
The association between the interdelivery interval and early postpartum urinary incontinence in women who had consecutive vaginal deliveries: a retrospective cohort study
Highlight box
Key findings
• In this cohort study of 2,492 parous women, the prevalence of early postpartum urinary incontinence (UI) was found to vary depending on the interdelivery interval (IDI), which was defined as the time from the previous delivery to the next delivery. The risk curves for UI were U-shaped, with a nadir near 72 months. The association between the IDI and UI was more pronounced in the younger women (<35 years) and the women with a pre-pregnancy body mass index of <25 kg/m2.
What is known and what is new?
• The associations between UI and obstetric-related factors, such as vaginal delivery, a prolonged second stage of labor, maternal obesity, and high infant weight, are well established.
• However, the association between the timing of deliveries and UI remains unclear. We conducted a preliminary evaluation of the association between the IDI and postpartum UI in a cohort of parous women who had vaginal deliveries.
What is the implication, and what should change now?
• Parous women who give birth again within 41 months should receive additional screening or treatment.
Introduction
Urinary incontinence (UI) is defined as any complaint of involuntary loss (leakage) of urine (1). The condition occurs in both sexes but is much more frequent in women (2). According to robust studies in the United States, Europe, and Asia, the prevalence of UI is approximately 30% (3-5). Over half of the women affected by UI have reported that UI is bothersome and that its treatment represents a considerable economic burden (6).
Stress urinary incontinence (SUI) causes predictable urine loss during activities that increase intra-abdominal pressure (e.g., exercise, laughing, and sneezing). Urgent urinary incontinence (UUI) is mainly characterized by urgency, followed by involuntary loss of urine, and increased urinary frequency or nocturia (2). Different mechanisms cause different types of UI; however, the association between UI and obstetric-related factors is well established. Vaginal delivery (7,8), forceps-assisted delivery (9,10), a prolonged second stage of labor (11), maternal obesity (12,13), and a high infant weight (14) can cause severe injury to the supporting structures of the pelvic floor, further contributing to postpartum UI. Mechanical injury during labor increases with the number of deliveries. However, it is not yet clear whether mechanical injury during labor varies with the length of the interdelivery interval (IDI; the time from the previous delivery to next delivery).
The short IDI is associated with a number of adverse maternal-fetal outcomes (15-18). Preterm birth, pregnancy complications, and maternal-fetal mortality are often the primary factors affecting the timing of the next delivery (18,19), while pelvic floor disorders (e.g., postpartum UI) are often overlooked. Further, transient UI in the early postpartum period can develop into persistent UI (20). Thus, Early identification of risk factors for UI and early rehabilitation of patients with UI is critical. We conducted a preliminary evaluation of the association between the IDI and postpartum UI in a cohort of parous women who had vaginal deliveries. This study is reported according to the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4684/rc).
Methods
Design, setting, and participants
The protocol was approved by the Ethics Committee of the International Peace Maternity and Child Health Hospital (IPMCH), Shanghai Jiao Tong University School of Medicine (No. 2016-55), and the requirement for individual consent was waived. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
A retrospective cohort study of postpartum screening data was conducted at IPMCH from September 2019 to May 2022. The data included the pelvic floor-related examination data of women at 42 to 60 days postpartum that were collected during maternity appointments at the Institutional Pelvic Floor Rehabilitation Center. The physicians performed pelvic floor muscle function measurements and consulted with each woman about pelvic floor disorders. The results were recorded in an electronic health system and matched to the maternal baseline data in the electronic medical system using a unique hospitalization number. Only parous women who delivered vaginally were included to avoid confounding by the mode of delivery. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had a preterm birth; (II) had a twin birth; (III) had postpartum visits beyond 60 days; and (IV) had no available baseline data (e.g., height, weight, or labor summary data).
Primary exposure: IDI
The IDI was defined as the period between the previous pregnancy delivery date and the last pregnancy delivery date, which was expressed in months as the unit of measurement. As there were no relevant studies to use as references, the participants were divided into the following 4 groups based on their IDI quartiles: the Quartile 1 (<41 months) group, the Quartile 2 (41–62 months) group, the Quartile 3 (63–90 months) group, and the Quartile 4 (≥91 months) group.
Primary outcome: early postpartum UI
The primary outcome of this study was UI, which was defined by the International Continence Society and International Urogynecological Association as any involuntary urine leakage (1). If the participants self-reported symptoms of urine leakage after delivery, they were further assessed by a physician using the International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form (ICIQ-UI-SF) (21). The type of UI was determined primarily by section 6 of the questionnaire. The participants who chose the response of “leaks when you cough or sneeze” or “leaks, if you are physically active/exercising” from the list of options, were diagnosed with SUI, and those who chose “leaks before you can get to the toilet” were diagnosed with UUI, and those who chose both symptoms, were diagnosed with mixed urinary incontinence (MUI). The ICIQ-UI-SF is now available in Chinese, and its test validity and accuracy have been validated (22). In addition, the severity of urinary loss was based on the scoring system developed by Sandvik et al. (23). Mild to severe UI was the final study outcome.
