
Narrative review of roles of astrocytes in subarachnoid hemorrhage
Introduction
Subarachnoid hemorrhage (SAH) is a devastating stroke subtype with more than 50% mortality and morbidity, and often leads to permanent neurological and cognitive deficits for survivors. Compared to other stroke subtypes, SAH seems to occur in the younger populations, half of the SAH patients are under 55 years old at the time of onset. SAH also mostly concerns females. The incidence ratio of female versus male is 70%:30%. In total, 85% of SAH cases are caused by intracranial aneurysms rupture, and 10% of those SAH patients die before intervention because of the sudden attack and severe conditions of the disease (1). The long-term outcome after SAH remains poor. Early brain injury (EBI) and delayed cerebral ischemia (DCI) are considered the most important factors contributing to the poor outcome of SAH. EBI occurs within the first 72 h following SAH. The severity of EBI is associated with bleeding volume, level of consciousness, neuroinflammation and brain edema. According to a recent prospective, observational, multicenter, cohort, phase III diagnostic trial of spreading depolarizations (SD) in SAH patients, the strongest predictor of long-term outcome was total focal brain damage detected by neuroimaging two weeks after the initial hemorrhage. The most important causes of focal brain damage associated with SAH are initial intracerebral hemorrhage (ICH) and infarction due to either early cerebral ischemia (ECI) (2) or DCI. Longitudinal neuroimaging found that the average patient admitted to the intensive care unit after aneurysm treatment had already lost 46±73 mL of brain tissue to ICH and ECI and lost an additional 36±80 mL to delayed ischemic infarcts in the following 14 days. Importantly, in contrast to EBI, DCI is a potentially modifiable cause of lesions during intensive care, as it allows treatment with a neuroprotective intervention before the possible insult or shortly thereafter. Furthermore, association analyses were performed for manually segmented (I) EBI; (II) DCI damage; and (III) total brain damage (EBI + DCI). Each of the three multiple regression models included a SD variable and the median Glasgow Coma Scale (GCS) score from early phase, late phase, or total phase neuromonitoring, suggesting that both SD variables and GCS are currently the strongest predictors of focal brain damage after SAH (3). For decades, people have focused on the intense investigation of cerebral vasospasm (CVS) as the main contributor to poor outcome, yet clinical trials towards mitigating CVS have shown to be a disappointment. Under this circumstances, further investigation into other mechanisms of brain injury following SAH is urgently needed.
As glial cells of neural progenitor origin, astrocytes ubiquitously exist in the central nervous system (CNS), and interact with all kinds of cell types including neurons, microglia, oligodendrocytes, oligodendrocyte progenitor cells, perivascular cells as well as meningeal fibroblasts and circulating immune cells to form the neuron-glia system. In healthy brain, astrocytes participate in many fundamental activities. Astrocytes uptake glutamate to regulate neuronal metabolism, maintain extracellular environment via ion and water channels, regulate cerebral blood flow, stabilize cell-cell communications, synthetize neurotransmitter and defend against oxidative stress (Figure 1).
Compared to the housekeeping functions in normal brains, astrocytes exhibit a quite different response in pathological conditions known as astrocyte reactivity that was considered homogenous and functionally passive (4). Reactive astrocytes are characterized by the distinct morphological changes including cell body hypertrophy, glial fibrillary acidic protein (GFAP) expression upregulation. Reactive astrogliosis is seen virtually in all kinds of neurological diseases, including ischemic and hemorrhagic stroke, epilepsy, demyelination, traumatic brain injury, neurodegeneration disease and neoplastic disease (5-7). The function of reactive astrocytes is different than the normal ones, they serve important roles including axonal remodeling, glial scar formation, regulation of blood-brain barrier (BBB) permeability, mobilizing progenitors, synaptic remodeling, immunomodulation and neurite outgrowth. Experimental evidence suggests that astrocytes are involved in inverse neurovascular coupling and brain edema formation after SAH (8-10) (Figure 2). Furthermore, it seems that the severity of hemorrhage is related to the robustness of astrocytes response (2). However, there is still a lack of evidence to support whether astrocytic pathway after SAH is protective or detrimental.
This review aims at summarizing the role of astrocytes activation following SAH, and exploring the roles of astrocytes in neuroprotection, neuroinflammation, neurotoxicity, BBB disruption, vasospasm and cortical spreading depression (CSD) following SAH. We present the following article in accordance with the Narrative Review reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5486/rc).
Methods
The database PubMed was searched for studies up to 31 May 2022. The following key terms were used for searching: “subarachnoid hemorrhage” OR “SAH” AND “astrocytes”. The titles and abstracts were analyzed in the initial research. Included articles were reviewed in full text. Articles revealed the relationships between SAH and astrocytes related to neuroinflammation, neurotoxicity, brain edema or BBB disruption, vasospasm and cortical spreading depression were included in this review. Studies that are unrelated to SAH or astrocytes were excluded. Two authors YL and JC evaluated the quality and eligibility of each included study independently. The corresponding author GKW provided the consensus or discussion if there was a discrepancy. We found 198 articles using the searching keywords “subarachnoid hemorrhage” OR “SAH” AND “astrocytes”. A total of 156 articles were eliminated due to unrelated topic. Forty-two articles were reviewed in full text and 12 articles were excluded because of our selection criteria. We selected the 30 relevant articles and we also checked relevant references in each article to generate the below systemic review (Table 1).
Table 1
| Items | Specifications |
|---|---|
| Date of search | 31/5/2022 |
| Databases and other sources | PubMed |
| Search term used | “Subarachnoid hemorrhage”; “SAH”; “astrocytes” |
| Timeframe | From 1998 to May 2022 |
| Inclusion and exclusion criteria | Inclusion criteria: focus on the relationship between subarachnoid hemorrhage and astrocytes in terms of neuroinflammation, neurotoxicity, brain edema, vasospasm and spreading depression |
| Exclusion criteria: main topic not related to subarachnoid hemorrhage or astrocytes; main topic not related to neuroinflammation, neurotoxicity, brain edema, vasospasm or spreading depression | |
| Selection process | Two authors evaluated the quality and eligibility of each included study independently. The corresponding author provides the consensus or discussion if there was a discrepancy |
SAH, subarachnoid hemorrhage.
