
Efficacy and safety of anlotinib plus camrelizumab in treating retroperitoneal soft tissue sarcomas: a single-center retrospective cohort study
Highlight box
Key findings
• The combination of anlotinib and camrelizumab demonstrated efficacy and safety in the treatment of retroperitoneal sarcomas with an ORR of 26.3%.
What is known and what is new?
• A few mono tyrosine kinase inhibitors, including anlotinib and pazopanib, had minimally beneficial effects on sarcomas.
• Anlotinib plus programmed cell death protein 1 (PD-1) inhibitor showed promising efficacy and safety in retroperitoneal sarcomas for first-line therapy, particularly for non-L-sarcomas.
What is the implication, and what should change now?
• Anlotinib plus PD-1 inhibitor may be an alternate treatment for retroperitoneal sarcomas, both for perioperative therapy and unresectable sarcomas. Biomarkers should be investigated in order to identify sensitive patients.
Introduction
Retroperitoneal soft tissue sarcomas (RSTs) are a heterogeneous group of rare tumors arising in the retroperitoneum and account for approximately 10% of all soft tissue sarcomas (STSs) (1). Complete and extended surgical resection is the only potentially curative treatment (2,3). Once advanced RSTs are contiguous with vital organs, radical surgical resection is rarely performed. Similar to advanced STSs from the trunk and extremities, doxorubicin, either alone or in combination with ifosfamide, is the first-line standard treatment for all histologic subtypes, but only a few patients achieve an objective response (4). In particular, liposarcoma (LPS) and leiomyosarcoma (LMS), the most common subtypes of RST, are less sensitive to conventional chemotherapies (5-7). Downsizing RST as a candidate for radical resection may prolong survival, but the relevant treatment outcome is still poor.
Some new agents, including trabectedin, eribulin, tyrosine kinase inhibitors (TKIs), and immune checkpoint inhibitors (ICIs), have shown therapeutic effects against sarcomas. However, only specific subtypes of sarcomas can benefit from these drugs, and the objective response rate (ORR) remains unsatisfactory.
A randomized phase III trial that compared eribulin and dacarbazine revealed that eribulin could prolong progression-free survival (PFS) and overall survival (OS) in patients with LPS, while the ORR was only 1.4% (8). A phase II study showed insufficient evidence for pazopanib in the treatment of LPS (9). Pazopanib demonstrated efficacy for metastatic non-adipocytic STS in the PALETTE trial, but with an ORR of only 6% (10). The data showed that these drugs were ineligible for preoperative treatment.
Anlotinib is a novel TKI that targets multiple receptors, including vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptors (FGFRs), and c-kit, covering tumor proliferation, vasculature, and the tumor microenvironment (11). A phase II study demonstrated its value in a spectrum of STSs with an ORR of 13% (12). Pembrolizumab has been proven to yield a favorable ORR in undifferentiated pleomorphic sarcoma (UPS) and alveolar soft part sarcoma (13,14). However, these histological subtypes rarely originate from the retroperitoneal cavity.
TKIs combined with ICIs are an innovative regimen owing to their synergistic effects in cancer treatment (15). This combination could normalize vascular-immune crosstalk to potentiate cancer immunity (16). In a range of refractory malignancies, such as hepatocellular carcinoma, cholangiocarcinoma and lung cancer, the combination of lenvatinib and pembrolizumab has been shown to achieve a significantly improved OS and ORR (17-20). Whether sarcomas can benefit from this combination therapy remains unknown.
Sun et al. explored the therapeutic effect of anlotinib combined with ICIs in previously treated metastatic sarcomas. The ORR was 34.4% in a small sample size (21). You et al. found TKI-ICI treatment has antitumor activity in soft tissue sarcomas, with a favorable ORR of 36.6% in dedifferentiated liposarcoma (DDLPS) subgroup (22). Although this treatment was well tolerated by majority patients, the clinical effect needs to be explored with large sample size in sarcomas from retroperitoneal site.
In this retrospective study, RST patients underwent anlotinib combined with camrelizumab were collected. The data were analyzed to evaluate the efficacy and safety of this regimen. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-23-460/rc).