Covariates and definitions
All the data were obtained from electronic case database. The demographic and pregnancy characteristics included age, pre-pregnancy body mass index (BMI; calculated based on early pregnancy weight and height), gravidity, parity, weight gain in pregnancy (calculated as prenatal weight minus early pregnancy weight), complications (hypertensive disorders, gestational diabetes mellitus/pre-pregnancy gestational diabetes mellitus, anemia, impaired liver and kidney function, and abnormal thyroid function), gestation age, induction of labor (including oxytocin, prostaglandin, and cervix balloon mechanical induction of labor), and infant weight. The delivery characteristic variables included the second stage of labor (the time from the cervix being fully dilated to the complete delivery of the fetus), presence of perineal lacerations, episiotomy, and use of instruments during birth (including low forceps and vacuum extractors).
Statistical analysis
Logistic regression models were used to calculate the odds ratios (ORs) and 95% CIs and to test differences in the risk of early postpartum UI across the IDI quartiles. The following 3 multivariate models were used: Model 1 (in which age and pre-pregnancy BMI were adjusted); Model 2 (to which gravidity, infant weight, weight gain in pregnancy, and complications, such as hypertensive disorders, diabetes, and others, were added; and Model 3 (to which the induction of labor, epidural anesthesia, the second stage of labor, instrumental birth, perineal lacerations, and episiotomy were added). The P values for the trends were calculated using the tertile median value as a quasi-continuous variable in the model.
The associations between the quartiles of IDI and UI were evaluated on a continuous scale using restricted cubic spline curves based on the logistic regression models. To balance best fit and overfitting in the main splines for UI, the number of knots was chosen as the lowest value for the Akaike information criterion (knots =4 at the 5th, 35th, 65th, and 95th percentiles of IDI).
Given the possible confounding causalities of UI history and to minimize the potential effects of the risk factors (e.g., a parity ≥3, macrosomia, complications during pregnancy, instrumental birth, and episiotomy) reported by previous studies (9,12-14), we conducted a sensitivity analysis to exclude any individuals with these characteristics. We also tested the interactions between the IDI and age (<35 and ≥35 y) and pregnancy BMI (<25 and ≥25 kg/m2) in the fully adjusted model.
The statistical analyses and graphing were performed with SPSS 26.0 (IBM Corp, Armonk, NY, USA) and R version 4.1.3 (R Core Team, Vienna, Austria). All the tests were 2-sided, and a P value <0.05 was considered statistically significant.
Results
A total of 29,530 women delivered in the IPMCH from September 2019 to May 2022. Among these 29,530 women, 27,038 were excluded for the following reasons: 23,755 were primiparous women; 2,896 had a history of cesarean delivery; 104 had a history of preterm delivery; 6 had a history of twin births; 148 attended postpartum visits beyond 60 days; and 129 had missing baseline data (Figure 1).
Thus, the analytic sample ultimately comprised 2,492 participants with a mean [standard deviation (SD)] age of 33.47 (3.51) years. The median [interquartile range (IQR)] IDI of the entire cohort was 62 [40–90] months, while that of the Quartile 1 group was 29 (range, 14–40; IQR, 24–36) months, that of the Quartile 2 group was 50 (range, 41–62; IQR, 46–57) months, that of the Quartile 3 group was 74 (range, 63–90; IQR, 68–82) months, and that of the Quartile 4 group was 118 (range, 91–281; IQR, 101–144.5) months. The pre-pregnancy BMI was similar between the IDI groups. Age, weight gain in pregnancy, and certain pregnancy complications (gestational/pregestational diabetes) increased as the IDI increased. In addition, the incidence of induction of labor, instrument-assisted labor, and episiotomies during labor increased as the IDI increased (Table 1).