Results
The concept of reactive astrocytes
Reactive astrocytes are defined as the process by which astrocytes change in response to brain insult. GFAP is a major constituent of astrocytes intermediate filaments and is the most commonly used marker for reactive astrocytes. The expression level of GFAP showed a regional difference, hippocampal astrocytes exhibited higher GFAP level than striatal, cortical, and thalamic astrocytes populations (11). Up-regulation of GFAP expression level is a prominent feature of reactive astrocytes, it occurs in various disorders in the CNS and it is an early response to injury. Moreover, the degree of GFAP expression level in reactive astrocytes parallels the severity of the injury under most circumstances (4). In addition to the GFAP upregulation, reactive astrocytes also show prominent morphological changes. Hypertrophy of soma and main processes, process polarization toward a lesion, ramification changes have been seen in reactive astrocytes. Recent studies also revealed that astrocytes undergo massive transcriptional changes which involves hundreds of genes being up-regulated or down-regulated. For instance, Serpina3n and Lcn2 were significantly induced by lipopolysaccharides (LPS) injection or middle cerebral artery occlusion (MCAO) (12). In contrast, some genes associated with important astrocytes functions such as glutamate transporter-1 (GLT-1), glutamine synthase (GS) and potassium channel KIR4.1 are down-regulated in multiple diseases (13,14).
The debate over whether reactive astrocytes are protective or harmful remains controversial. Both good and bad effects of reactive astrocytes have been observed in the past. Reactive astrocytes are able to produce pro-inflammatory cytokines that exacerbate spinal cord injury, and inhibit the axon regeneration after brain injury (15,16). Also, reactive astrocytes have shown to be crucial for withstanding insult and promoting recovery after cerebral ischemia as well as experimental autoimmune encephamyelitis (EAE) (17,18). Therefore, it is important to identify and categorize the “good” and “bad” reactive astrocytes. Though multiple schemes have been brought out to categorize this diversity, until now, the concept of reactive astrocytes subtypes and how to discriminate amongst them still need to be developed. Due to the advances in RNA sequencing and proteomics, we are able to assess cells from a detailed molecular expression level. Similar to the normal healthy CNS, transcriptional analysis has already revealed diversified clusters of astrocytes in different CNS disease models (19,20). In LPS-induced neuroinflammation and MCAO stroke models, reactive astrocytes display two phenotypes “A1” and “A2” respectively, indicating that the phenotypes of reactive astrocyte strongly depended on the type of the inducing injury. In the MCAO model, reactive astrocytes exhibited the neuroprotective “A2” phenotype, based on the fact that “A2” phenotype up-regulate many neurotrophic factors and promote neuronal survival as well as tissue repair. In contrast, reactive astrocytes induced by LPS injection exhibited the neurotoxic “A1” phenotype, “A1” phenotype up-regulate many classical complement cascade genes which is known to be destructive to synapses and secrete a neurotoxin that induces rapid death of neurons and oligodendrocytes (12). They have also shown that activated microglia play a role in inducing “A1” neurotoxic reactive astrocyte via secretion of various inflammatory cytokines in vitro and in vivo (21). Furthermore, “A1” phenotype has been found in many different diseases including Alzheimer’s disease, prion disease, Parkinson’s disease, Huntington’s disease, glioblastoma and amyotrophic lateral sclerosis (22-24). However, though the definitions of “A1” and “A2” phenotypes are useful, it’s an oversimplification for the distinguish of reactive astrocytes. Reactive astrocytes don’t simply polarize into binary phenotypes as we mentioned “A1”, “A2” or neuroprotective, neurotoxic. Instead, reactive astrocytes may adopt multiple functional states based on the pathology, with only a small portion of common changes among those different states. In the process when the astrocytes become reactive, they loss certain homeostatic functions, at the same time gain some protective as well as detrimental functions. Whether the overall impact of reactive astrocytes on the particular disease is protective or detrimental will depend on the balance and nature of gained and lost function and the relative abundance of different astrocytes phenotypes. In addition to distinguish and categorize reactive astrocytes from transcriptional changes, we can also distinguish them based on their structure, proliferative state, the types of cells they are able to interact with, as well as the tissue architecture to which they contributed (25). There have been two fundamentally different subtypes proposed based on the criteria we mentioned above, including proliferative, border-forming reactive astrocytes and non-proliferative reactive astrocytes. The border-forming reactive astrocytes work as a limitans borders that separate and isolate inflamed, damaged and fibrotic tissue from adjacent viable tissues, thus protecting and preserving adjacent tissues (26,27). Newly proliferated reactive astrocytes can be deriving from both existing astrocytes and periventricular neural progenitors (28). Newly proliferated reactive astrocytes are types of cells who located in neural tissue that is not overtly damaged that maintain its tissue architecture to responds to injury (29). Non-proliferative reactive astrocytes react to injury and exhibit multiple changes in molecular expression and cellular hypertrophy. Cellular hypertrophy is frequently used to describe morphological changes in reactive astrocytes, however, study has found that reactive astrocytes show hypertrophy of their intermediate filament-rich main cellular processes but seem to remain their unique “tiled” territories (30). Thus, non-proliferative reactive astrocytes are likely to interact with the same elements that they interact with in the healthy brain, such as neurons and synapses.
Several SAH induced molecular changes in the expression of reactive astrocytes have been reported and are summarized in Table 2 (31-37).
Table 2
| Molecular changes in astrocytes | Remarks | Outcome | References |
|---|---|---|---|
| GFAP | A constituent of intermediate filaments | A marker of reactive astrocytes | (9) |
| ET-1 | A vasoconstrictor mediator | Higher expression level indicates a higher risk of delayed ischemia | (31) |
| AQP4 | Water channel protein | Increased level causes hydrocephalus | (32) |
| MMP-9 | Matrix metalloproteinases | Cause BBB disruption | (33) |
| S100B | A calcium-binding protein | Higher level predicts poor long-term outcome | (34) |
| GLT-1 | Glutamate-transporter 1 | Downregulated after SAH | (35) |
| HDAC2 | Histone deacetylase 2 | Inhibition of HDAC2 improved DCI | (35) |
| TLR3, TLR4 | Toll-like receptor | Activation of TLR leads to synthesis of pro-inflammatory cytokines | (36) |
| HO-1 | Heme oxygenase | Alleviate neuronal cell death and cognitive impairment | (37) |
SAH, subarachnoid hemorrhage; GFAP, glial fibrillary acidic protein; ET-1, endothelin-1; AQP4, aquaporin-4; MMP-9, metalloproteinases-9; BBB, blood-brain barrier; GLT-1, glutamate-transporter-1; HDAC2, histone deacetylase 2; DCI, delayed cerebral ischemia; TLR, Toll-like receptor; HO-1, heme oxygenase-1.
