
Prolonged cannulation time is an independent risk factor for moderate-to-severe post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis: a large cohort study
Highlight box
Key findings
• In this large retrospective cohort study of nearly 7,000 patients undergoing endoscopic retrograde cholangiopancreatography (ERCP), we found that prolonged cannulation time significantly influenced the occurrence of moderate-to-severe (MS) pancreatitis.
What is known and what is new?
• The risk factors for developing post-ERCP pancreatitis (PEP) include a younger age, being female, suspected sphincter of Oddi dysfunction, normal liver function, and difficult cannulation.
• We found that a total cannulation time >15 min was an independent risk factor for MS pancreatitis.
What is the implication, and what should change now?
• Our findings indicate that endoscopists should try to avoid significantly prolonged cannulation times (e.g., >15 min). The risks for MS PEP can be reduced in a number of ways, including by the early switching to precut sphincterotomy, or seeking help from a more experienced endoscopist.
Introduction
Acute pancreatitis is the most frequent complication of endoscopic retrograde cholangiopancreatography (ERCP) (1). The clinical outcomes of post-ERCP pancreatitis (PEP) patients vary widely depending on the different severity. Mild PEP is mostly self-limiting, and has a rapid recovery and short hospitalization time. However, some patients may develop moderate-to-severe PEP (MS PEP), resulting in prolonged hospitalization, intensive care unit admission, and an increased use of hospital resources. These patients have significant morbidity and mortality (2). Previous studies have shown that the incidence of PEP ranges from 3–15%, and the incidence of severe PEP ranges from 0.13–5% (3-8). As MS PEP is more likely to cause substantial damage to patients, studying it should be a priority in PEP research. However, to date, few studies have been conducted on MS PEP.
The correct identification of patients at risk of PEP is important, as it can help to prevent the occurrence of PEP and reduce its harmful consequences. Previous studies have identified numerous risk factors for developing PEP (1,9,10). These factors can mainly be categorized into patient-related and procedure-related factors (11). Patient-related factors that have been reported include being of a younger age, being female, suspected sphincter of Oddi dysfunction (SOD), and normal liver function. Procedure-related factors include difficult cannulation [e.g., a cannulation time >5 min, >5 cannulation attempts, and >1 inadvertent pancreatic duct (PD) cannulation], and frequent contrast injections into the PD (1,9). A recent study also reported that a fatty pancreas is a risk factor in the development of PEP (12). However, the majority of previous studies have not attempted to distinguish between the risk factors associated with overall PEP and those of MS PEP. As mild PEP accounts for the majority of cases of PEP, the risk factors mentioned above mainly represent the outcomes of cases of mild PEP. Several studies have investigated the risk factors associated with MS PEP (10,13-15). However, these studies have been limited by their small sample sizes or the inclusion of inadequate factors (Table S1).
As the incidence rates and the clinical consequences of mild and MS PEP differ, we hypothesized that the risk factors for PEP of various severities might also differ. We conducted a retrospective study to investigate whether the risk factors of MS PEP were distinct to those of mild PEP. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4697/rc).
Methods
Patients
Consecutive patients with native papilla who had undergone elective ERCP from January 2010 to February 2022 at Xijing Hospital in China were enrolled in this retrospective study. Patient-related and procedure-related information was retrieved from a prospectively maintained database, as previously described (16). Patients were excluded from the study if they met any of the following exclusion criteria: (I) had been diagnosed with acute pancreatitis within 7 days before the procedure; (II) had previous episodes of acute pancreatitis with existing local complications; and/or (III) had organ failure before undergoing ERCP. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Xijing Hospital (No. KY20201005-F-3) and individual consent for this retrospective analysis was waived.
ERCP procedure
For the ERCP, each patient lay in the left lateral position, and the initial cannulation was performed using a sphincterotome with a guidewire. If the initial cannulation was unsuccessful or difficulties were experienced (e.g., if the guidewire entered the PD), the double-wire technique (DWT) could be used for further cannulation. Precut sphincterotomy techniques, including the needle-knife and transpancreatic precut sphincterotomy, were also applied to complete the cannulation as appropriate. The success of deep-biliary cannulation was judged by the direction of guidewire travel or contrast injection. Therapeutic manipulation (e.g., a sphincterotomy, balloon dilation, stone extraction, and stenting) were performed as appropriate (7). To prevent PEP, rectal indomethacin (100 mg) before or after ERCP was administered and/or a phylactic plastic stent was implanted, both of which were performed at the discretion of the endoscopists. Aggressive hydration with lactated ringer solution was not routinely administered unless PEP was suspected after ERCP. Trainees were allowed to perform the initial selective cannulation with standard cannulation methods for 10 min, as described previously (7). After undergoing ERCP, each patient temporarily fasted, and gradually resumed their diet if they displayed no obvious symptoms for 6 hours. Each patient’s vital signs, such as abdominal symptoms and cardiopulmonary function, were observed. Serum amylase assays were routinely performed 24 hours after the patients underwent ERCP.