Table 1
| Characteristics | Total (n=2,492) | Quartile of IDI | |||
|---|---|---|---|---|---|
| Quartile 1 (n=623) | Quartile 2 (n=640) | Quartile 3 (n=606) | Quartile 4 (n=623) | ||
| IDI, mean (SD), month | 70.78 (7.23) | 29.65 (6.97) | 51.53 (6.41) | 75.16 (8.07) | 127.45 (12.97) |
| Age, mean (SD), y | 33.47 (3.51) | 31.39 (3.06) | 32.52 (3.10) | 33.79 (2.69) | 36.23 (3.19) |
| Pre-pregnancy BMI, mean (SD), kg/m2 |
26.99 (2.87) | 27.19 (2.99) | 27.04 (2.84) | 26.92 (2.79) | 26.82 (2.84) |
| Gravidity, median [IQR] | 2 [2–3] | 2 [2–3] | 2 [2–3] | 3 [2–3] | 3 [2–4] |
| Parity, n (%) | |||||
| 2 | 2,421 (97.2) | 605 (97.1) | 629 (98.3) | 596 (98.3) | 591 (94.9) |
| ≥3 | 71 (2.8) | 18 (2.9) | 11 (1.7) | 10 (1.7) | 32 (5.1) |
| Gestational age, median [IQR], week | 39 [38–39] | 39 [38–39] | 39 [38–39] | 39 [38–40] | 39 [38–40] |
| Infant weight, mean (SD), g | 3,419.73 (365.85) | 3,392.28 (346.54) | 3,416.76 (368.57) | 3,446.25 (365.98) | 3,424.43 (380.32) |
| Weight gain in pregnancy, mean (SD), kg |
12.78 (3.79) | 11.70 (3.13) | 12.53 (3.32) | 13.16 (3.90) | 13.73 (4.41) |
| Complications, n (%) | |||||
| Hypertensive disorders | 93 (3.7) | 25 (4.0) | 22 (3.4) | 20 (3.3) | 26 (4.2) |
| Diabetes (gestational/pregestational) | 425 (17.1) | 89 (14.3) | 96 (15.0) | 99 (16.3) | 141 (22.6) |
| Other complications | 412 (16.5) | 99 (15.9) | 96 (15.0) | 117 (19.3) | 100 (16.1) |
| Induction of labor, n (%) | 906 (36.4) | 208 (33.4) | 212 (33.1) | 223 (36.8) | 263 (42.2) |
| Epidural anesthesia, n (%) | 744 (29.9) | 178 (28.6) | 182 (28.4) | 185 (30.5) | 199 (31.9) |
| The second stage of labor, mean (SD), min | 32.53 (20.45) | 32.06 (19.85) | 31.94 (20.21) | 32.52 (20.51) | 33.62 (21.22) |
| Instrumental birth, n (%) | 56 (2.2) | 4 (0.6) | 8 (1.3) | 17 (2.8) | 27 (4.3) |
| Perineal lacerations, n (%) | 1518 (60.9) | 384 (61.6) | 397 (62.0) | 389 (64.2) | 348 (55.9) |
| Episiotomy, n (%) | 116 (4.7) | 9 (1.4) | 20 (3.1) | 37 (6.1) | 50 (8.0) |
| UI, n (%) | 709 (28.5) | 246 (39.5) | 163 (25.5) | 136 (22.4) | 164 (26.3) |
| SUI | 445 (17.9) | 147 (23.6) | 105 (16.4) | 90 (14.9) | 103 (16.5) |
| UUI | 132 (5.3) | 51 (8.2) | 27 (4.2) | 22 (3.6) | 32 (5.1) |
| MUI | 132 (5.3) | 48 (7.7) | 31 (4.8) | 24 (4.0) | 29 (4.7) |
Quartile 1: <41 months; Quartile 2: 41–62 months; Quartile 3: 63–90 months; Quartile 4: ≥91 months. IDI, interdelivery interval; SD, standard deviation; IQR, interquartile range; BMI, body mass index; UI, urinary incontinence; SUI, stress urinary incontinence; UUI, urgency urinary incontinence; MUI, mixed urinary incontinence.
After adjusting for all potential confounders, we observed that a longer IDI was associated with lower risk of UI compared to the participants in Quartile 1 [Quartile 2: lower adjusted odds ratio (aOR) 0.48 (95% CI: 0.36–0.63); Quartile 3: aOR 0.37 (95% CI: 0.27–0.49); Quartile 4: aOR 0.40 (95% CI: 0.28–0.57); the P value for the trend was <0.001]. Notably, the participants in the Quartile 3 group had the lowest risk of UI, even though the participants in this group did not have the longest IDIs. Each subtype of UI was analyzed separately, and similar results were obtained for SUI and MUI (Table 2).