Astrocytes and neuroinflammation
Astrocytes play a controversial role in neuroinflammation, that is to say, astrocytes can be both protective and detrimental to neuroinflammation (38-43). Compelling evidence have shown that astrocytes responses to various cytokines, hormones and growth factors seems to be beneficial, it is supported by the fact that the absence of astrocytes exacerbates brain injury (44). There are several protective pathways in astrocytes. One is mediated by the glycoprotein gb130, an essential signal transducer for members of the interleukin-6 (IL-6) cytokine family. Astrocytic gp130 signaling pathway is essential for glial cell to survive. GFAPcre-gp130fl/fl mice showed increased apoptosis of astrocyte, worsened mortality rate after toxoplasma encephalitis (TE) infection, and deteriorated EAE scores (38,45). Ligand binding to the gp130 receptor activates mitogen-activated protein kinase (MAPK) (SHP2/Ras/ERK) signaling cascades and signal transducer and activator of transcription (STAT) 1/3, study has observed mice with defective SHP2/Ras/ERK signaling pathway increased EAE severity, indicating that gb130-mediated SHP2/Ras/ERK activation limits neuroinflammation (38,39).
transforming growth factor-β (TGF-β) signaling is another signaling inside of astrocytes strongly upregulated after brain injury (46,47). Mice with defective TGF-β pathway in astrocytes showed aggravated neuroinflammation as well as augmented myeloid cell activation after ischemic stroke (40). TGF-β signaling inhibits nuclear translocation of the proinflammatory transcription factor nuclear factor κB (NF-κB) activation, thus inhibiting NF-κB-mediated neuroinflammation (48). Interferon (IFN)-γ plays dual roles in neuroinflammation, as it provides neuroprotection by negatively regulate the neutrophil and T cell accumulation and promote macrophage and microglia activation (49). Mice with deficient IFN-γ pathway in astrocytes display enhanced leukocyte infiltration, upregulation of inflammatory genes transcription, including CCL2, CXCL10, iNOS, and downregulation of anti-inflammatory cytokines such as IL-10 and IL-27 in EAE disease (41).
In addition, there are several detrimental signaling pathway in astrocytes. During neuroinflammation process, IL-17 receptor is upregulated in astrocytes (50). As a very important inflammatory cytokine, IL-17 works as an inducer of the activation and mobilization of neutrophils to inflammation sites. Study had found that IL 17 signaling blocking in astrocytes via Act1 knocking down effectively reduce infiltrating cell numbers in the brain, such as Th1 and Th17 cells, and show therapeutic effect in EAE without affecting the peripheral immune system (51). Sphingolipids is another important detrimental signaling pathway. Sphingosine-1-phosphate (S1P) and S1P receptors (S1PR) are expressed ubiquitously in the brain. In both innate and adaptive immune systems, S1P-S1PR signaling in involved with immune trafficking and activation (51). Mice with deficient S1P1 signaling in astrocytes displayed decreased demyelination and axonal loss, indicating the pathogenic effect of S1P signaling pathway in astrocytes (42). As another lipid mediator, LacCer triggers astrogliosis and inflammation. LacCer in astrocytes activates interferon regulatory factor 1 (IRF-1) and NF-κB transcription factors, which in turn recruit Cfs2 (GM-CSF) promoter (43).
Reactive astrocytes are able to generate and release different molecules such as cytokines, chemokines, growth factors and neurotrophic factors, which potentially aggravate or ameliorate neuroinflammation upon CNS injury. Activated astrocytes release several mediators regulated by NF-κB, such as CXCL10, CCL2, vascular endothelial growth factor (VEGF) (52,53). In vivo study shows that astrocytes CCL2 enhance leukocyte recruitment into the brain parenchyma, specific depletion of CCL2 in astrocytes shows immune cell infiltration decrease, demyelination, and axonal loss reduction (36). Moreover, deletion of CXCL10 in astrocytes displays similar effects. Astrocytes also produce neuroprotective mediator such as BDNF, elimination of BDNF production in astrocytes leads to a more severe EAE course with exacerbated axonal damage (33). Heme oxygenase-1 (HO-1) is an enzyme found in neuron, microglia and astrocytes (54). HO-1 metabolize free heme released into the subarachnoid space during SAH thus alleviate brain injury following SAH (55). Nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response has been proved to be a crucial anti-inflammatory pathway in SAH. Depletion of Nrf2 in astrocytes tremendously worsens inflammation by activation of NF-κB (56).
Besides GFAP, S100B is also a biomarker of reactive astrocytes, and the elevation of S100B expressions features the CNS pathologies as well. In SAH patients, the expression of GFAP and S100B levels upregulated in both cerebral spinal fluid (CSF) and serum, and higher S100B levels in CSF is associated with poor 1-year clinical outcome (57). In animal SAH models, GFAP and astrocytic S100B levels were also increased significantly. After SAH, astrocytic polarization towards both A1 and A2 phenotypes had been seen in rats SAH models (58). Unlike microglia, whose dynamics of polarization and transcriptomic diversity have been well studied after SAH, more work need to be done concerning the astrocytic polarization and subsets in SAH (34,59,60).