Data collection
The following patient-related data were collected from the database: age, gender, body mass index (BMI), indications for ERCP [e.g., common bile duct stones (CBDS)], malignant biliary strictures, benign or indeterminate biliary strictures, suspected SOD, and pancreatic disease), laboratory test results [including alanine aminotransferase (ALT), total bilirubin (TBIL), and alkaline phosphatase (ALP)], prior history of surgery, and comorbidities. The following procedure-related information was also collected: the cannulation method, the number of cannulation attempts, the cannulation time, the number of inadvertent PD cannulations, pancreatic contrast injection, and trainee involvement.
Outcomes and definitions
The primary outcome of this study was the incidence of PEP. According to Cotton criteria, PEP was diagnosed when newly onset or worsening abdominal pain emerged that was associated with an elevation in serum amylase of at least 3 times the normal range 24 hours after ERCP and hospitalization for at least 48 hours (17). Under Cotton criteria, the severity of the pancreatitis was graded as mild, moderate, and severe when hospitalization was prolonged by 2 to 3 days, 4 to 10 days, and >10 days, respectively. Under Cotton criteria, severe pancreatitis was also characterized by the occurrence of hemorrhagic pancreatitis, pancreatic necrosis, pseudocysts, or the need for interventional operations. According to the revised Atlanta criteria (18), the severity of pancreatitis was classified into the following categories: mild (in which there was no organ failure, local or systemic complications); moderate (in which there was transient organ failure and/or local complications); and severe (in which there was persistent organ failure, possibly causing death). The total cannulation time was counted from the beginning of contact with the papilla to the deep cannulation of the common bile duct. A cannulation attempt was defined as the sphincterotome touching the papilla for at least 5 seconds.
Statistical analysis
The results for the continuous variables are expressed as the mean ± standard deviation, or the median and interquartile range (IQR) and were analyzed using the Student’s t-test or non-parametric test as appropriate. The categorical variables are expressed as the frequency or percentage and were analyzed using the chi-square test or Fisher’s exact test. A univariate logistic regression analysis was conducted to analyze the possible risk factors for PEP. To assess the risk factors associated with PEP, a multivariate logistic analysis with the forward stepwise method was conducted using variables with a P value <0.1 from the univariate logistic analysis. To reduce any bias caused by the small sample size, a Firth (19) logistic regression analysis was conducted to identify the risk factors associated with Atlanta-related MS PEP. The regression analysis results are expressed as the odds ratio (OR) value and the 95% confidence interval (CI). The results of the Firth logistic regression analysis are presented as the relative risk (RR) and 95% CI. The statistical analyses were performed using SPSS 24.0 (IBM, Armonk, NY, USA). A two-sided P value >0.05 indicated a statistically significant difference.
Results
Patient characteristics
A total of 7,387 patients with native papilla who had undergone ERCP from January 2010 to February 2022 were considered for this study. A total of 443 patients were excluded due to recent pancreatitis, the presence of pancreatitis-related complications, or organ failure before ERCP. Thus, 6,944 patients were ultimately included in the analysis. As Table 1 shows, the patients had a median age of 61.0 (IQR, 49.0–72.0) years, about half of the patients were female, 36.0% (2,497/6,944) of the patients had previously undergone a cholecystectomy, and the most common ERCP indication was CBDS (63.1%, 4,385/6,944).