Table 2
| Outcomes | Quartile of IDI, [median], month | P value for the trend | |||
|---|---|---|---|---|---|
| Quartile 1 [29], (n=623) | Quartile 2 [50], (n=640) | Quartile 3 [74], (n=606) | Quartile 4 [118], (n=623) | ||
| UI | |||||
| Model 1 | 1 (Reference) | 0.49 (0.38–0.62) | 0.39 (0.30–0.50) | 0.41 (0.31–0.55) | <0.001 |
| Model 2 | 1 (Reference) | 0.48 (0.38–0.62) | 0.37 (0.29–0.49) | 0.40 (0.29–0.53) | <0.001 |
| Model 3 | 1 (Reference) | 0.48 (0.36–0.63) | 0.37 (0.27–0.49) | 0.40 (0.28–0.57) | <0.001 |
| SUI | |||||
| Model 1 | 1 (Reference) | 0.49 (0.38–0.62) | 0.39 (0.30–0.50) | 0.41 (0.31–0.55) | <0.001 |
| Model 2 | 1 (Reference) | 0.48 (0.38–0.62) | 0.37 (0.29–0.49) | 0.40 (0.29–0.53) | <0.001 |
| Model 3 | 1 (Reference) | 0.48 (0.36–0.63) | 0.37 (0.27–0.49) | 0.40 (0.28–0.57) | 0.001 |
| UUI | |||||
| Model 1 | 1 (Reference) | 0.50 (0.31–0.81) | 0.44 (0.25–0.74) | 0.66 (0.38–1.12) | 0.181 |
| Model 2 | 1 (Reference) | 0.51 (0.31–0.82) | 0.43 (0.25–0.74) | 0.67 (0.37–1.18) | 0.046 |
| Model 3 | 1 (Reference) | 0.48 (0.27–0.83) | 0.44 (0.23–0.80) | 0.65 (0.33–1.24) | 0.089 |
| MUI | |||||
| Model 1 | 1 (Reference) | 0.56 (0.34–0.89) | 0.41 (0.24–0.68) | 0.40 (0.23–0.69) | 0.002 |
| Model 2 | 1 (Reference) | 0.53 (0.33–0.86) | 0.37 (0.22–0.63) | 0.34 (0.19–0.62) | 0.001 |
| Model 3 | 1 (Reference) | 0.56 (0.33–0.95) | 0.37 (0.20–0.68) | 0.33 (0.16–0.67) | 0.002 |
Data were presented as adjusted OR (95% CI). Quartile 1: <41 months; Quartile 2: 41–62 months; Quartile 3: 63–90 months; Quartile 4: ≥91 months. Model 1: adjusted for age and pre-pregnancy BMI. Model 2: adjusted for variables included in Model 1 + gravidity, parity, infant weight, weight gain in pregnancy, and complications (hypertensive disorders, diabetes, and others). Model 3: adjusted for variables included in Model 2 + induction of labor, epidural anesthesia, the second stage of labor, instrumental birth, perineal lacerations, and episiotomy. IDI, interdelivery interval; UI, urinary incontinence; MUI, mixed urinary incontinence; SUI, stress urinary incontinence; UUI, urgency urinary incontinence; MUI, mixed urinary incontinence; OR, odds ratio.
In addition, the restricted cubic splines showed a U-curved association between the IDI and incidence of early postpartum UI. The results of the multivariable-adjusted analyses showed that the IDI associated with the lowest risk of having postpartum UI was 72 months in the overall population (Figure 2).
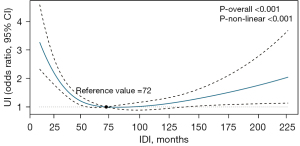
The Exclusion of participants with UI during pregnancy, those who delivered macrosomia, those with complications during pregnancy, those with instrumental birth, and those with prolonged second stage of labor did not substantially alter the observed association between the IDI and early postpartum UI (both P values for the trend were <0.001; Table 3). We observed significant interactions between BMI (the P value for the interaction was <0.001) and age (the P value for the interaction =0.002) on the relationship between the IDI and early postpartum UI. Specifically, the association between the IDI and early postpartum UI was more pronounced among younger women (<35 y) and women with a lower pre-pregnancy BMI (<25 kg/m2) (Table 4).