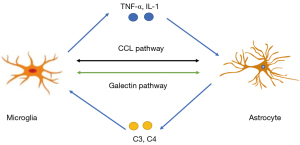
A recent study has firstly reported the detailed single-cell transcriptomic characterization of microglia after SAH (60). It revealed some interactions between microglia and astrocytes in CC-motif chemokine ligand (CCL) signaling and Galectin signaling pathway. CCL family play many important roles in neurodegenerative and neuroinflammatory processes. After SAH, microglia act as the main sender and receiver of CCL pathway while astrocytes act as a mediator. As glycan-binding proteins, Galectins act as endogenous modulators of inflammatory response in the brain. Galectin signaling pathway mainly depended on microglia after SAH, while astrocytes work as an influencer in that pathway. This particular study gives us more evidence about the fact that astrocytes are important inflammation modulators in SAH (Figure 3). However, more clear and evident interactions between microglia and astrocytes need to be clarified in the future.
Astrocytes and neurotoxicity
Elevated glutamate concentrations in both CSF and blood have been shown to correlate with a worse neurological outcome in various neurodegenerative processes. After stroke, excessive glutamate over activates glutamate receptors, which in turn lead to intracellular Ca2+ overload and excitotoxicity (32). Clinical studies have shown that the concentration of glutamate in CSF significantly increased in aneurysmal subarachnoid hemorrhage (aSAH) patients with neurologic deficits and is associated with a higher occurrence of CVS, edema and DCI (61). The mechanism which may cause the high interstitial glutamate concentration is not fully understood. In humans, ischemic events after SAH caused failing glutamine synthesis during energy crisis as evidenced by microdialysis measurement. Despite the failing glutamine synthesis, another possibility to cause high concentration of interstitial glutamate is the release of glutamine by platelet. The activation of platelets also leads to the downregulation of surface glutamate receptor 2, a marker of excitotoxity exposure and a possible mechanism of neuronal dysfunction (62).
Astrocytic glutamate transporters, known as excitatory amino acid transporters (EAATs), are essential in glutamate homeostasis in the brain. The brain has several mechanisms by which excess glutamate is eliminated to prevent neurotoxicity. EAATs take up glutamate from extracellular space into the cytosol, where glutamate convert into glutamine in astrocytes. Glutamate could also be oxidatively metabolized to α-ketoglutarate used for ATP synthesis.
A study showed that excessive glutamate causes GluN1/GluN2B NMDA receptors and mGluR1 over-stimulation, which leads to the calcium overload and cell apoptosis (59). Moreover, blockage of GluN1/GluN2B NMDA receptors and mGluR1 prevents glutamate-mediated Ca2+ overloading and cell deaths in primary neurons in experimental SAH (63). The inhibition of EAAT gene transcription is associated with histone modification, especially histone acetylation. After SAH, it was found that interstitial glutamate accumulated in the hippocampus and glutamate-transporter-1 (GLT-1) decreased in astrocytes. Astrocytic histone deacetylase 2 (HDAC2) expression was significantly increased after SAH, HDAC2 inhibition not only restore GLT-1 expression level by transcription regulation but also effectively improved DCI in SAH mice (64).
Excessive ferritin following SAH appear to be related to neurotoxic cascades. Dysregulated iron homeostasis and downstream oxidative events lead to both neurotoxicity and vasospasm (65). Astrocytes are strategically located to acquire nutrients from the circulating blood such as iron. Therefore, astrocytes participate in brain iron homeostasis and prevent neurotoxic cascades after SAH (66).
Astrocytes and brain edema, BBB breakdown
Vasogenic edema causes pathological cell swelling in SAH. Aquaporin-4 (AQP4) is the predominant water channel protein expressed in astrocytes and mediates water homeostasis across the BBB. AQP1 and AQP4 expression levels were significantly increased in human brain after SAH (67). Following SAH in rats, the expression level of AQP1 and AQP4 markedly increased in the ventricle region. Furthermore, a positive correlation between AQP1 and AQP4 expression levels and lateral ventricle area was seen (68). After SAH, there were some morphological changes of hippocampal astrocytes including cell body swelling, processes retracting and AQP4 positive capillary coverage reducing (69). Atorvastatin, an inhibitor of the 3-hydroxy-3-methylglutaryl A (HMG-CoA) reductase, reduced AQP4 expression, ameliorated hydrocephalus and EBI after experimental SAH (35).
The glymphatic system consists of peri-arterial CSF inflow running in the same direction as blood flow, propelled by the pulsatility of the arterial wall. The glymphatic system clears key proteins involved in neurodegeneration, and conversely, inhibition of glymphatic-lymphatic transport accelerates protein accumulation and cognitive decline in mouse models of Alzheimer’s disease, traumatic brain injury, and Parkinson’s disease (70). Recently, the role of glymphatic system after SAH has brought certain attention. Following brain injury, AQP4 and cation channel protein sulfonylurea receptor 1 (SUR1) form an ion/water channel complex which may responsible for the bulk water influx in swollen astrocytes as well as impaired CSF movement (71). Impaired glymphatic system is associated with AQP4 polarization loss, and leads to impaired brain macromolecular substances elimination (72,73). Surgical blockade of cerebral lymphatic drainage worsens brain edema and cerebral ischemia during after SAH (31,74). After SAH, a long-lasting glymphatic malfunction has been reported, which may be associated with abnormal aggregation of blood cells and blood components within the brain perivascular spaces as well as activated astrocytes with loss of AQP4 polarization (75).
Astrocytes is crucial in maintaining the structural integrity of BBB (76). Matrix metalloproteinases-9 (MMP-9) is associated with tight junctions and basal lamina degradation that cause BBB integrity break down (77). MMP-9 in particular has been shown to play key roles in the pathophysiology of SAH in both animal and patient studies (78). Astrocytes and microglia secrete MMP-9 when stimulated with a gram-negative cell wall product, LPS (79). Astrocyte derived neurotrophic factor (MANF) protein exerts BBB protection effects by suppressing MMP-9 expression after SAH (80). Reactive astrocytes also express osteopontin which is known to reduce BBB disruption after SAH (81). Therefore, astrocytes may be protective in maintaining the integrity of BBB after SAH.
