the expression of survivin and vimentin in Eca109 and TE1 cells. At the same time, E-cadherin was up-regulated, especially in cells infected with oncolytic virus containing AdSProE1a-shSRVN + shOCT4. It was shown that AdSProE1a-shSRVN + shOCT4, AdSProE1a-shOCT4, and Ad-shSRVN + shOCT4 could down-regulate the expression of OCT4, especially AdSProE1a-shSRVN + shOCT4; AdSProE1a-shSRVN did not affect the expression of OCT4.
In MRC-5 cells, survivin was barely detected, and OCT4 was negatively expressed. All of AdSProE1a-shSRVN + shOCT4, AdSProE1a-shSRVN, and AdSProE1a-shOCT4 could down-regulate the expression of survivin in MRC-5 cells yet survivin was completely negative in MRC-5 cells transfected with oncolytic virus containing AdSProE1a-shSRVN + shOCT4. All oncolytic viruses had no significant effect on the expression of E-cadherin and vimentin in MRC-5 cells (Figure 3A). Confocal immunofluorescence staining was used to locate and quantify survivin and OCT4. Compared to the control group, AdSProE1a-shSRVN + shOCT4 significantly reduced the expression of survivin and OCT4 in Eca109 cells, while AdSProE1a-shNC and Ad-shSRVN + shOCT4 had no significant effect on the expression of survivin and OCT4 in cells (Figure 3B).

The effect of oncolytic adenovirus on the proliferation and cell cycle of ESCC cells
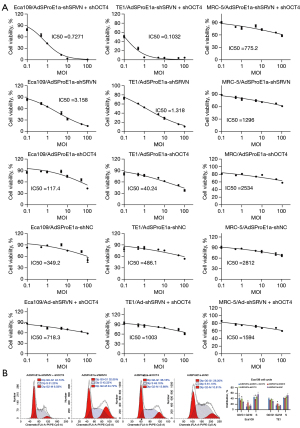
Cell proliferation ability after transfection was detected by the CCK-8 method. The cell survival rate was 100% at the starting point of culture (0 h), and the viability of Eca109 and TE1 cells in each group gradually decreased with the increase in the MOI value, while MRC-5 cells had no apparent response to the oncolytic virus. The IC50 value was used to evaluate the cytotoxicity of the oncolytic virus, and it was found that the knockdown of survivin or OCT4 significantly reduced the IC50 value. The IC50 of cancer cells transfected with AdSProE1a-shSRVN decreased significantly more than in any other group, except for the dual knockdown group. The cytotoxicity of AdSProE1a-shNC on cells originated from the oncolysis of viral replication, hence the inhibitory effect mediated by the replication-type oncolytic adenovirus was stronger than that mediated by the replication-deficient adenovirus (Figure 4A). Both replication-type oncolytic adenoviruses and replication-deficient adenoviruses have high IC50 values for MRC-5 inhibition. This study confirmed that the dual knockdown of survivin and OCT4 mediated by replicative oncolytic adenovirus not only strengthened the cytotoxicity of the cancer cells, but also improved the specificity of therapy against the cancer cells. Cell cycle identification showed that in Eca109 and TE1 cells, knockdown of OCT4 with AdSProE1a-shOCT4 mainly caused G0/G1 phase arrest, and AdSProE1a-shSRVN knockdown of survivin mainly caused G2/M phase arrest. The double knockdown of survivin and OCT4 by AdSProE1a-shSRVN + shOCT4 was still characterized by G0/G1 arrest (Figure 4B).
Oncolytic adenovirus-mediated dual knockdown of survivin and OCT4 inhibited xenograft growth in vivo
A xenograft model was established by subcutaneous injection of Eca109 cells followed by intratumoral injection of the virus. As a result, compared to the control group, the tumor inhibition rates of the AdSProE1a-shSRVN + shOCT4, AdSProE1a-shSRVN, AdSProE1a-shOCT4, AdSProE1a-shNC, Ad-shSRVN + shOCT4, and Ad-EGFP groups were 89.93% (P<0.0001), 81.43% (P<0.0001), 51.62% (P<0.0001), 35.94% (P<0.0001), 18.22% (P=0.0209), and 4.86% (P=0.5936), respectively. The results showed that the inhibitory effect of dual knockdown was significantly better than that of single-gene intervention, and the therapeutic effect mediated by replication-type oncolytic adenovirus was significantly stronger than that of a replication-deficient adenovirus vector, confirming that the oncolytic adenovirus with survivin and OCT4 dual knockdown could produce significant anticancer effects and improve ESCC treatment effects (Figure 5A).