Table 3
| Models | Quartile of IDI | P value for the trend | |||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Model 1 | <0.001 | ||||
| Case/n | 214/528 | 141/530 | 106/511 | 140/506 | |
| OR (95% CI) | 1 (Reference) | 0.47 (0.35–0.64) | 0.30 (0.22–0.43) | 0.41 (0.28–0.60) | |
| Model 2 | <0.001 | ||||
| Case/Total | 239/605 | 161/629 | 134/596 | 154/591 | |
| OR (95% CI) | 1 (Reference) | 0.49 (0.37–0.64) | 0.37 (0.27–0.50) | 0.39 (0.27–0.55) | |
| Model 3 | <0.001 | ||||
| Case/Total | 237/595 | 157/598 | 128/571 | 152/584 | |
| OR (95% CI) | 1 (Reference) | 0.49 (0.37–0.65) | 0.37 (0.27–0.50) | 0.38 (0.27–0.55) | |
| Model 4 | <0.001 | ||||
| Case/Total | 178/450 | 120/453 | 95/402 | 104/399 | |
| OR (95% CI) | 1 (Reference) | 0.51 (0.37–0.70) | 0.41 (0.28–0.59) | 0.37 (0.24–0.57) | |
| Model 5 | <0.001 | ||||
| Case/Total | 245/619 | 161/632 | 133/589 | 161/596 | |
| OR (95% CI) | 1 (Reference) | 0.47 (0.36–0.62) | 0.36 (0.26–0.49) | 0.39 (0.28–0.56) | |
| Model 6 | <0.001 | ||||
| Case/Total | 244/614 | 158/620 | 128/569 | 154/573 | |
| OR (95% CI) | 1 (Reference) | 0.46 (0.35–0.61) | 0.35 (0.25–0.47) | 0.38 (0.26–0.54) | |
Quartile 1: <41 months; Quartile 2: 41–62 months; Quartile 3: 63–90 months; Quartile 4: ≥91 months. †, adjusted for age, pre-pregnancy body mass index, gravidity, parity (except for Model 2), infant weight (except for Model 3), weight gain in pregnancy, complications (hypertensive disorders, diabetes, and others) (except for Model 4), induction of labor, epidural anesthesia, the second stage of labor, instrumental birth (except for Model 5), perineal lacerations, and episiotomy (except for Model 6). Model 1: excluding participants with a self-reported history of UI. Model 2: excluding participants with parity ≥3. Model 3: excluding participants with macrosomia. Model 4: excluding participants with complications during pregnancy. Model 5: excluding participants with an instrumental birth. Model 6: excluding participants with an episiotomy. IDI, interdelivery interval; UI, urinary incontinence; OR, odds ratio.
Table 4
| Characteristics | Statistic | Quartile of IDI | P for interaction | |||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||
| Age | 0.002 | |||||
| <35 years | Case/Total | 208/535 | 116/492 | 74/360 | 33/181 | |
| OR (95% CI) | 1 (Reference) | 0.43 (0.31–0.58) | 0.33 (0.23–0.48) | 0.33 (0.20–0.55) | ||
| ≥35 years | Case/Total | 38/88 | 47/148 | 62/246 | 131/442 | |
| OR (95% CI) | 1 (Reference) | 0.66 (0.35–1.24) | 0.42 (0.23–0.77) | 0.52 (0.29–0.94) | ||
| Pre-pregnancy BMI | <0.001 | |||||
| <25 kg/m2 | Case/Total | 66/159 | 30/160 | 25/156 | 30/167 | |
| OR (95% CI) | 1 (Reference) | 0.25 (0.14–0.45) | 0.21 (0.11–0.40) | 0.20 (0.10–0.42) | ||
| ≥25 kg/m2 | Case/Total | 180/464 | 133/480 | 111/450 | 134/456 | |
| OR (95% CI) | 1 (Reference) | 0.59 (0.43–0.80) | 0.44 (0.31–0.62) | 0.50 (0.34–0.75) | ||
Quartile 1: <41 months; Quartile 2: 41–62 months; Quartile 3: 63–90 months; Quartile 4: ≥91 months. †, adjusted for age, pre-pregnancy BMI, gravidity, parity, infant weight, weight gain in pregnancy, complications (hypertensive disorders, diabetes, and others), induction of labor, epidural anesthesia, the second stage of labor, instrumental birth, perineal lacerations, and episiotomy. IDI, interdelivery interval; UI, urinary incontinence; OR, odds ratio; BMI, body mass index.
Discussion
The present study investigated the association between the IDI and the risk of developing early postpartum UI using the data of 2,492 parous women. We found that a longer IDI was independently associated with early postpartum UI after adjusting for potential confounding factors, and that the Quartile 3 group had the lowest risk, even though the participants in this group did not have the longest IDIs. The risk of early postpartum UI related to changes in the IDI showed a U-shaped curve pattern. The exclusion of participants with characteristics affecting the outcomes from the analysis did not materially change the results. Further, we observed that the strength of the association between the IDI and postpartum UI varied when the participants were stratified according to age and pre-pregnancy BMI.
For parous women, choosing the optimal IDI to have another baby is important. Both short and long IDIs appear to be associated with increased risks of adverse maternal-fetal outcomes, including preterm delivery (24), small-for-gestational-age birth (25), and infant mortality (26-29). Clinical postpartum practice and public health guidelines recommend an IDI of at least 18 to 24 months and no more than 60 months (19,30-32). However, in relation to postpartum pelvic floor disorders in women, there is a lack of evidence to support the views expressed in the guidelines. Indeed, to date, no previous research appears to have been conducted on the association between the IDI and postpartum UI.