Astrocytes and vasospasm
CVS is a feared complication of SAH and it typically occurs within 3 to 15 days after SAH (82). The mechanism involved in the development of CVS include pathways for nitric oxide (NO), endothelin, hypoxia-induced factors, and inflammatory markers (e.g., TNF, IL-1, IL-6) (83). Vasoconstriction activator endothelin-1 (ET-1) upregulate, as well as hypercontractility of vascular smooth muscle cell have been reported to be involved in CVS (84). ET1 is essential in the initiation and maintenance of CVS (85). The level of ET-1 in the CSF significantly elevated in SAH patients (86,87). Higher level s of ET-1 in the CSF is associated with higher risk of CVS. The expression level of ET-1 peaked at 3–5 days and stayed high level until 10 days following SAH onset (88). After SAH, astrocytes predominantly express ET-1 and high level of ET-1 is related to worse outcome in mice (89). There are two major receptors, ETA and ETB, mediate the ET-1 induced constriction (90). The expression levels of ETA and ETB increased after experimental animal models of SAH (37,91). Furthermore, the expression levels of ETA and ETB mRNA significantly elevated in arteries incubated with hemorrhagic CSF (88). ET-1 receptor antagonists effectively prevent and relieve SAH-induced CVS (92). However, according to the recent study, CVS has shown no correlation with outcome after SAH, which indicates that CVS is unlikely the principal pathomechanism of DCI, although it seems to be involved in it as a modulating factor (3).
Astrocytes and SD
SD is described as a propagating wave of depolarization in neurons and glial cells in cerebral gray matter (93). This pathophysiologic phenomenon of CSD has been observed in various diseases including SAH, ischemic stroke and ICH (94,95). CSD occur along a continuum from short-lasting harmless to terminal deleterious events, all of which are observed in patients with SAH (96). Prolonged CSD is correlate to worse outcomes in SAH patients (97,98). After SAH, CSD will lead to vasoconstriction and secondary brain injury through mechanisms involving decreased blood flow, increased energy demand, cytokine release, and BBB disruption. Moreover, CSD has been implicated in the mechanism involving cytotoxic, ionic, and vasogenic edema (9,99-101). Furthermore, CSD triggered CSF influx by the glymphatic pathway (70). The key feature of SD is the near-complete breakdown of the transmembrane ion gradients associated with the influx of water into neurons (8,102). Thus, SD is the mechanism initiating the cytotoxic edema of neurons (9). In normal tissue, astrocytes remain functional and support neuronal recovery from SD. That neurons lead and astrocytes follow is exemplified by changes in intracellular calcium which rises first in neurons, then in astrocytes (103). Moreover, SD and the associated neuronal calcium wave remain unaffected when the astrocytic calcium wave is blocked by the depletion of internal calcium stores (104). Importantly, SD induces tone alterations in resistance vessels, causing either transient hyperperfusion in healthy tissue; or severe hypoperfusion in tissue at risk for progressive damage. In 1998, SD-induced spreading ischemia was discovered in a rat model mimicking conditions present following SAH in which artificial cerebrospinal fluid (aCSF) was applied topically on the brain containing an increased K+ concentration ([K+]aCSF) and either the NO scavenger hemoglobin or the NO synthase (NOS) inhibitor L-NNA (105). It was proposed that SD-induced spreading ischemia may be the pathophysiological correlate of DCI after SAH. The important role of NO deficiency in this model agrees well with the increasingly recognized hypothesis, originally proposed by Furchgott and colleagues, that clot-related factors cause NO deficiency after SAH (106-109). NO deficiency leads to vasoconstriction both directly and indirectly through the lack of its permissive effect on other vasodilators (8,108,110). NO deficiency also increases susceptibility to SD both in vivo and in brain slices (111). Translating this model to the human condition required several steps. This started with the discovery that clusters of SD occur in SAH patients and are associated with initially reversible, waxing and waning episodes of delayed neurological deficits (112). The development of optoelectrode strips for subdural implantation in patients and the introduction of true direct current (DC)-electrocorticography (ECoG) then enabled to capture the entire continuum from normal spreading hyperemia to the highly pathologic spreading ischemia in response to SD after SAH (113). Meanwhile, the whole sequence of progressively prolonged SD-induced spreading ischemia and the transition from clustered SD to a negative ultraslow potential was demonstrated to be the pathophysiological correlate of delayed infarction development in humans after SAH when optoelectrodes were placed directly over a newly developing delayed cerebral infarct as revealed by longitudinal neuroimaging (114). Thus, the phenomenology of changes in electrical potential, regional cerebral blood flow, and oxygen in brain tissue observed during infarct development after SAH in humans are exactly the same as in the model of spreading ischemia in the rat, which mimics conditions after SAH (105). Several lines of evidence suggest that astrocytes are importantly involved in the inverse neurovascular response to SD as previously discussed in directly showed that the calcium wave in astrocytes associated with SD can lead to constriction of blood vessels via the astrocytic end-feet (10,103). In general, astrocytes are of paramount importance for the orderly process of SD. Astrocyte-directed inactivation of connexin 43 decreased astrocytic gap junctional communication and increased tissue susceptibility to SD and the propagation speed of SD (115). Selective intoxication of astrocytes caused SD that initiated a shallow negative ultraslow potential as neurons began to die (116). Astrocytic dysfunction may accelerate neuronal death under ischemic conditions (117). Accordingly, disturbed astrocyte function can abolish the typical slow spread of SD in the ischemic zone and can accelerate cellular dying (118). Astrocytes play key roles in the regulation of CSD and subsequently reactive astrocytes could potentially be involved in SAH-associated CSD. CSD enhances the expression of TLR3 and TLR4 in astrocytes, given that TLR3 and TLR4 play important role in neuroinflammatory process in the brain, we can infer that astrocytic TLR receptors may be involved in mechanism of CSD-related neuroinflammation (119). Impaired K+ and glutamate clearance by astrocytes cause propagation of the CSD (120). At the onset of CSD, astrocytes exhibit concurrent Ca2+ waves that are temporally and spatially associated with the propagation of CSD and neuronal depolarization (104,121). Moreover, after the initial concurrent Ca2+ waves, CSD further promoted an extended period astrocytic activity that involved enhanced Ca2+ oscillations during the recovery phase. CSD-induced astrocytic Ca2+ oscillations were temporally associated with elevated gliotransmission, which in turn induced slow inward currents (SICs) in pyramidal neurons (122).