Methods
Study design
Based on a perioperative clinical trial (ChiCTR2100054019) at Peking University Cancer Hospital Sarcoma Center, a group of patients with RST underwent preoperative treatment with TKIs and ICIs. Partial data were collected to retrospectively evaluate the antitumor activity of anlotinib and camrelizumab in this cohort. The primary objective of this study was to identify the ORR and PFS based on the investigator’s assessments. The secondary objective was to evaluate treatment-related adverse events (TRAEs) according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Review Board of Peking University Cancer Hospital (approval No. 2021KT43). Written informed consent was obtained from all study participants.
Patients, treatments and follow-up
Eligible patients were adults (≥18 years old) with histologically proven primary or recurrent sarcoma that was not suitable for complete surgical resection. As requested for safety, the patients were required to have adequate renal, hepatic, and bone marrow reserve function, as well as normal endocrine function. Patients with gastrointestinal stromal tumors, perivascular epithelioid cell tumors, and inflammatory myofibroblastic tumors, which have reliable drug targets, were excluded from this study (23-25). Baseline clinical characteristics were recorded in detail before treatment.
Anlotinib was administered at 12 mg every day for 2 weeks on and 1 week off, and 200 mg of camrelizumab was transfused every 3 weeks. According to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) (26), response assessment with enhanced computed tomography or magnetic resonance imaging was conducted every 3 cycles (9 weeks) of treatment. Patients who had at least 1 response evaluation were analyzed. All patients were continuously followed up. PFS was calculated from baseline examination to the date of documented progression or death (of any cause). Patients who were still progression-free were censored at the time of their last follow-up. OS was defined as the interval from baseline examination to patient death from any cause or last follow-up.
Statistical methods
All data processing was performed using JMP (v14.3.0; SAS Institute, Cary, NC, USA). Pearson chi-squared test was used to describe and assess the differences in the subgroups. OS rates and PFS rates were analyzed by Kaplan-Meier survival tests, and P values were calculated using log-rank tests to evaluate the correlations between prognosis and subtype or tumor grade. Univariate and multivariate survival analyses were performed with Cox proportional hazard regression model to identify independent parameters affecting OS. Results with P values of less than 0.05 were considered significant.
Results
Patient characteristics
A total of 65 patients underwent anlotinib plus camrelizumab treatment in our department between July 2019 and April 2022. Five patients were excluded because they received only one cycle of treatment due to the COVID-19 pandemic. One patient was excluded because a diagnosis of sarcomatoid carcinoma. Two patients were lost to follow-up. Our final analysis included 57 patients with RST (35 males, 61.4%; 22 females, 38.6%), with a median age of 55 years (range, 18–81 years). Additionally, 19 (33.3%) patients had initially unresectable sarcomas. The remaining 38 patients had relapsed sarcomas after the prior surgery. Among them, 39 patients with primary local advanced disease and 18 with postoperative recurrence had distal metastases to the peritoneum, lymph nodes, liver, and lungs.
The major histological subtypes included 30 (52.6%) cases of LPS and 8 (14.0%) cases of LMS, which are well known as L-sarcomas. Of the 19 (33.3%) non-L-sarcomas in our cohort, there were 10 different sarcoma types, with 3 cases being high-grade sarcomas without a specific diagnosis. According to the Fédération Nationale des Centres de Lutte Contre Le Cancer (FNCLCC) grading system (27), G1, G2, and G3 retroperitoneal sarcomas accounted for 19.3%, 42.1%, and 38.6% of the cases, respectively. Details are presented in Table 1.