The mechanisms underlying the pathogenesis of UI are still unclear. Hypermobility of the urethra, weakness of the urinary sphincter, and physiological perturbations to the bladder function are now considered direct causes of SUI or UUI (2,6). The impairment of these structures or functions increases with the number of deliveries, as does the risk of postpartum UI (33,34). The relaxation of pelvic floor support structures due to changes in hormone levels during pregnancy is another significant predisposing factor for postpartum UI (35). The pelvic floor function of women after childbirth has a certain ability to self-repair (1). However, short-term repetitive mechanical injury or high levels of hormone exposure may have more severe adverse effects on pelvic floor support structures. The present study found that IDI ≥41 months was associated with a lower risk of postpartum UI compared to IDI <41 months.
When quartile grouping was used, the highest quartile IDI (>90 months) did not show a significant reduction in UI risk compared to the lowest quartile IDI (<41 months). Several studies have proposed the theory of “physiological regression” (36,37), which suggests that women undergo remodeling of the pelvic floor support structures after vaginal delivery, which makes it easier for the infant to pass through the birth canal on the next delivery. It may that a longer IDI causes pelvic floor remodeling to rebound, and the injury to the supporting structures of the pelvic floor caused during another delivery is further exacerbated. To determine the optimal IDI, we used a restricted cubic spline curve, and found that the IDI with the lowest risk of postpartum UI was 72 months (the lowest point of the U-shaped curve), which is a IDI well above that which is generally considered the optimal IDI (19,26,29,36). However, the association between the IDI and other adverse maternal-fetal outcomes needs to be considered in clinical practice, and parous mothers often do not deliver again within an optimal IDI (38-40). Thus, postpartum screening and the rehabilitation of postpartum pelvic floor dysfunction need to be prioritized by clinicians.
We observed an interaction between age and IDI. In the United States, women who have their first birth at the age of 35 years or older had a shorter IDI compared to those who began bearing children at younger ages (40). The opposite pattern was observed in our study. Due to differences between the 2 countries in terms of economy, culture, and fertility intention, we cannot provide a reasonable explanation for these different results. However, the association between the IDI and risk of postpartum UI was certainly stronger in the younger women (<35 y) than the older women (≥35 y). Aging has been reported to be associated with the prevalence of UI (8,41). The effects of exercise or natural recovery from UI may be more pronounced in younger women than in older women (42). A long-term longitudinal cohort study showed that the difference in the incidence of postpartum UI due to the mode of delivery was not statistically significant in a 40-year-old population (10). This finding may be due to the effects of aging in the pathogenesis of UI with advancing age.
We observed a stronger association between the IDI and postpartum UI in women with a BMI <25 kg/m2 than in women with a BMI ≥25 kg/m2. Previous studies have shown that obesity leads to a persistent increase in abdominal pressure (12,13), which induces abnormal bladder and urethral positioning and further contributes to UI (14). We speculate that lower pre-pregnancy weight and an optimal IDI have a synergistic effect on reducing postpartum UI. However, any such association requires further investigation.
This study had a large sample size, but it still had several limitations. First, the UI symptoms were self-reported and were thus subject to recall bias. Second, other factors during the interval periods (e.g., a history of functional pelvic floor exercises, and the intensity of daily physical activity) might not have been taken into account. Third, as the present study was a retrospective cohort study, the causality of the observed associations could not be established. We also cannot exclude the possibility of reverse causality; that is, the IDI of the participants changed because of UI related to the previous delivery. Fourth, we did not apply the restricted cubic spline model to each subtype of UI, as we considered the over-modeling problem caused by the limitation of the sample size. Finally, almost all of the participants in this study were residents of Shanghai, China, an economically more developed region of China. Thus, our results may not be generalizable to all Chinese people. Further studies need to be conducted to replicate our findings in a nationally representative sample.
Conclusions
In conclusion, we found that the IDI was independently associated with the incidence of early postpartum UI. The risk curves were U-shaped for the outcomes, with a nadir near 72 months. Moreover, the association between the IDI and the incidence of early postpartum UI was significantly stronger in the younger women and the women who were not overweight in our cohort. Our findings indicated that the optimal IDI is longer than previously thought (36–60 months) for women. However, in clinical practice, indicators, such as preterm birth and maternal–fetal mortality, and pelvic floor disorders are often overlooked. Our findings indicate that additional screening or treatment should be provided in the postpartum period to women who do not deliver at the optimal interval.
Acknowledgments
We would like to sincerely thank all the women who participated in this study and the medical staff at the Pelvic Floor Rehabilitation Medical Center.