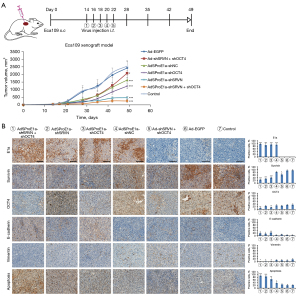
In addition, tumor specimens were taken for IHC examination. It was revealed that E1a was strongly expressed in AdSProE1a-shSRVN + shOCT4, AdSProE1a-shSRVN, AdSProE1a-shOCT4, and the AdSProE1a-shNC group and negative expression was observed in Ad-shSRVN + shOCT4, Ad-EGFP, and the control group. Survivin decreased in AdSProE1a-shSRVN + shOCT4, AdSProE1a-shSRVN, AdSProE1a-shOCT4, and in the Ad-shSRVN + shOCT4 group; OCT4 decreased in the AdSProE1a-shSRVN + shOCT4 and the AdSProE1a-shOCT4 group. However, E-cadherin and vimentin in tumors from AdSProE1a-shSRVN + shOCT4, AdSProE1a-shSRVN, and the AdSProE1a-shOCT4 group were up-regulated and down-regulated, respectively. The results of TUNEL revealed that except for the Ad-shSRVN + shOCT4 and Ad-EGFP group, the apoptosis rate of cancer cells in all groups, especially the AdSProE1a-shSRVN + shOCT4 group, significantly promoted apoptosis in cancer cells compared to the control group (Figure 5B).
Discussion
Our previous study found that survivin was expressed in 62% of ESCC tissues and 8% of adjacent normal esophageal tissues, suggesting that survivin might regulate the malignant biological behavior of ESCC cells and could be negatively correlated with the prognosis. Besides, inhibition of survivin could induce apoptosis, lead to cell cycle arrest, and inhibit the proliferation of cancer cells (13,19). We further explored the underlying mechanism and found that survivin was positively regulated by OCT4, and patients with a high expression of OCT4 and survivin showed a worse prognosis (13).
Oncolytic virus therapy has become an efficient therapy for malignant tumors (20). Our previous study confirmed that the surviving promoter-containing oncolytic adenovirus could achieve an effective anti-tumor effect. Replicability of oncolytic viruses eliminates the disadvantages of low expression of transfected genes and tumor-targeting inability, which is completely different from traditional chemotherapy, radiotherapy, and other adjuvant therapies (21). For EC, due to the high malignancy rate and the rapid proliferation of cancer cells, especially for tumor stem cells, single-gene intervention therapy was not sufficient to inhibit tumor growth and metastasis, which requires us to further optimize the treatment strategy. Therefore, we considered a gene therapy method combining oncolytic virus therapy and double-gene intervention, hoping to achieve a synergistic effect. Based on the high expression of survivin in EC and that it has been shown to be regulated by OCT4, we synthesized a specific replicative oncolytic adenovirus containing shRNA of survivin and OCT4 to realize dual knockdown, with the intention to establish an effective targeted therapy strategy. In detail, the expression of survivin increased with the proliferation of cancer cells, which initiated replication of the oncolytic virus and shRNAs targeting survivin and OCT4, resulting in cancer cell death. Furthermore, once the cancer cells were eliminated by the oncolytic virus and the expression of survivin was diminished, the virus stopped replicating and functioning. The entire feedback mechanism endowed the therapeutic system with higher specificity and safety, and the presence of the virus appeared to prevent the recurrence and metastasis of cancer cells and sustain an anti-tumor effect.
By detecting the expression of survivin and OCT4 in ESCC cells and the replication ability of the oncolytic adenovirus in cancer cells, we found that survivin was significantly upregulated in cancer cells, which was positively correlated with the replication ability of the oncolytic adenovirus. The massive replication of the oncolytic adenovirus enabled an efficient increase in the expression of EGFP and shRNA while having little effect on normal cells, such as MRC-5. Therefore, the oncolytic adenovirus regulated by the survivin-promoter could achieve higher specificity and safety in treatment used for ESCC.