Table 1
| Characteristics | N=57 (%) |
|---|---|
| Sex | |
| Male | 35 (61.4) |
| Female | 22 (38.6) |
| Age (years) | |
| Median [range] | 55 [18–81] |
| <60 | 39 (68.4) |
| 60–70 | 15 (26.3) |
| >70 | 3 (5.3) |
| Histology | |
| Liposarcoma | 30 (52.6) |
| Leiomyosarcoma | 8 (14.0) |
| Rhabdomyosarcoma | 3 (5.3) |
| Fibrosarcoma | 2 (3.5) |
| Aggressive fibromatosis | 2 (3.5) |
| DSRCT | 2 (3.5) |
| MPNST | 2 (3.5) |
| SMFT | 2 (3.5) |
| UPS | 1 (1.8) |
| Synovial sarcoma | 1 (1.8) |
| Malignant paraganglioma | 1 (1.8) |
| High-grade sarcomas (NOS) | 3 (5.3) |
| FNCLCC grade | |
| G1 | 11 (19.3) |
| G2 | 24 (42.1) |
| G3 | 22 (38.6) |
| Type of disease | |
| Local advanced | 39 (68.4) |
| Metastases [peritoneum/LN/liver/lung] | 18 (31.6) [10/1/4/8] |
| Prior surgery | |
| Yes | 38 (66.7) |
| No | 19 (33.3) |
| Treatment | |
| First-line therapy | 53 (93.0) |
| Second-line therapy | 4 (7.0) |
Data shown are numbers and percentage of patients or median and range values with available data. DSRCT, desmoplastic small round cell tumor; MPNST, malignant peripheral nerve sheath tumor; SMFT, solitary malignant fibrous tumor; UPS, undifferentiated pleomorphic sarcoma; NOS, not otherwise specified; FNLCC, Fédération Nationale des Centres de Lutte Contre le Cancer; LN, lymph node.
Treatment exposure and adverse events
Patients underwent a median of 6 cycles of treatment (range, 3–28), 19 patients (33.3%) were treated for ≥9 cycles, 53 patients (93.0%) received anlotinib and camrelizumab as first-line treatments, and 4 patients (7.0%) received second-line treatment due to disease progression under doxorubicin-based chemotherapy.
Table 2 summarizes the TRAEs. TRAEs occurred in 28 (49.1%) patients. Hypertension (24.6%), hypothyroidism (19.3%), palmar-plantar erythrodysesthesia syndrome (12.3%), and oral mucositis (8.8%) were the most common TRAEs. There were no grade 4 TRAEs, but 13 (22.8%) patients had grade 3 TRAEs, including 8 (14.0%) with hypertension, 4 (7.0%) with oral mucositis, 2 (3.5%) with palmar-plantar erythrodysesthesia syndrome, and 1 (1.8%) with pituititis. Additionally, 2 patients experienced anlotinib dose reduction, 3 patients experienced an interruption in anlotinib, and 1 patient diagnosed with pituititis underwent hormone replacement therapy, which was terminated with camrelizumab.
Table 2
| AEs | Total, n (%) | Grade 1–2, n (%) | Grade 3–4, n (%) |
|---|---|---|---|
| Hypertension | 14 (24.6) | 6 (10.5) | 8 (14.0) |
| Hypothyroidism | 11 (19.3) | 11 (19.3) | 0 (0.0) |
| PPE | 7 (12.3) | 5 (8.8) | 2 (3.5) |
| Mucositis oral | 5 (8.8) | 1 (1.8) | 4 (7.0) |
| Proteinuria | 4 (7.0) | 4 (7.0) | 0 (0.0) |
| Hyperthyroidism | 3 (5.3) | 3 (5.3) | 0 (0.0) |
| Hoarseness | 3 (5.3) | 3 (5.3) | 0 (0.0) |
| Fatigue | 3 (5.3) | 3 (5.3) | 0 (0.0) |
| Neutropenia | 2 (3.5) | 2 (3.5) | 0 (0.0) |
| RCCEP | 1 (1.8) | 1 (1.8) | 0 (0.0) |
| Pituititis | 1 (1.8) | 0 (0.0) | 1 (1.8) |
AE, adverse event; PPE, palmar-plantar erythrodysesthesia syndrome; RCCEP, reactive cutaneous capillary endothelial proliferation.
Efficacy
According to RECIST v1.1, CR and PR were recorded as objective responses in 2 (3.5%) and 13 (22.8%) patients, respectively, and 31 (54.4%) patients had stable disease (SD) for a disease control rate of 80.7%. The remaining 11 (19.3%) had disease progression (PD).