Funding: This work was supported by the Interdisciplinary Program of Shanghai Jiao Tong University (No. YG2019ZDA05) and the Shanghai Municipal Key Clinical Specialty, Shanghai, China (No. GFY1808004).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4684/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4684/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4684/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol was approved by the Ethics Committee of the International Peace Maternity and Child Health Hospital (IPMCH), Shanghai Jiao Tong University School of Medicine (No. 2016-55), and the requirement for individual consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bo K, Frawley HC, Haylen BT, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Int Urogynecol J 2017;28:191-213. [Crossref] [PubMed]
- Aoki Y, Brown HW, Brubaker L, et al. Urinary incontinence in women. Nat Rev Dis Primers 2017;3:17042. [Crossref] [PubMed]
- Zhang L, Zhu L, Xu T, et al. A Population-based Survey of the Prevalence, Potential Risk Factors, and Symptom-specific Bother of Lower Urinary Tract Symptoms in Adult Chinese Women. Eur Urol 2015;68:97-112. [Crossref] [PubMed]
- Minassian VA, Stewart WF, Wood GC. Urinary incontinence in women: variation in prevalence estimates and risk factors. Obstet Gynecol 2008;111:324-31. [Crossref] [PubMed]
- Cerruto MA, D'Elia C, Aloisi A, et al. Prevalence, incidence and obstetric factors' impact on female urinary incontinence in Europe: a systematic review. Urol Int 2013;90:1-9. [Crossref] [PubMed]
- Hu JS, Pierre EF. Urinary Incontinence in Women: Evaluation and Management. Am Fam Physician 2019;100:339-48. [PubMed]
- Cheng H, Gong F, Shen Y, et al. A nomogram model predicting the risk of postpartum stress urinary incontinence in primiparas: A multicenter study. Taiwan J Obstet Gynecol 2022;61:580-4. [Crossref] [PubMed]
- Blomquist JL, Muñoz A, Carroll M, et al. Association of Delivery Mode With Pelvic Floor Disorders After Childbirth. JAMA 2018;320:2438-47. [Crossref] [PubMed]
- Chang SR, Lin WA, Chang TC, et al. Risk factors for stress and urge urinary incontinence during pregnancy and the first year postpartum: a prospective longitudinal study. Int Urogynecol J 2021;32:2455-64. [Crossref] [PubMed]
- Tähtinen RM, Cartwright R, Vernooij RWM, et al. Long-term risks of stress and urgency urinary incontinence after different vaginal delivery modes. Am J Obstet Gynecol 2019;220:181.e1-8. [Crossref] [PubMed]
- Wang Q, Yu X, Sun X, et al. Does epidural anesthesia influence pelvic floor muscle endurance and strength and the prevalence of urinary incontinence 6 weeks postpartum? Int Urogynecol J 2020;31:577-82. [Crossref] [PubMed]
- Chang SR, Lin WA, Lin HH, et al. Cumulative incidence of urinary incontinence and associated factors during pregnancy and after childbirth: a cohort study. Int Urogynecol J 2022;33:1451-61. [Crossref] [PubMed]
- Arrue Gabilondo M, Ginto L, Zubikarai M, et al. Risk factors associated with stress urinary incontinence 12 years after first delivery. Int Urogynecol J 2021;32:3061-7. [Crossref] [PubMed]
- Wang K, Xu X, Jia G, et al. Risk Factors for Postpartum Stress Urinary Incontinence: a Systematic Review and Meta-analysis. Reprod Sci 2020;27:2129-45. [Crossref] [PubMed]
- Swaminathan A, Fell DB, Regan A, et al. Association between interpregnancy interval and subsequent stillbirth in 58 low-income and middle-income countries: a retrospective analysis using Demographic and Health Surveys. Lancet Glob Health 2020;8:e113-22. [Crossref] [PubMed]
- Tessema GA, Pereira G. Challenging the assumption that interpregnancy interval causes stillbirth in low-income and middle-income countries. Lancet Glob Health 2020;8:e16-7. [Crossref] [PubMed]
- Liu C, Snowden JM, Lyell DJ, et al. Interpregnancy Interval and Subsequent Severe Maternal Morbidity: A 16-Year Population-Based Study From California. Am J Epidemiol 2021;190:1034-46. [Crossref] [PubMed]
- Shifti DM, Chojenta C, G, Holliday E, et al. Individual and community level determinants of short birth interval in Ethiopia: A multilevel analysis. PLoS One 2020;15:e0227798. [Crossref] [PubMed]
- ACOG Committee Opinion No. 736: Optimizing Postpartum Care. Obstet Gynecol 2018;131:e140-50. [Crossref] [PubMed]
- MacArthur C, Wilson D, Herbison P, et al. Urinary incontinence persisting after childbirth: extent, delivery history, and effects in a 12-year longitudinal cohort study. BJOG 2016;123:1022-9. [Crossref] [PubMed]