In our study, it was found that the oncolytic adenovirus-mediated knockdown of survivin or OCT4 could inhibit the proliferative ability of cancer cells and induce cell cycle arrest. Compared to other groups, the inhibitory effect was more pronounced in the dual knockdown group. Target therapy mediated by the replicative oncolytic adenovirus was significantly more effective than that mediated by the replication-deficient adenovirus. It was also found in the experiment that the knockdown of OCT4 could down-regulate the expression of survivin, while the knockdown of survivin had no effect on OCT4, suggesting that OCT4 is an upstream regulator of survivin. The knockdown of survivin and OCT4 also influenced the cell cycle; OCT4 knockdown mainly caused G0/G1 phase arrest, and survivin knockdown mainly caused G2/M phase arrest.
In order to further explore the synergistic effect of the oncolytic adenovirus-mediated dual knockdown of survivin and OCT4, we constructed a xenograft model by subcutaneous injection of Eca109 cells. After intratumoral injection of virus treatment, we found that the curative effect of dual knockdown therapy was significantly better than single-gene intervention. The therapeutic effect mediated by the replication-type oncolytic adenovirus was significantly stronger than the replication-deficient adenovirus vector, confirming that the survivin and OCT4 dual knockdown oncolytic adenovirus therapeutic strategy could produce multiple effects and significantly improve the anti-tumor effect. Since EMT is a common molecular event in EC and is closely related to its recurrence and metastasis, we detected the expression levels of EMT-related molecules in ESCC cell lines and nude mice xenografts. Oncolytic adenovirus-mediated dual knockdown of OCT4 and survivin upregulated E-cadherin and downregulated vimentin in cancer cells and tumor tissues, suggesting the reversal of EMT in ESCC after treatment, which was considered one of the important mechanisms to achieve a therapeutic effect. It was recognized that EC cells highly expressed OCT4 and survivin, which endowed the cells with high proliferative and metastatic abilities by inducing EMT. The process of EMT was reversed after simultaneous interference by OCT4 and survivin led to the inhibition of tumor progression. However, there are some limitations in our study. First of all, the replication ability of oncolytic adenovirus in vivo and the corresponding virus titer displaying anti-tumor effect were not discussed. Besides, the consistency and stability of anti-tumor effect of the oncolytic adenovirus need to be explored in the following study. At last, researches about the side-effects of oncolytic adenovirus-mediated therapy in vivo and in vitro were not presented.
Conclusions
In conclusion, our study established an oncolytic adenovirus-mediated target therapy against EC. The replicative oncolytic adenovirus-mediated dual knockdown strategy, which was characterized by the survivin promoter as the core regulatory sequence, exerted an inhibitory effect on EC (Figure S1). Our study demonstrated that the dual target design strategy ensured the efficacy and safety of the treatment system and provided a novel and effective adjuvant target therapy for EC. In our future study, we will continue to explore the consistency and stability of anti-tumor effect of the oncolytic adenovirus. Besides, the underlying mechanism, especially the relevant signaling pathways, will be discussed and the side-effect of oncolytic adenovirus-mediated dual knockdown of survivin and OCT4 in vitro and in vivo will be explored.
Acknowledgments
We would like to thank the Editors of the AME Editing Service for their help in polishing our paper.
Funding: This work was sponsored by Natural Science Foundation of Shanghai (No. 19ZR1456400), 2020 “Hospital New Star” Young Medical Talent Program of Shanghai to CGL, the National Natural Science Foundation of China (No. 82172756) and National Key Research and Development Program of China (No. 2021YFC2501005).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4499/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4499/dss