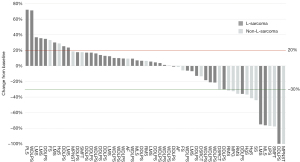
Among the 38 L-sarcoma cases, only 1 (2.6%) and 4 (10.5%) patients had CR and PR, respectively. There were 25 (65.8%) and 8 (21.1%) patients with SD and PD, respectively. In contrast, of the 19 non-L-sarcoma patients, there were 1 (5.3%), 9 (47.4%), 6 (31.6%), and 3 (15.8%) patients with CR, PR, SD, and PD, respectively. There was a significant difference in ORR between L-sarcoma and non-L-sarcoma patients [13.2% (5/38) vs. 52.6% (10/19); P=0.0031].
In subgroup analysis based on the FNCLCC grading system, none of the G1 sarcomas had an objective response, while the ORR of the G2 and G3 groups were similar (33.3% vs. 36.4%, respectively; Table 3).
Table 3
| Groups | CR | PR | SD | PD | ORR (%) | DCR (%) | P (for ORR) |
|---|---|---|---|---|---|---|---|
| Total | 2 | 13 | 31 | 11 | 26.3 | 80.7 | |
| Sarcoma type | 0.0031 | ||||||
| L-sarcoma | 1 | 4 | 25 | 8 | 13.2 | 78.9 | |
| Non-L-sarcoma | 1 | 9 | 6 | 3 | 52.6 | 84.2 | |
| FNCLCC grade | 0.0486 | ||||||
| G1 | 0 | 0 | 10 | 1 | 0.0 | 90.9 | |
| G2 | 2 | 5 | 11 | 3 | 33.3 | 85.7 | |
| G3 | 0 | 8 | 10 | 4 | 36.4 | 81.9 |
RECIST, response evaluation criteria in solid tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate; FNCLCC, Fédération Nationale des Centres de Lutte Contre le Cancer.
The 2 CR patients were diagnosed with dedifferentiated liposarcoma (DDLPS) and MDM2-amplified malignant peripheral nerve sheath tumor (MPNST), respectively. Both CRs were achieved during the first response evaluation. The DDLPS case received only 3 cycles of treatment and was interrupted owing to the COVID-19 pandemic. The patient’s CR status persisted for 29 months (Figure 1). The patient with MPNST underwent 17 cycle of combination therapy and remained in CR for 28 months. The 13 patients with PR were diagnosed with DDLPS (n=2), MLS (n=1), LMS (n=1), rhabdomyosarcoma (RMS; n=2), UPS (n=1), desmoplastic small round cell tumors (DSRCT; n=1), solitary malignant fibrous tumor (SMFT; n=1), synovial sarcoma (n=1), malignant paraganglioma (n=1), and nonspecific high-grade sarcomas (n=2). Detailed data are presented in Table 4 and Figure 2.
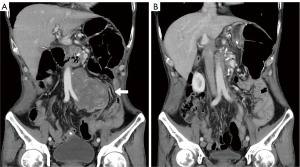
Table 4
| RST | CR | PR | SD | PD | ORR (%) |
|---|---|---|---|---|---|
| Liposarcoma | 1 | 3 | 22 | 4 | 13.3 |
| WDLPS | 0 | 0 | 12 | 1 | 0.0 |
| DDLPS | 1 | 2 | 9 | 2 | 21.4 |
| MLS | 0 | 1 | 1 | 0 | 50.0 |
| PLS | 0 | 0 | 0 | 1 | 0.0 |
| Leiomyosarcoma | 0 | 1 | 3 | 4 | 12.5 |
| Rhabdomyosarcoma | 0 | 2 | 1 | 0 | 66.7 |
| Fibrosarcoma | 0 | 0 | 1 | 1 | 0.0 |
| Aggressive fibromatosis | 0 | 0 | 2 | 0 | 0.0 |
| DSRCT | 0 | 1 | 1 | 0 | 50.0 |
| MPNST | 1 | 0 | 0 | 1 | 50.0 |
| SMFT | 0 | 1 | 1 | 0 | 50.0 |
| UPS | 0 | 1 | 0 | 0 | 100.0 |
| Synovial sarcoma | 0 | 1 | 0 | 0 | 100.0 |
| Malignant paraganglioma | 0 | 1 | 0 | 0 | 100.0 |
| High-grade sarcomas (NOS) | 0 | 2 | 0 | 1 | 66.7 |
RST, retroperitoneal soft tissue sarcoma; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; WDLPS, well-differentiated liposarcoma; DDLPS, dedifferentiated liposarcoma; MLS, myxoid liposarcoma; PLS, pleomorphic liposarcoma; DSRCT, desmoplastic small round cell tumor; MPNST, malignant peripheral nerve sheath tumor; SMFT, solitary malignant fibrous tumor; UPS, undifferentiated pleomorphic sarcoma; NOS, not otherwise specified.