Conclusions
We summarized the response of astrocytes induced by SAH. Both beneficial and detrimental effects of astrocytes are implicated in neuroinflammation, neurotoxicity, brain edema, vasospasm and cortical SD after SAH. The effects of reactive astrocytes on neurological recovery and functional outcome after SAH designate astrocytes as a potential promising therapeutic target of pharmacological and cell-based approaches. A better understanding of the astrocytic activity after SAH can help develop new therapeutics options for SAH. Indeed, preclinical studies have already shown that therapeutic targeting the astrocytes response could have a beneficial effect in reducing neuronal damage and cognitive impairment after SAH. Translational stroke researches are urgently needed to determine the precise mechanism of astrocyte-induced brain damage and repair following SAH and, most importantly, to develop treatments to improve patient prognosis.
Acknowledgments
Funding: The study was supported by the CUHK Direct Grant for Research 2021/2022 (Reference No. 2021.078).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5486/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5486/coif). The authors have no conflicts of interest to declare.
Ethical Statement: All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Claassen J, Park S. Spontaneous subarachnoid haemorrhage. Lancet 2022;400:846-62. [Crossref] [PubMed]
- Kooijman E, Nijboer CH, van Velthoven CT, et al. Long-term functional consequences and ongoing cerebral inflammation after subarachnoid hemorrhage in the rat. PLoS One 2014;9:e90584. [Crossref] [PubMed]
- Dreier JP, Winkler MKL, Major S, et al. Spreading depolarizations in ischaemia after subarachnoid haemorrhage, a diagnostic phase III study. Brain 2022;145:1264-84. [Crossref] [PubMed]
- Escartin C, Guillemaud O, Carrillo-de Sauvage MA. Questions and (some) answers on reactive astrocytes. Glia 2019;67:2221-47. [Crossref] [PubMed]
- Price BR, Johnson LA, Norris CM. Reactive astrocytes: The nexus of pathological and clinical hallmarks of Alzheimer's disease. Ageing Res Rev 2021;68:101335. [Crossref] [PubMed]
- Linnerbauer M, Rothhammer V. Protective Functions of Reactive Astrocytes Following Central Nervous System Insult. Front Immunol 2020;11:573256. [Crossref] [PubMed]
- Williamson MR, Fuertes CJA, Dunn AK, et al. Reactive astrocytes facilitate vascular repair and remodeling after stroke. Cell Rep 2021;35:109048. [Crossref] [PubMed]
- Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med 2011;17:439-47. [Crossref] [PubMed]
- Dreier JP, Lemale CL, Kola V, et al. Spreading depolarization is not an epiphenomenon but the principal mechanism of the cytotoxic edema in various gray matter structures of the brain during stroke. Neuropharmacology 2018;134:189-207. [Crossref] [PubMed]
- Major S, Petzold GC, Reiffurth C, et al. A role of the sodium pump in spreading ischemia in rats. J Cereb Blood Flow Metab 2017;37:1687-705. [Crossref] [PubMed]
- Chai H, Diaz-Castro B, Shigetomi E, et al. Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron 2017;95:531-549.e9. [Crossref] [PubMed]
- Zamanian JL, Xu L, Foo LC, et al. Genomic analysis of reactive astrogliosis. J Neurosci 2012;32:6391-410. [Crossref] [PubMed]
- Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int 2007;51:333-55. [Crossref] [PubMed]
- Nwaobi SE, Cuddapah VA, Patterson KC, et al. The role of glial-specific Kir4.1 in normal and pathological states of the CNS. Acta Neuropathol 2016;132:1-21. [Crossref] [PubMed]
- Alilain WJ, Horn KP, Hu H, et al. Functional regeneration of respiratory pathways after spinal cord injury. Nature 2011;475:196-200. [Crossref] [PubMed]
- Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol 2008;209:294-301. [Crossref] [PubMed]
- Bush TG, Puvanachandra N, Horner CH, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 1999;23:297-308. [Crossref] [PubMed]
- Voskuhl RR, Peterson RS, Song B, et al. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci 2009;29:11511-22. [Crossref] [PubMed]
- Tassoni A, Farkhondeh V, Itoh Y, et al. The astrocyte transcriptome in EAE optic neuritis shows complement activation and reveals a sex difference in astrocytic C3 expression. Sci Rep 2019;9:10010. [Crossref] [PubMed]
- Adams KL, Gallo V. The diversity and disparity of the glial scar. Nat Neurosci 2018;21:9-15. [Crossref] [PubMed]
- Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017;541:481-7. [Crossref] [PubMed]
- Wu T, Dejanovic B, Gandham VD, et al. Complement C3 Is Activated in Human AD Brain and Is Required for Neurodegeneration in Mouse Models of Amyloidosis and Tauopathy. Cell Rep 2019;28:2111-2123.e6. [Crossref] [PubMed]
- Sun S, Sun Y, Ling SC, et al. Translational profiling identifies a cascade of damage initiated in motor neurons and spreading to glia in mutant SOD1-mediated ALS. Proc Natl Acad Sci U S A 2015;112:E6993-7002. [Crossref] [PubMed]
- Smith HL, Freeman OJ, Butcher AJ, et al. Astrocyte Unfolded Protein Response Induces a Specific Reactivity State that Causes Non-Cell-Autonomous Neuronal Degeneration. Neuron 2020;105:855-866.e5. [Crossref] [PubMed]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 2009;32:638-47. [Crossref] [PubMed]
- Katsouri L, Birch AM, Renziehausen AWJ, et al. Ablation of reactive astrocytes exacerbates disease pathology in a model of Alzheimer's disease. Glia 2020;68:1017-30. [Crossref] [PubMed]
- Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014;81:229-48. [Crossref] [PubMed]