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4499/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4499/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animal experiments were approved by the Ethics Committee of Shanghai Chest Hospital, in compliance with guidelines for the care and use of animals of Shanghai Jiao Tong University School of Medicine.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Chen Y, Zhu SM, Xu XL, et al. Expression levels of HER2 and MRP1 are not prognostic factors of long-term survival in 829 patients with esophageal squamous cell carcinoma. Oncol Lett 2016;11:745-52. [Crossref] [PubMed]
- Chen Y, Bieerkehazhi S, Li X, et al. Survivin Regulates Bad Gene Expression by Binding to Its Promoter and Modulates Cell Cycle and Apoptosis in Esophageal Carcinoma Cell. J Oncol 2021;2021:1384289. [Crossref] [PubMed]
- Santarelli A, Mascitti M, Lo Russo L, et al. Survivin-Based Treatment Strategies for Squamous Cell Carcinoma. Int J Mol Sci 2018;19:971. [Crossref] [PubMed]
- Puskas R, Bikov A, Horvath P, et al. Circulating Survivin Protein Levels in Lung Cancer Patients Treated With Platinum-Based Chemotherapy. Pathol Oncol Res 2021;27:631969. [Crossref] [PubMed]
- Shao Q, Xu J, Deng R, et al. The expressions of YAP1, β-catenin and survivin in colon cancer tissues and their clinical significance. Int J Clin Exp Pathol 2018;11:6032-8. [PubMed]
- Chang WH, Nguyen TT, Hsu CH, et al. KRAS-dependent cancer cells promote survival by producing exosomes enriched in Survivin. Cancer Lett 2021;517:66-77. [Crossref] [PubMed]
- Miyao T, Koike H, Sekine Y, et al. YM155 Reverses Cabazitaxel Resistance in Castration-resistant Prostate Cancer by Reducing Survivin Expression. Anticancer Res 2020;40:5091-5. [Crossref] [PubMed]
- Li K, Liu T, Chen J, et al. Survivin in breast cancer-derived exosomes activates fibroblasts by up-regulating SOD1, whose feedback promotes cancer proliferation and metastasis. J Biol Chem 2020;295:13737-52. [Crossref] [PubMed]
- Ahmad B, Gamallat Y, Su P, et al. Alantolactone induces apoptosis in THP-1 cells through STAT3, survivin inhibition, and intrinsic apoptosis pathway. Chem Biol Drug Des 2021;97:266-72. [Crossref] [PubMed]
- Vallböhmer D, Peters JH, Oh D, et al. Survivin, a potential biomarker in the development of Barrett's adenocarcinoma. Surgery 2005;138:701-6; discussion 706-7. [Crossref] [PubMed]
- Hu H, Li Z, Chen J, et al. P16 reactivation induces anoikis and exhibits antitumour potency by downregulating Akt/survivin signalling in hepatocellular carcinoma cells. Gut 2011;60:710-21. [Crossref] [PubMed]
- Li C, Yan Y, Ji W, et al. OCT4 positively regulates Survivin expression to promote cancer cell proliferation and leads to poor prognosis in esophageal squamous cell carcinoma. PLoS One 2012;7:e49693. [Crossref] [PubMed]
- Li Z, Li X, Li C, et al. Transcription factor OCT4 promotes cell cycle progression by regulating CCND1 expression in esophageal carcinoma. Cancer Lett 2014;354:77-86. [Crossref] [PubMed]
- van Vloten JP, Matuszewska K, Minow MAA, et al. Oncolytic Orf virus licenses NK cells via cDC1 to activate innate and adaptive antitumor mechanisms and extends survival in a murine model of late-stage ovarian cancer. J Immunother Cancer 2022;10:e004335. [Crossref] [PubMed]
- Nelson A, Gebremeskel S, Lichty BD, et al. Natural killer T cell immunotherapy combined with IL-15-expressing oncolytic virotherapy and PD-1 blockade mediates pancreatic tumor regression. J Immunother Cancer 2022;10:e003923. [Crossref] [PubMed]
- Khalique H, Baugh R, Dyer A, et al. Oncolytic herpesvirus expressing PD-L1 BiTE for cancer therapy: exploiting tumor immune suppression as an opportunity for targeted immunotherapy. J Immunother Cancer 2021;9:e001292. [Crossref] [PubMed]
- Hamdan F, Ylösmäki E, Chiaro J, et al. Novel oncolytic adenovirus expressing enhanced cross-hybrid IgGA Fc PD-L1 inhibitor activates multiple immune effector populations leading to enhanced tumor killing in vitro, in vivo and with patient-derived tumor organoids. J Immunother Cancer 2021;9:e003000. [Crossref] [PubMed]
- Li C, Li Z, Zhu M, et al. Clinicopathological and prognostic significance of survivin over-expression in patients with esophageal squamous cell carcinoma: a meta-analysis. PLoS One 2012;7:e44764. [Crossref] [PubMed]
- Cui C, Wang X, Lian B, et al. OrienX010, an oncolytic virus, in patients with unresectable stage IIIC-IV melanoma: a phase Ib study. J Immunother Cancer 2022;10:e004307. [Crossref] [PubMed]
- Zhang Y, Fang L, Zhang Q, et al. An oncolytic adenovirus regulated by a radiation-inducible promoter selectively mediates hSulf-1 gene expression and mutually reinforces antitumor activity of I131-metuximab in hepatocellular carcinoma. Mol Oncol 2013;7:346-58. [Crossref] [PubMed]