Follow-up and survival
After a median follow-up of 15.8 months (range, 2.9–30.2 months), a total of 30 progressive events were recorded for a median PFS (mPFS) of 9.1 months (95% confidence interval: 5.6–16.8 months), with 3- and 6-month PFS rates of 83.6% and 60.8%, respectively. Non-L-sarcoma patients had a significantly longer mPFS than did L-sarcoma patients (11.1 vs. 6.3 months; P=0.0256), while the mPFS was similar between the FNCLCC grade subgroups (G1 vs. G2 vs. G3: 5.9 vs. 13.5 vs. 6.8 months, respectively; P=0.6361; Figure 3).

Thirteen PR patients had an mPFS of 13.9 months (range, 6.5–30.2 months). Three of these patients had tumor progression at last follow-up, with PFS ranging from 7.7–16.8 months. One individual discontinued treatment in PR status due to adverse events (AEs) and another abandoned treatment. As of this writing, the 8 remaining patients maintain a PR status.
Only 14 deaths have been recorded in our cohort thus far, and the median OS has not been reached.
Discussion
This retrospective study covers all patients with RST who received anlotinib and camrelizumab at our single center. Although this was a retrospective study, patients underwent treatment and evaluation using a prospective method. Moreover, very few patients were lost to follow-up. An ORR of 26.3% attested to the efficacy of anlotinib plus camrelizumab in the treatment of RSTs. Our results are comparable with the outcomes of clinical trials (EORTC 62012), in which the ORR was 26.5% in patients treated with doxorubicin and ifosfamide (AI). However, LPS and LMS, well-known chemotherapy-insensitive sarcomas, accounted for only 14% and 26% of the cohort, respectively, in the phase III randomized study (28).
In our study, LPS and LMS accounted for more than two-thirds of cases and were the most common histological subtypes of the retroperitoneal site. Unfortunately, the ORR of targeted immunotherapy was only 13.2% (5/38) in L-sarcoma, which did not show a superior effect to conventional chemotherapy. Accurate data of ORR (ranging from 8% to 12%) in LPS cases treated with an anthracycline-containing regimen were all derived from retrospective studies (6,29,30). The data were similar to our results of 13.3% (4/30) for LPS. Furthermore, our study indicated that anlotinib plus camrelizumab showed better antitumor activity in DDLPS, with an ORR of 21.4%. However, nearly half of the LPS cases were well-differentiated liposarcoma (WDLPS), and none of them had a tumor response in assessment. Although nearly all WDLPS cases were evaluated as SD, this was mainly due to the slow growth characteristics of the sarcoma and not the therapeutic effect of the treatment. This is also the reason that G1 sarcomas, which were mainly WDLPS, showed no tumor response on radiological evaluation.
LPS has four subtypes. Myxoid liposarcoma (MLS) is rare but chemotherapy sensitive, with an ORR of 43.2% (31). Other regimens, such as doxorubicin plus dacarbazine or trabectedin, have comparable ORRs of 44% and 46–51%, respectively (32-34). Katz et al. speculated that the high efficacy of dose-intensive AI chemotherapy in MLS appears not to be a feature unique to this regimen, but rather a feature of the chemotherapy sensitivity of MLS (31). Interestingly, our study showed that MLS had a similar ORR of 50% under targeted immunotherapy although in a small number of cases.