- Avery K, Donovan J, Peters TJ, et al. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn 2004;23:322-30. [Crossref] [PubMed]
- Huang L, Zhang SW, Wu SL, et al. The Chinese version of ICIQ: a useful tool in clinical practice and research on urinary incontinence. Neurourol Urodyn 2008;27:522-4. [Crossref] [PubMed]
- Sandvik H, Seim A, Vanvik A, et al. A severity index for epidemiological surveys of female urinary incontinence: comparison with 48-hour pad-weighing tests. Neurourol Urodyn 2000;19:137-45. [Crossref] [PubMed]
- Schummers L, Hutcheon JA, Hernandez-Diaz S, et al. Association of Short Interpregnancy Interval With Pregnancy Outcomes According to Maternal Age. JAMA Intern Med 2018;178:1661-70. [Crossref] [PubMed]
- Marinovich ML, Regan AK, Gissler M, et al. Associations between interpregnancy interval and preterm birth by previous preterm birth status in four high-income countries: a cohort study. BJOG 2021;128:1134-43. [Crossref] [PubMed]
- Nisha MK, Alam A, Islam MT, et al. Risk of adverse pregnancy outcomes associated with short and long birth intervals in Bangladesh: evidence from six Bangladesh Demographic and Health Surveys, 1996-2014. BMJ Open 2019;9:e024392. [Crossref] [PubMed]
- Merklinger-Gruchala A, Jasienska G, Kapiszewska M. Short interpregnancy interval and low birth weight: A role of parity. Am J Hum Biol 2015;27:660-6. [Crossref] [PubMed]
- Lin J, Liu H, Wu DD, et al. Long interpregnancy interval and adverse perinatal outcomes: A retrospective cohort study. Sci China Life Sci 2020;63:898-904. [Crossref] [PubMed]
- McKinney D, House M, Chen A, et al. The influence of interpregnancy interval on infant mortality. Am J Obstet Gynecol 2017;216:316.e1-9. [Crossref] [PubMed]
- Regan AK, Gissler M, Magnus MC, et al. Association between interpregnancy interval and adverse birth outcomes in women with a previous stillbirth: an international cohort study. Lancet 2019;393:1527-35. [Crossref] [PubMed]
- Zhang L, Shen S, He J, et al. Effect of Interpregnancy Interval on Adverse Perinatal Outcomes in Southern China: A Retrospective Cohort Study, 2000-2015. Paediatr Perinat Epidemiol 2018;32:131-40. [Crossref] [PubMed]
- Rapaport Pasternak H, Sheiner E, Goldbart A, et al. Short and long interpregnancy interval and the risk for pediatric obstructive sleep apnea in the offspring. Pediatr Pulmonol 2021;56:1085-91. [Crossref] [PubMed]
- Liang CC, Wu MP, Lin SJ, et al. Clinical impact of and contributing factors to urinary incontinence in women 5 years after first delivery. Int Urogynecol J 2013;24:99-104. [Crossref] [PubMed]
- Bergman I, Westergren Söderberg M, Lundqvist A, et al. Associations Between Childbirth and Urinary Incontinence After Midurethral Sling Surgery. Obstet Gynecol 2018;131:297-303. [Crossref] [PubMed]
- Bodner-Adler B, Bodner K, Kimberger O, et al. Role of serum steroid hormones in women with stress urinary incontinence: a case-control study. BJU Int 2017;120:416-21. [Crossref] [PubMed]
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA 2006;295:1809-23. [Crossref] [PubMed]
- Zhu BP, Rolfs RT, Nangle BE, et al. Effect of the interval between pregnancies on perinatal outcomes. N Engl J Med 1999;340:589-94. [Crossref] [PubMed]
- Harrison MS, Montes SB, Rivera C, et al. Interpregnancy Interval in a Rural Guatemalan Population: Results from a Quality Improvement Database. Matern Child Health J 2020;24:1038-46. [Crossref] [PubMed]
- Shifti DM, Chojenta C, Holliday EG, et al. Socioeconomic inequality in short birth interval in Ethiopia: a decomposition analysis. BMC Public Health 2020;20:1504. [Crossref] [PubMed]
- Harrison MS. Interpregnancy interval in lower versus higher human development index countries: a hypothesis about pregnancy spacing. Int Health 2021;13:208-10. [Crossref] [PubMed]
- Cagnacci A, Palma F, Carbone MM, et al. Association between urinary incontinence and climacteric symptoms in postmenopausal women. Menopause 2017;24:77-84. [Crossref] [PubMed]
- Sigurdardottir T, Steingrimsdottir T, Geirsson RT, et al. Can postpartum pelvic floor muscle training reduce urinary and anal incontinence?: An assessor-blinded randomized controlled trial. Am J Obstet Gynecol 2020;222:247.e1-8. [Crossref] [PubMed]
(English Language Editors: C. Mullens and L. Huleatt)