Table 1
| Variables | All patients (n=6,944) | Patients complicated with PEP | |||||
|---|---|---|---|---|---|---|---|
| No (n=6,582) | Yes (n=362) | ||||||
| Cotton criteria | Revised Atlanta criteria | ||||||
| Mild (n=286) | MS (n=76) | Mild (n=345) | MS (n=17) | ||||
| Age (years), medium (IQR) | 61.0 (49.0–72.0) | 61.0 (49.0–72.0) | 60.0 (48.8–70.0) | 63.0 (51.0–71.0) | 60.0 (49.3–70.0) | 64.0 (49.5–80.5) | |
| BMI (kg/m2), median (IQR) | 22.5 (20.2–25.8) | 22. (20.2–24.8) | 22.5 (20.5–24.2) | 22.7 (20.0–25.4) | 22.5 (20.3–24.6) | 23.2 (21.3–25.8) | |
| Female, n (%) | 3,450 (49.7) | 3,226 (49.0) | 174 (60.8) | 50 (65.8) | 216 (62.6) | 8 (47.1) | |
| Comorbidities, n (%) | |||||||
| Diabetes | 637 (9.2) | 608 (9.2) | 25 (8.7) | 4 (5.3) | 27 (7.8) | 2 (11.8) | |
| Hypertension^ | 1,472 (21.2) | 1,411 (21.4) | 52 (18.2) | 9 (11.8) | 59 (17.1) | 2 (11.8) | |
| Cirrhosis | 90 (1.3) | 88 (1.3) | 2 (0.7) | 0 | 2 (0.6) | 0 | |
| Cholecystectomy | 2,497 (36.0) | 2,369 (36.0) | 104 (36.4) | 24 (31.6) | 122 (35.4) | 6 (35.3) | |
| Digestive tract reconstruction | 68 (1.0) | 61 (0.9) | 6 (2.1) | 1 (1.3) | 6 (1.7) | 1 (5.9) | |
| Prior history of pancreatitis | 442 (6.4) | 424 (6.4) | 15 (5.2) | 3 (3.9) | 18 (5.2) | 0 | |
| Indications, n (%) | |||||||
| CBDS | 4,385 (63.1) | 4,165 (63.3) | 172 (60.1) | 48 (63.2) | 210 (60.9) | 10 (58.8) | |
| Malignant BS | 1,219 (17.6) | 1,154 (17.5) | 53 (18.5) | 15 (19.7) | 64 (18.6) | 4 (23.5) | |
| Benign or indeterminate BS | 398 (5.7) | 384 (5.8) | 11 (3.8) | 3 (3.9) | 13 (3.8) | 1 (5.9) | |
| Pancreatic diseases | 206 (3.0) | 195 (3.0) | 9 (3.1) | 2 (2.6) | 11 (3.2) | 0 | |
| Suspected SOD | 265 (3.8) | 251 (3.7) | 12 (4.2) | 1 (1.3) | 13 (3.8) | 1 (5.9) | |
| Others | 521 (7.5) | 486 (7.4) | 29 (10.1) | 6 (7.9) | 34 (9.9) | 1 (5.9) | |
| Laboratory tests, n (%) | |||||||
| TBIL <1× ULN | 2,266 (32.6) | 2,188 (32.2) | 121 (42.3) | 27 (35.5) | 141 (40.9) | 7 (5.9) | |
| ALP <1× ULN | 1,535 (22.1) | 1,439 (21.9) | 81 (28.3) | 15 (19.7) | 91 (26.4) | 5 (29.4) | |
| ALT <1× ULN | 2,180 (31.4) | 2,036 (30.9) | 117 (40.9) | 27 (35.5) | 136 (39.4) | 8 (47.1) | |
^, hypertension was diagnosed if the blood pressure reading was ≥140/90 mmHg. A diagnosis of hypertension was usually based on the average of 2 or more readings taken on separate occasions. (NICE Guidelines, Hypertension in Adults: Diagnosis and Management, 2022 version). PEP, post-endoscopic retrograde cholangiopancreatography pancreatitis; IQR, interquartile range; MS, moderate-to-severe; BMI, body mass index; CBDS, common bile duct stone; SOD, sphincter of Oddi dysfunction; BS, biliary stricture; TBIL, total bilirubin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; ULN, upper limit of normal; NICE, National Institute for Health and Care Excellence.
The overall incidence of PEP was 5.2% (362/6,944). According to the Cotton criteria, 76 (1.1%) patients had MS PEP, while according to the revised Atlanta criteria, 17 (0.2%) patients had MS PEP (Table 1). The calculation of the incidence of PEP excluded patients with pancreatic head cancers (Table S2).
ERCP procedure variables among patients with different severities of PEP
The procedure-related characteristics are shown in Table 2. The overall cannulation success rate was 97.1% (6,744/6,944), which was primarily achieved by standard cannulation (83.9%, 5,828/6,944). The median cannulation time was 4.4 (1.2–12.2) min. The median cannulation attempts, and inadvertent PD cannulations were 3.0 (1.0–6.0) and 0 (0.0–1.0) times, respectively (Table 2).