Another issue worth discussing is the 3 RMS cases. The first 2 patients with subtype RMS were evaluated as PR in first-line treatment; however, the third patient with embryonal subtype RMS had tumor progression under anlotinib plus camrelizumab as second-line treatment, but this patient’s first-line AI chemotherapy resulted in partial tumor remission. Patients with chemotherapy-responsive sarcomas may also benefit from TKIs and PD-1 inhibitors. It is difficult to determine whether targeted immunotherapy is a supplementary regimen or merely a substitution for conventional chemotherapy. Further studies need to be designed with greater-than-second-line treatment and that concentrate on sarcoma subtypes. Nevertheless, previous studies have revealed that multiple sarcomas, including LPS, seem to benefit more from anlotinib than from other multikinase inhibitors (9,10,12). The combination of anlotinib and ICIs may have indispensable value.
In contrast, anlotinib plus camrelizumab has advantages in clinical administration, AE management, and long-term response once it takes effect. Anlotinib is an orally administered drug. Camrelizumab is a PD-1 inhibitor similar to pembrolizumab and may be transfused and extended once every 6 weeks (35,36). Anlotinib rarely causes proteinuria compared with lenvatinib and has fewer AEs than does pazopanib (37-39). Common AEs associated with hypertension can be self-monitored and symptomatic. Severe palmar-plantar erythrodysesthesia syndrome and oral mucositis always lead to interruption of anlotinib, but the grade 3–4 incidence is low (only 3.5–7.0% in our study). Most patients resume the standard dose after discontinuation. Camrelizumab caused 19.3% events of hypothyroidism in our cohort, with 6 of the 11 afflicted patients being well-controlled with thyroxine replacement therapy. Discontinuation of PD-1 inhibitors due to severe immune-related AEs is rare, with only 1 RMS patient discontinuing treatment due to hypophysitis in our study. The patient subsequently received AI chemotherapy; however, the tumor remained stable without further shrinkage.
TKIs plus ICIs could provide a long-term major response in those with hepatocellular carcinoma, and the same phenomenon was also observed in patients with RST (19). Among the 15 patients with the best response, only 3 have tumor progression at present. The others had ongoing tumor alleviation ranging from 6.5 months to at least 30.2 months. The efficacy and safety characteristics of anlotinib combined with camrelizumab support this combination’s use in the first-line treatment of RSTs, especially for those who have worse performance status and cannot tolerate conventional chemotherapies.
Our study revealed that anlotinib combined with camrelizumab had a superior therapeutic effect in non-L-sarcomas, and over half of the patients achieved CR or PR. Patients with RMS, DSRCT, MPNST, SMFT, UPS, synovial sarcoma, malignant paraganglioma, and high-grade sarcoma without a specified diagnosis may benefit from this regimen, as well as those with DDLPS and MLS. Recently, TKIs and ICIs in combination with chemotherapy have been widely studied in a variety of malignancies and have shown favorable responses (40,41). The effectiveness and safety of our findings demonstrate the feasibility of this 3-drug combination therapy in the future. Exploratory studies are worth conducting, particularly for potentially resectable RSTs.
Conclusions
The combination of anlotinib and camrelizumab demonstrated promising efficacy and safety in the treatment of RSTs, especially for non-L-sarcomas. However, L-sarcoma requires further investigation in this context.
Acknowledgments
The authors thank all of the colleagues who contributed to this study.