- Benner EJ, Luciano D, Jo R, et al. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature 2013;497:369-73. [Crossref] [PubMed]
- Burda JE, Bernstein AM, Sofroniew MV. Astrocyte roles in traumatic brain injury. Exp Neurol 2016;275:305-15. [Crossref] [PubMed]
- Wilhelmsson U, Bushong EA, Price DL, et al. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci U S A 2006;103:17513-8. [Crossref] [PubMed]
- Sun BL, Xie FM, Yang MF, et al. Blocking cerebral lymphatic drainage deteriorates cerebral oxidative injury in rats with subarachnoid hemorrhage. Acta Neurochir Suppl 2011;110:49-53. [Crossref] [PubMed]
- Dong XX, Wang Y, Qin ZH. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin 2009;30:379-87. [Crossref] [PubMed]
- Mills Ko E, Ma JH, Guo F, et al. Deletion of astroglial CXCL10 delays clinical onset but does not affect progressive axon loss in a murine autoimmune multiple sclerosis model. J Neuroinflammation 2014;11:105. [Crossref] [PubMed]
- Zheng ZV, Lyu H, Lam SYE, et al. The Dynamics of Microglial Polarization Reveal the Resident Neuroinflammatory Responses After Subarachnoid Hemorrhage. Transl Stroke Res 2020;11:433-49. [Crossref] [PubMed]
- Chen JH, Yang LK, Chen L, et al. Atorvastatin ameliorates early brain injury after subarachnoid hemorrhage via inhibition of AQP4 expression in rabbits. Int J Mol Med 2016;37:1059-66. [Crossref] [PubMed]
- Huang DR, Wang J, Kivisakk P, et al. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med 2001;193:713-26. [Crossref] [PubMed]
- Itoh S, Sasaki T, Asai A, et al. Prevention of delayed vasospasm by an endothelin ETA receptor antagonist, BQ-123: change of ETA receptor mRNA expression in a canine subarachnoid hemorrhage model. J Neurosurg 1994;81:759-64. [Crossref] [PubMed]
- Haroon F, Drögemüller K, Händel U, et al. Gp130-dependent astrocytic survival is critical for the control of autoimmune central nervous system inflammation. J Immunol 2011;186:6521-31. [Crossref] [PubMed]
- Tebbutt NC, Giraud AS, Inglese M, et al. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med 2002;8:1089-97. [Crossref] [PubMed]
- Cekanaviciute E, Fathali N, Doyle KP, et al. Astrocytic transforming growth factor-beta signaling reduces subacute neuroinflammation after stroke in mice. Glia 2014;62:1227-40. [Crossref] [PubMed]
- Hindinger C, Bergmann CC, Hinton DR, et al. IFN-γ signaling to astrocytes protects from autoimmune mediated neurological disability. PLoS One 2012;7:e42088. [Crossref] [PubMed]
- Choi JW, Gardell SE, Herr DR, et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci U S A 2011;108:751-6. [Crossref] [PubMed]
- Mayo L, Trauger SA, Blain M, et al. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat Med 2014;20:1147-56. [Crossref] [PubMed]
- Michinaga S, Koyama Y. Pathophysiological Responses and Roles of Astrocytes in Traumatic Brain Injury. Int J Mol Sci 2021;22:6418. [Crossref] [PubMed]
- Drögemüller K, Helmuth U, Brunn A, et al. Astrocyte gp130 expression is critical for the control of Toxoplasma encephalitis. J Immunol 2008;181:2683-93. [Crossref] [PubMed]
- Buckwalter MS, Wyss-Coray T. Modelling neuroinflammatory phenotypes in vivo. J Neuroinflammation 2004;1:10. [Crossref] [PubMed]
- Doyle KP, Cekanaviciute E, Mamer LE, et al. TGFβ signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J Neuroinflammation 2010;7:62. [Crossref] [PubMed]
- Cho ML, Min SY, Chang SH, et al. Transforming growth factor beta 1(TGF-beta1) down-regulates TNFalpha-induced RANTES production in rheumatoid synovial fibroblasts through NF-kappaB-mediated transcriptional repression. Immunol Lett 2006;105:159-66. [Crossref] [PubMed]
- Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity 2009;31:539-50. [Crossref] [PubMed]
- Colombo E, Di Dario M, Capitolo E, et al. Fingolimod may support neuroprotection via blockade of astrocyte nitric oxide. Ann Neurol 2014;76:325-37. [Crossref] [PubMed]
- Kang Z, Altuntas CZ, Gulen MF, et al. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity 2010;32:414-25. [Crossref] [PubMed]
- Lund IV, Hu Y, Raol YH, et al. BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci Signal 2008;1:ra9. [Crossref] [PubMed]
- Islam O, Loo TX, Heese K. Brain-derived neurotrophic factor (BDNF) has proliferative effects on neural stem cells through the truncated TRK-B receptor, MAP kinase, AKT, and STAT-3 signaling pathways. Curr Neurovasc Res 2009;6:42-53. [Crossref] [PubMed]
- Matz P, Turner C, Weinstein PR, et al. Heme-oxygenase-1 induction in glia throughout rat brain following experimental subarachnoid hemorrhage. Brain Res 1996;713:211-22. [Crossref] [PubMed]
- Gomes JA, Selim M, Cotleur A, et al. Brain iron metabolism and brain injury following subarachnoid hemorrhage: iCeFISH-pilot (CSF iron in SAH). Neurocrit Care 2014;21:285-93. [Crossref] [PubMed]
- Pan H, Wang H, Zhu L, et al. Depletion of Nrf2 enhances inflammation induced by oxyhemoglobin in cultured mice astrocytes. Neurochem Res 2011;36:2434-41. [Crossref] [PubMed]
- Balança B, Ritzenthaler T, Gobert F, et al. Significance and Diagnostic Accuracy of Early S100B Serum Concentration after Aneurysmal Subarachnoid Hemorrhage. J Clin Med 2020;9:1746. [Crossref] [PubMed]
- Ma M, Li H, Wu J, et al. Roles of Prokineticin 2 in Subarachnoid Hemorrhage-Induced Early Brain Injury via Regulation of Phenotype Polarization in Astrocytes. Mol Neurobiol 2020;57:3744-58. [Crossref] [PubMed]