Table 2
| Variables | All patients (n=6,944) | Patients complicated with PEP | |||||
|---|---|---|---|---|---|---|---|
| No (n=6,582) | Yes (n=362) | ||||||
| Cotton criteria | Revised Atlanta criteria | ||||||
| Mild (n=286) | MS (n=76) | Mild (n=345) | MS (n=17) | ||||
| Cannulation success rate, n (%) | 6,744 (97.1) | 6,396 (97.2) | 275 (96.2) | 73 (96.1) | 332 (96.2) | 16 (94.1) | |
| Cannulation method, n (%) | |||||||
| Standard, n (%) | 5,828 (83.9) | 5,574 (84.7) | 200 (69.9) | 54 (71.1) | 246 (71.3) | 8 (47.1) | |
| Double-wire technique, n (%) | 271 (3.9) | 249 (3.8) | 17 (5.9) | 5 (6.6) | 19 (5.5) | 3 (17.6) | |
| Precut sphincterotomy*, n (%) | 845 (12.2) | 759 (11.5) | 69 (24.1) | 17 (22.3) | 80 (23.2) | 6 (35.3) | |
| Cannulation time (min), median (IQR) | 4.4 (1.2–12.2) | 4.3 (1.2–12.0) | 8.2 (3.0–18.0) | 8.7 (2.6–20.1) | 7.90 (2.7–18.3) | 19.2 (7.8–36.8) | |
| Cannulation attempts (times), median (IQR) | 3.0 (1.0–6.0) | 3.0 (1.0–6.0) | 4.0 (2.0–8.0) | 5.0 (2.0–9.3) | 4.0 (2.0–8.0) | 7.0 (4.5–16.0) | |
| Inadvertent PD cannulation (times), median (IQR) | 0.0 (0.0–1.0) | 1.0 (0.0–1.0) | 1.0 (0.0–2.0) | 1.0 (0.0–2.5) | 1.0 (0.0–2.0) | 1.5 (0.0–2.5) | |
| Pancreatic contrast injection, n (%) | 201 (2.9) | 179 (2.7) | 17 (5.9) | 5 (6.6) | 21 (6.1) | 1 (5.9) | |
| Trainee involvement, n (%) | 3,746 (53.9) | 3,562 (54.1) | 150 (52.4) | 34 (44.7) | 172 (49.9) | 12 (70.6) | |
| Prevention method, n (%) | |||||||
| Prophylactic PD stent | 654 (9.4) | 602 (9.1) | 41 (14.3) | 11 (14.5) | 46 (13.3) | 6 (35.3) | |
| Indomethacin | 1,689 (24.3) | 1,588 (24.1) | 83 (29.0) | 18 (23.7) | 97 (28.1) | 4 (23.5) | |
*, including patients undergoing precut sphincterotomy following DWT. PEP, post-endoscopic retrograde cholangiopancreatography pancreatitis; MS, moderate-to-severe; IQR, interquartile range; PD, pancreatic duct; DWT, double-wire technique.
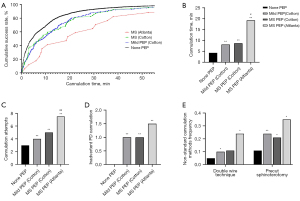
As Figure 1 shows, compared to patients without PEP, those with mild or MS PEP had a longer cannulation time, more cannulation attempts, more inadvertent PD cannulations, and more uses of non-standard cannulation techniques (all P<0.05). Further, the cannulation times gradually increased in patients with non-PEP, Cotton-related mild PEP, Cotton-related MS PEP, and Atlanta-related MS PEP (all P<0.001 among the 4 groups). The number of cannulation attempts, and inadvertent PD cannulations also increased progressively across the 4 groups. The use of the DWT (24% vs. 5–11%) and precut sphincterotomy (35% vs. 11–21%) in patients with Atlanta-related MS PEP was significantly higher than that in the other 3 groups.
Univariate logistic regression analysis for different severities of PEP
In relation to overall and mild PEP, among the 14 patient-related variables, the univariate logistic regression analysis revealed that being female and having normal liver function were high-risk factors (all P<0.05). Among the 9 procedure-related, the cannulation method (i.e., DWT and precut sphincterotomy), a cannulation time >15 min, cannulation attempts ≥8 times, inadvertent PD cannulation ≥1 time, and a prophylactic PD stent were identified as risk factors associated with both overall and mild PEP (all P<0.05).
In relation to both Cotton-related and Atlanta-related MS PEP, precut sphincterotomy, a cannulation time >15 min, cannulation attempts ≥8 times, and inadvertent PD cannulation ≥1 time were found to be high-risk factors (all P<0.05). Further, some factors, including being female, having hypertension, and benign or undetermined biliary stricture, were associated with Cotton-related MS PEP alone, while a prophylactic PD stent was a potential risk factor for Atlanta-related MS PEP alone (all P<0.05; Table 3).