Funding: This study was supported by the Capital Health Research and Development of Special Funds (approval No. 2020-1-1021), the Beijing Municipal Natural Science Foundation (approval No. Z190022), the Science Foundation of Peking University Cancer Hospital (approval No. 2022-4), and the Beijing Municipal Administration of Hospital’s Ascent Plan (approval No. DFL20181104).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-460/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-460/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-460/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Review Board of Peking University Cancer Hospital (approval No. 2021KT43). Written informed consent was obtained from all study participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swallow CJ, Strauss DC, Bonvalot S, et al. Management of Primary Retroperitoneal Sarcoma (RPS) in the Adult: An Updated Consensus Approach from the Transatlantic Australasian RPS Working Group. Ann Surg Oncol 2021;28:7873-88. [Crossref] [PubMed]
- Rossi CR, Varotto A, Pasquali S, et al. Patient outcome after complete surgery for retroperitoneal sarcoma. Anticancer Res 2013;33:4081-7. [PubMed]
- Wang J, Grignol VP, Gronchi A, et al. Surgical management of retroperitoneal sarcoma and opportunities for global collaboration. Chin Clin Oncol 2018;7:39. [Crossref] [PubMed]
- Constantinidou A, Jones RL. Systemic therapy in retroperitoneal sarcoma management. J Surg Oncol 2018;117:87-92. [Crossref] [PubMed]
- Gahvari Z, Parkes A. Dedifferentiated Liposarcoma: Systemic Therapy Options. Curr Treat Options Oncol 2020;21:15. [Crossref] [PubMed]
- Italiano A, Toulmonde M, Cioffi A, et al. Advanced well-differentiated/dedifferentiated liposarcomas: role of chemotherapy and survival. Ann Oncol 2012;23:1601-7. [Crossref] [PubMed]
- Balti M, Ayari J, Ben Azaiez M, et al. Retroperitoneal leiomyosarcoma in adult. Presse Med 2019;48:77-9. [Crossref] [PubMed]
- Schöffski P, Chawla S, Maki RG, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet 2016;387:1629-37. [Crossref] [PubMed]
- Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol 2009;27:3126-32. [Crossref] [PubMed]
- Cesne AL, Bauer S, Demetri GD, et al. Safety and efficacy of Pazopanib in advanced soft tissue sarcoma: PALETTE (EORTC 62072) subgroup analyses. BMC Cancer 2019;19:794. [Crossref] [PubMed]
- Sun Y, Niu W, Du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol 2016;9:105. [Crossref] [PubMed]
- Chi Y, Fang Z, Hong X, et al. Safety and Efficacy of Anlotinib, a Multikinase Angiogenesis Inhibitor, in Patients with Refractory Metastatic Soft-Tissue Sarcoma. Clin Cancer Res 2018;24:5233-8. [Crossref] [PubMed]
- Wilky BA, Trucco MM, Subhawong TK, et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: a single-centre, single-arm, phase 2 trial. Lancet Oncol 2019;20:837-48. [Crossref] [PubMed]
- Groisberg R, Hong DS, Behrang A, et al. Characteristics and outcomes of patients with advanced sarcoma enrolled in early phase immunotherapy trials. J Immunother Cancer 2017;5:100. [Crossref] [PubMed]
- Yi M, Jiao D, Qin S, et al. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer 2019;18:60. [Crossref] [PubMed]
- Lee WS, Yang H, Chon HJ, et al. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med 2020;52:1475-85. [Crossref] [PubMed]
- Finn RS, Ikeda M, Zhu AX, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol 2020;38:2960-70. [Crossref] [PubMed]
- Rizzo A, Dadduzio V, Ricci AD, et al. Lenvatinib plus pembrolizumab: the next frontier for the treatment of hepatocellular carcinoma? Expert Opin Investig Drugs 2022;31:371-8. [Crossref] [PubMed]
- Lin J, Yang X, Long J, et al. Pembrolizumab combined with lenvatinib as non-first-line therapy in patients with refractory biliary tract carcinoma. Hepatobiliary Surg Nutr 2020;9:414-24. [Crossref] [PubMed]