Table 3
| Variables | Overall PEP | Cotton criteria | Revised Atlanta criteria | |||
|---|---|---|---|---|---|---|
| Mild PEP | MS PEP | Mild PEP | MS PEP | |||
| Patient-related factors | ||||||
| Female | 1.67 (1.34–2.07)#*** | 1.58 (1.24–2.01)#*** | 2.03 (1.26–3.28)#** | 1.70 (1.36–2.12)#*** | 0.88 (0.34–2.28) | |
| Hypertension | 0.74 (0.56–0.98)#* | 0.81 (0.60–1.11) | 0.50 (0.25–0.99)#* | 0.76 (0.59–1.01) | 0.60 (0.12–1.93) | |
| ERCP indications | ||||||
| CBDS | Reference | Reference | Reference | Reference | Reference | |
| Malignant BS, | 1.48 (1.09–2.01)#* | 1.39 (0.99–1.97) | 1.80 (0.97–3.36) | 1.43 (1.05–1.96)#* | 2.78 (0.78–8.71) | |
| Benign or indeterminate BS | 1.70 (1.10–2.62)#* | 1.41 (0.85–2.37) | 2.73 (1.26–5.94)#* | 1.70 (1.09–2.64)#* | 2.42 (0.25–11.13) | |
| Suspected SOD | 1.76 (0.98–3.15) | 1.85 (0.98–3.48) | 1.34 (0.32–5.61) | 1.53 (0.82–2.90) | 1.53 (0.01–33.66) | |
| Pancreatic diseases | 1.00 (0.49–2.07) | 1.10 (0.51–2.38) | 0.64 (0.09–4.69) | 1.05 (0.51–2.16) | 8.08 (1.50–30.50) | |
| Others | 1.37 (0.94–1.98) | 1.26 (0.82–1.93) | 1.78 (0.85–3.71) | 1.34 (0.91–1.96) | 2.36 (0.44–8.84) | |
| Laboratory test results | ||||||
| TBIL <1× ULN | 1.48 (1.19–1.84)#** | 1.53 (1.21–1.95)#** | 1.17 (0.67–2.04) | 1.48 (1.18–1.85)#** | 1.42 (0.53–3.61) | |
| ALP <1× ULN | 1.63 (1.22–2.19)#* | 1.67 (1.22–2.28)#** | 1.34 (0.68–2.65) | 1.63 (1.22–2.19)#** | 1.44 (0.44–4.54) | |
| ALT <1× ULN | 1.62 (1.29–2.05)#*** | 1.71 (1.33–2.20)#*** | 1.18 (0.67–2.08) | 1.62 (1.28–2.04)#*** | 2.64 (0.91–7.61) | |
| Procedure-related factors | ||||||
| Cannulation methods | ||||||
| Standard cannulation | Reference | Reference | Reference | Reference | Reference | |
| Double-wire technique | 1.94 (1.23–3.05)#** | 1.77 (1.07–32.95)#* | 1.90 (0.75–4.78) | 1.61 (0.99–2.61)#* | 6.84 (1.29–24.94)#* | |
| Precut sphincterotomy | 2.49 (1.93–3.21)#*** | 2.50 (1.88–3.32)#*** | 2.19 (1.26–3.80)#** | 2.37 (1.82–3.09)#*** | 5.33 (1.82–14.82)#* | |
| Cannulation time >15 min | 2.13 (1.67–2.67)#*** | 1.95 (1.51–2.52)#*** | 2.79 (1.76–4.42)#*** | 2.01 (1.59–2.53)#*** | 5.73 (2.18–15.09)#*** | |
| Cannulation attempts ≥8 | 1.61 (1.25–2.01)#*** | 1.56 (1.18–2.06)#*** | 1.78 (1.06–2.98)#* | 1.56 (1.21–2.02)#** | 3.50 (1.24–9.15)#* | |
| Inadvertent PD cannulation ≥1 | 3.07 (2.40–3.93)#*** | 3.03 (2.30–4.01)#*** | 2.90 (1.73–4.87)#*** | 3.02 (2.35–3.89)#*** | 4.35 (1.12–16.84)#* | |
| Trainee involvement, | 0.86 (0.69–1.06) | 0.92 (0.73–1.17) | 0.67 (0.43–1.06) | 0.82 (0.66–1.02) | 1.89 (0.71–5.71) | |
| Pancreatic contrast injection ≥1 | 2.32 (1.47–3.65)#*** | 2.22 (1.33–3.71)#** | 2.40 (0.96–6.00) | 2.31 (1.45–3.68)#*** | 2.94 (0.32–11.88) | |
| Prevention methods | ||||||
| Prophylactic PD stent | 1.67 (1.23–2.26)#*** | 1.66 (1.18–2.34)#** | 1.64 (0.86–3.12) | 1.52 (1.10–2.09)#* | 4.93 (1.63–13.14)#** | |
| Indomethacin | 1.22 (0.96–1.54) | 1.29 (0.99–1.67) | 0.97 (0.57–1.64) | 1.23 (0.97–1.57) | 1.11 (0.33–3.07) | |
The data are presented as the odds ratio (95% CI) or relative risk (95% CI). #, P<0.1; *, P<0.05; **, P<0.01; ***, P<0.001. PEP, post-endoscopic retrograde cholangiopancreatography pancreatitis; MS, moderate-to-severe; BMI, body mass index; ERCP, endoscopic retrograde cholangiopancreatography; CBDS, common bile duct stone; SOD, sphincter of Oddi dysfunction; BS, biliary stricture; TBIL, total bilirubin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; PD, pancreatic duct; ULN, upper limit of normal; CI, confidence interval.
Multivariate regression analysis for different severities of PEP
A multivariate analysis was performed based on the results of the univariate analysis when the P value was <0.10. Being female, an ALT less than the upper limit of normal (ULN) (<1× ULN), and inadvertent PD cannulation were found to be independent risk factors for overall and mild PEP. In relation to Cotton-related MS PEP, the multivariate regression analysis revealed that being female (OR, 2.48; 95% CI: 1.36–4.51), inadvertent PD cannulation ≥1 time (OR, 2.17; 95% CI: 1.23–3.82), and a total cannulation time >15 min (OR, 2.07; 95% CI: 1.14–3.77) were independent risk factors (all P<0.05). Conversely, the Firth logistic regression analysis revealed that a cannulation time >15 min (OR, 3.80; 95% CI: 1.07–14.03) was the only independent risk factor for Atlanta-related MS PEP (Table 4). The results of the regression analysis based on the 5-5-1 criteria are also set out in Tables S3,S4 (1).
Table 4
| Variables | Overall PEP | Cotton criteria | Revised Atlanta criteria | |||
|---|---|---|---|---|---|---|
| Mild PEP | MS PEP | Mild PEP | MS PEP | |||
| Female | 2.06 (1.30–3.29)** | 1.95 (1.18–3.22)** | 2.48 (1.36–4.51)** | 2.28 (1.41–3.71)** | – | |
| ALT <1× ULN | 1.80 (1.15–2.81)* | 1.64 (1.10–2.67)* | – | 1.77 (1.20–2.79)* | – | |
| Inadvertent PD cannulation | 4.96 (3.15–7.81)*** | 4.45 (2.74–7.25)*** | 2.17 (1.23–3.82)** | 5.10 (3.20–8.12)*** | – | |
| Cannulation time >15 min | – | – | 2.07 (1.14–3.77)* | – | 3.80 (1.07–14.03)* | |
The data are presented as the odds ratio (95% CI) or relative risk (95% CI). *, P<0.05; **, P<0.01; ***, P<0.001. PEP, post-endoscopic retrograde cholangiopancreatography pancreatitis; MS, moderate-to-severe; ALT, alanine aminotransferase; PD, pancreatic duct; ULN, upper limit of normal; CI, confidence interval.
Discussion
The most common complication after ERCP is pancreatitis, which has an incidence of approximately 3–15% (1,3,20). The incidence of pancreatitis after ERCP is associated with differences in the susceptibility factors and intraoperative mechanical or chemical injury of patients (20). The incidence rate of PEP in this study was 5.4%. The incidence rates of Cotton-related and Atlanta-related MS PEP were 1.1% and 0.2%, respectively. These results were similar to or lower than those reported previously (1,3). To investigate whether the risk factors for MS PEP were distinct from those for mild PEP, 2 classification criteria were chosen in this study. Our study revealed that the proportions and the independent risk factors related to MS PEP, especially as defined by the revised Atlanta criteria, were quite different to those defined by the Cotton criteria. To the best of our knowledge, this was the first large retrospective cohort study to reveal that prolonged cannulation time significantly affected the occurrence of MS PEP. Injuries caused by MS PEP are substantial and harmful; thus, endoscopists should pay close attention to difficulties with cannulation.
In the present study, being female, inadvertent PD cannulation, and ALT <1× ULN were found to be independent risk factors for overall PEP. This finding has been reported in many previous studies and guidelines (1,3,21-23). Conversely, other reported risk factors, such as being aged <50 years, suspected SOD, previous PEP, and pancreatic injection, were not significantly associated with PEP in our study. The reason for this finding might be due to the different characteristics of the enrolled patients at the teaching hospital. Our study only enrolled patients with native papilla, and the proportion of patients with a suspected SOD indication was low (2.4%). Pancreatic injections were rarely performed due to the risk of PEP at our center. As we expected, the independent risk factors for mild PEP were similar to those for overall PEP, which might be a result of the overrepresentation of mild PEP in all patients with PEP.