- Yang X, Zhu C, Zhao H. Immune checkpoint inhibitors combined with tyrosine kinase inhibitors is the treatment option of previously treated advanced non-small cell lung cancer harboring EGFR or ALK genetic aberration. Transl Lung Cancer Res 2022;11:2164-6. [Crossref] [PubMed]
- Sun X, Xu J, Xie L, et al. Effectiveness and Tolerability of Anlotinib Plus PD-1 Inhibitors for Patients with Previously Treated Metastatic Soft-Tissue Sarcoma. Int J Gen Med 2022;15:7581-91. [Crossref] [PubMed]
- You Y, Guo X, Zhuang R, et al. Activity of PD-1 Inhibitor Combined With Anti-Angiogenic Therapy in Advanced Sarcoma: A Single-Center Retrospective Analysis. Front Mol Biosci 2021;8:747650. [Crossref] [PubMed]
- Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626-32. [Crossref] [PubMed]
- Sanfilippo R, Fabbroni C, Fucà G, et al. Addition of Antiestrogen Treatment in Patients with Malignant PEComa Progressing to mTOR Inhibitors. Clin Cancer Res 2020;26:5534-8. [Crossref] [PubMed]
- Butrynski JE, D'Adamo DR, Hornick JL, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med 2010;363:1727-33. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Stoeckle E, Coindre JM, Bonvalot S, et al. Prognostic factors in retroperitoneal sarcoma: a multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer 2001;92:359-68. [Crossref] [PubMed]
- Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol 2014;15:415-23. [Crossref] [PubMed]
- Jones RL, Fisher C, Al-Muderis O, et al. Differential sensitivity of liposarcoma subtypes to chemotherapy. Eur J Cancer 2005;41:2853-60. [Crossref] [PubMed]
- Gronchi A, De Paoli A, Dani C, et al. Preoperative chemo-radiation therapy for localised retroperitoneal sarcoma: a phase I-II study from the Italian Sarcoma Group. Eur J Cancer 2014;50:784-92. [Crossref] [PubMed]
- Katz D, Boonsirikamchai P, Choi H, et al. Efficacy of first-line doxorubicin and ifosfamide in myxoid liposarcoma. Clin Sarcoma Res 2012;2:2. [Crossref] [PubMed]
- Patel SR, Burgess MA, Plager C, et al. Myxoid liposarcoma. Experience with chemotherapy. Cancer 1994;74:1265-9. [Crossref] [PubMed]
- Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol 2007;8:595-602. [Crossref] [PubMed]
- Gronchi A, Bui BN, Bonvalot S, et al. Phase II clinical trial of neoadjuvant trabectedin in patients with advanced localized myxoid liposarcoma. Ann Oncol 2012;23:771-6. [Crossref] [PubMed]
- Lala M, Li M, Sinha V, et al. A Six-Weekly (Q6w) Dosing Schedule for Pembrolizumab Based on an Exposure-Response (Er) Evaluation Using Modeling and Simulation. J Clin Oncol 2018;36:abstr 3062.
- Goldstein DA, Ratain MJ, Saltz LB. Weight-Based Dosing of Pembrolizumab Every 6 Weeks in the Time of COVID-19. JAMA Oncol 2020;6:1694-5. [Crossref] [PubMed]
- Sasaki R, Fukushima M, Haraguchi M, et al. Impact of lenvatinib on renal function compared to sorafenib for unresectable hepatocellular carcinoma. Medicine (Baltimore) 2022;101:e29289. [Crossref] [PubMed]
- Grünwald V, Karch A, Schuler M, et al. Randomized Comparison of Pazopanib and Doxorubicin as First-Line Treatment in Patients With Metastatic Soft Tissue Sarcoma Age 60 Years or Older: Results of a German Intergroup Study. J Clin Oncol 2020;38:3555-64. [Crossref] [PubMed]
- Que Y, Liang Y, Zhao J, et al. Treatment-related adverse effects with pazopanib, sorafenib and sunitinib in patients with advanced soft tissue sarcoma: a pooled analysis. Cancer Manag Res 2018;10:2141-50. [Crossref] [PubMed]
- Jian Z, Fan J, Shi GM, et al. Lenvatinib Plus Toripalimab as First-Line Treatment for Advanced Intrahepatic Cholangiocarcinoma: A Single-Arm, Phase 2 Trial. J Clin Oncol 2021;39:abstr 4099.
- Lam TC, Tsang KC, Choi HC, et al. Combination atezolizumab, bevacizumab, pemetrexed and carboplatin for metastatic EGFR mutated NSCLC after TKI failure. Lung Cancer 2021;159:18-26. [Crossref] [PubMed]
(English Language Editor: J. Gray)