Recently, several studies on the risk factors for MS PEP have been published. A large retrospective study from the United States based on an inpatient database showed that an older age, being male, and multiple comorbidities were the main independent predictors of MS PEP (10). Some reports have indicated that pancreatic volume, obesity, and the neutrophil-lymphocyte ratio might also be independent risk factors for MS PEP (13-15). However, these studies were limited by their small sample sizes (n<300). Due to the rarity of MS PEP, the sample of any relevant study needs to be sufficiently large to detect significant differences. Notably, the studies mentioned above did not include procedure-related variables, such as the cannulation time, the number of cannulation attempts, the number of inadvertent PD cannulations, and the application of advanced cannulation techniques, which have been shown to be closely related to the occurrence of PEP and might play additive or synergistic roles in the development of MS PEP (10,13,14).
In the present study, a total cannulation time >15 min was found to be an independent risk factor for both Cotton-related and Atlanta-related MS PEP. The median cannulation time in patients who developed Atlanta-related MS PEP was 19.2 (7.8–36.8) min, which was >4 times that of the time spent performing the general cannulation. A significant prolongation in cannulation time was often accompanied by an increasing number of cannulation attempts and the possibility of inadvertent PD cannulation, which can result in edema and damage to the PD sphincter and increase the possibility of MS PEP. Our results suggest that endoscopists should seek to avoid significantly prolonged cannulation (i.e., a cannulation time >15 min). Early switching to precut sphincterotomy or seeking help from more experienced endoscopists are options that could be applied to reduce the risks of MS PEP.
Endoscopists have used multiple pharmacological and procedural interventions to reduce the risk of PEP over the last few decades. Rectal nonsteroidal anti-inflammatory drugs are the mainstay of PEP prophylaxis (1,24,25). However, as Table S5 shows, the use of rectal indomethacin was not found to be associated with a lower incidence of PEP in this study. It may be that the risks of PEP differ between patients who receive indomethacin and those who do not. However, our data showed that the use of pre-procedure rectal indomethacin (vs. no use of indomethacin) tended to play protective role in preventing overall PEP (4.1% vs. 5.0%), Cotton-related MS PEP (0.7% vs. 1.1%), and Atlanta-related PEP (0.1% vs. 0.2%); however, the differences were not significant. Our results are consistent with those of Luo, who found that pre-procedure rectal indomethacin played a protective role in preventing overall PEP and Cotton-related MS PEP (25).
Prophylactic PD stents are an effective method for preventing PEP (1). PD stents were implanted in 654 patients in this study. As Table S6 shows, the patients who received PD stent implants had a higher incidence of overall PEP, Cotton-related MS PEP, and Atlanta-related PEP than those who did not receive stent implants. However, this finding could be explained conservatively, as the effects of PD stents on PEP can be confounded by difficult cannulation and unsuccessful PD stent placement. In the present study, patients with PD stents had a significantly higher rate of difficult cannulation than those without PD stents (90.5% vs. 60.7%). Further, due to the retrospective nature of this study, the data of patients undergoing unsuccessful placement of PD stents were unavailable. Unsuccessful attempts may increase the risks of PEP (26). Overall, and as several guidelines suggest, PD stents are still a good choice for the prevention of PEP, especially in patients undergoing unintended PD cannulation (24,27).
This study had some limitations. First, this study had a single-center retrospective design. Thus, its findings may be subject to change due to bias. However, the ERCP database is prospectively maintained, and nearly all of the variables related to PEP were recorded in detail. Additionally, the sample size of this study was large, the inclusion and exclusion criteria were strictly controlled, and patients with native papillae were selected to minimize bias. However, the potential risk factors identified in this study need to be verified by other large-scale prospective multicenter studies in the future. Second, the data collected in this study spanned a long period of >10 years. Rectal indomethacin and prophylactic PD stents were less frequently used in the early stage of this period, and these prophylactic methods might be associated with the incidence of MS PEP (Table S7).
Conclusions
This large cohort study confirmed that some traditional risk factors, such as female gender, inadvertent PD cannulation, and ALT <1× ULN, were independently associated with PEP and mild PEP. However, the risk factors for MS PEP (especially Atlanta-related MS PEP) differed from those for overall PEP. A prolonged cannulation time >15 min was also identified as an independent risk factor of MS PEP.
Acknowledgments
Funding: This work was supported in part by a grant from the National Natural Science Foundation of China (No. 81970557).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4697/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4697/dss
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4697/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4697/coif). All authors report that this work was supported by a grant from the National Natural Science Foundation of China (No. 81970557). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Xijing Hospital (No. KY20201005-F-3) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dumonceau JM, Kapral C, Aabakken L, et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2020;52:127-49. [Crossref] [PubMed]
- Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol 2007;102:1781-8. [Crossref] [PubMed]
- Kochar B, Akshintala VS, Afghani E, et al. Incidence, severity, and mortality of post-ERCP pancreatitis: a systematic review by using randomized, controlled trials. Gastrointest Endosc 2015;81:143-149.e9. [Crossref] [PubMed]
- Zhou W, Li Y, Zhang Q, et al. Risk factors for postendoscopic retrograde cholangiopancreatography pancreatitis: a retrospective analysis of 7,168 cases. Pancreatology 2011;11:399-405. [Crossref] [PubMed]
- Cheng CL, Sherman S, Watkins JL, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol 2006;101:139-47. [Crossref] [PubMed]
- Testoni PA, Mariani A, Aabakken L, et al. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2016;48:657-83. [Crossref] [PubMed]
- Pan Y, Zhao L, Leung J, et al. Appropriate time for selective biliary cannulation by trainees during ERCP--a randomized trial. Endoscopy 2015;47:688-95. [Crossref] [PubMed]
- Wang P, Li ZS, Liu F, et al. Risk factors for ERCP-related complications: a prospective multicenter study. Am J Gastroenterol 2009;104:31-40. [Crossref] [PubMed]
- Shimamura T, Miyahara K, Takamori A, et al. Risk Factors for Post-Endoscopic Retrograde Pancreatography Pancreatitis: A Retrospective Chart Review in a Regional Hospital in Japan. Digestion 2020;101:557-62. [Crossref] [PubMed]
- Abbas A, Sethi S, Vidyarthi G, et al. Predictors of postendoscopic retrograde cholangiopancreatography pancreatitis, analysis of more than half a million procedures performed nationwide over the last 15 years. JGH Open 2020;4:736-42. [Crossref] [PubMed]
- Wang X, Zhao J, Wang L, et al. Relationship between papilla-related variables and post endoscopic retrograde cholangiopancreatography pancreatitis: A multicenter, prospective study. J Gastroenterol Hepatol 2020;35:2184-91. [Crossref] [PubMed]
- Park CH, Chung MJ, Park DH, et al. Impact of pancreatic fat on the risk of post-endoscopic retrograde cholangiopancreatography pancreatitis. Surg Endosc 2022;36:5734-42. [Crossref] [PubMed]
- Maruyama H, Shiba M, Ishikawa-Kakiya Y, et al. Positive correlation between pancreatic volume and post-endoscopic retrograde cholangiopancreatography pancreatitis. J Gastroenterol Hepatol 2020;35:769-76. [Crossref] [PubMed]
- Kim EJ, Cho JH, Oh KY, et al. The Risk Factors for Moderately Severe and Severe Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis According to the Revised Atlanta Classification. Pancreas 2017;46:1208-13. [Crossref] [PubMed]
- Lee SH, Lee TY, Cheon YK. The Neutrophil-Lymphocyte Ratio as an Early Predictive Marker of the Severity of Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis. Medicina (Kaunas) 2021;58:13. [Crossref] [PubMed]
- Wang X, Luo H, Tao Q, et al. Difficult biliary cannulation in ERCP procedures with or without trainee involvement: a comparative study. Endoscopy 2022;54:447-54. [Crossref] [PubMed]
- Freeman ML. Understanding risk factors and avoiding complications with endoscopic retrograde cholangiopancreatography. Curr Gastroenterol Rep 2003;5:145-53. [Crossref] [PubMed]
- Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993;80:27-38. [Crossref]
- Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet 2015;386:85-96. [Crossref] [PubMed]
- Masci E, Mariani A, Curioni S, et al. Risk factors for pancreatitis following endoscopic retrograde cholangiopancreatography: a meta-analysis. Endoscopy 2003;35:830-4. [Crossref] [PubMed]
- Testoni PA, Mariani A, Giussani A, et al. Risk factors for post-ERCP pancreatitis in high- and low-volume centers and among expert and non-expert operators: a prospective multicenter study. Am J Gastroenterol 2010;105:1753-61. [Crossref] [PubMed]
- Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc 2001;54:425-34. [Crossref] [PubMed]
- Dumonceau JM, Andriulli A, Elmunzer BJ, et al. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - updated June 2014. Endoscopy 2014;46:799-815. [Crossref] [PubMed]
- Luo H, Zhao L, Leung J, et al. Routine pre-procedural rectal indometacin versus selective post-procedural rectal indometacin to prevent pancreatitis in patients undergoing endoscopic retrograde cholangiopancreatography: a multicentre, single-blinded, randomised controlled trial. Lancet 2016;387:2293-301. [Crossref] [PubMed]
- Freeman ML, Overby C, Qi D. Pancreatic stent insertion: consequences of failure and results of a modified technique to maximize success. Gastrointest Endosc 2004;59:8-14. [Crossref] [PubMed]
- ASGE Standards of Practice Committee. Adverse events associated with ERCP. Gastrointest Endosc 2017;85:32-47. [Crossref] [PubMed]
(English Language Editors: C.Mullens and L.Huleatt)