- Chen J, Zheng ZV, Lu G, et al. Microglia activation, classification and microglia-mediated neuroinflammatory modulators in subarachnoid hemorrhage. Neural Regen Res 2022;17:1404-11. [Crossref] [PubMed]
- Chen J, Sun L, Lyu H, et al. Single-cell analysis of microglial transcriptomic diversity in subarachnoid haemorrhage. Clin Transl Med 2022;12:e783. [Crossref] [PubMed]
- Wang HB, Wu QJ, Zhao SJ, et al. Early High Cerebrospinal Fluid Glutamate: A Potential Predictor for Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage. ACS Omega 2020;5:15385-9. [Crossref] [PubMed]
- Bell JD, Thomas TC, Lass E, et al. Platelet-mediated changes to neuronal glutamate receptor expression at sites of microthrombosis following experimental subarachnoid hemorrhage. J Neurosurg 2014;121:1424-31. [Crossref] [PubMed]
- Zhang Z, Liu J, Fan C, et al. The GluN1/GluN2B NMDA receptor and metabotropic glutamate receptor 1 negative allosteric modulator has enhanced neuroprotection in a rat subarachnoid hemorrhage model. Exp Neurol 2018;301:13-25. [Crossref] [PubMed]
- Tao K, Cai Q, Zhang X, et al. Astrocytic histone deacetylase 2 facilitates delayed depression and memory impairment after subarachnoid hemorrhage by negatively regulating glutamate transporter-1. Ann Transl Med 2020;8:691. [Crossref] [PubMed]
- Panther EJ, Zelmanovich R, Hernandez J, et al. Ferritin and Neurotoxicity: A Contributor to Deleterious Outcomes for Subarachnoid Hemorrhage. Eur Neurol 2022;85:415-23. [Crossref] [PubMed]
- Cheli VT, Correale J, Paez PM, et al. Iron Metabolism in Oligodendrocytes and Astrocytes, Implications for Myelination and Remyelination. ASN Neuro 2020;12:1759091420962681. [Crossref] [PubMed]
- Badaut J, Brunet JF, Grollimund L, et al. Aquaporin 1 and aquaporin 4 expression in human brain after subarachnoid hemorrhage and in peritumoral tissue. Acta Neurochir Suppl 2003;86:495-8. [Crossref] [PubMed]
- Long CY, Huang GQ, Du Q, et al. The dynamic expression of aquaporins 1 and 4 in rats with hydrocephalus induced by subarachnoid haemorrhage. Folia Neuropathol 2019;57:182-95. [Crossref] [PubMed]
- Anzabi M, Ardalan M, Iversen NK, et al. Hippocampal Atrophy Following Subarachnoid Hemorrhage Correlates with Disruption of Astrocyte Morphology and Capillary Coverage by AQP4. Front Cell Neurosci 2018;12:19. [Crossref] [PubMed]
- Mestre H, Du T, Sweeney AM, et al. Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science 2020;367:eaax7171. [Crossref] [PubMed]
- Stokum JA, Kwon MS, Woo SK, et al. SUR1-TRPM4 and AQP4 form a heteromultimeric complex that amplifies ion/water osmotic coupling and drives astrocyte swelling. Glia 2018;66:108-25. [Crossref] [PubMed]
- Kress BT, Iliff JJ, Xia M, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 2014;76:845-61. [Crossref] [PubMed]
- Iliff JJ, Chen MJ, Plog BA, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 2014;34:16180-93. [Crossref] [PubMed]
- Sun BL, Xia ZL, Wang JR, et al. Effects of blockade of cerebral lymphatic drainage on regional cerebral blood flow and brain edema after subarachnoid hemorrhage. Clin Hemorheol Microcirc 2006;34:227-32. [PubMed]
- Pu T, Zou W, Feng W, et al. Persistent Malfunction of Glymphatic and Meningeal Lymphatic Drainage in a Mouse Model of Subarachnoid Hemorrhage. Exp Neurobiol 2019;28:104-18. [Crossref] [PubMed]
- Giannoni P, Badaut J, Dargazanli C, et al. The pericyte-glia interface at the blood-brain barrier. Clin Sci (Lond) 2018;132:361-74. [Crossref] [PubMed]
- Lucke-Wold BP, Logsdon AF, Manoranjan B, et al. Aneurysmal Subarachnoid Hemorrhage and Neuroinflammation: A Comprehensive Review. Int J Mol Sci 2016;17:497. [Crossref] [PubMed]
- Hayman EG, Wessell A, Gerzanich V, et al. Mechanisms of Global Cerebral Edema Formation in Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care 2017;26:301-10. [Crossref] [PubMed]
- Jian Liu K, Rosenberg GA. Matrix metalloproteinases and free radicals in cerebral ischemia. Free Radic Biol Med 2005;39:71-80. [Crossref] [PubMed]
- Li T, Xu W, Gao L, et al. Mesencephalic astrocyte-derived neurotrophic factor affords neuroprotection to early brain injury induced by subarachnoid hemorrhage via activating Akt-dependent prosurvival pathway and defending blood-brain barrier integrity. FASEB J 2019;33:1727-41. [Crossref] [PubMed]
- Suzuki H, Hasegawa Y, Kanamaru K, et al. Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke 2010;41:1783-90. [Crossref] [PubMed]
- Zheng VZ, Wong GKC. Neuroinflammation responses after subarachnoid hemorrhage: A review. J Clin Neurosci 2017;42:7-11. [Crossref] [PubMed]
- Small C, Scott K, Smart D, et al. Microglia and Post-Subarachnoid Hemorrhage Vasospasm: Review of Emerging Mechanisms and Treatment Modalities. Clin Surg J 2022;3:INF1000213.
- Cossu G, Messerer M, Oddo M, et al. To look beyond vasospasm in aneurysmal subarachnoid haemorrhage. Biomed Res Int 2014;2014:628597. [Crossref] [PubMed]
- Ohkita M, Tawa M, Kitada K, et al. Pathophysiological roles of endothelin receptors in cardiovascular diseases. J Pharmacol Sci 2012;119:302-13. [Crossref] [PubMed]
- Kuruppu S, Chou SH, Feske SK, et al. Soluble and catalytically active endothelin converting enzyme-1 is present in cerebrospinal fluid of subarachnoid hemorrhage patients. Mol Cell Proteomics 2014;13:1091-4. [Crossref] [PubMed]
- Kanpolat Y. Information age publishing. Surg Neurol 2005;64:S1-2. [Crossref] [PubMed]
- Cheng YW, Li WJ, Dou XJ, et al. Role of endothelin-1 and its receptors in cerebral vasospasm following subarachnoid hemorrhage. Mol Med Rep 2018;18:5229-36. [Crossref] [PubMed]
- Yeung PK, Shen J, Chung SS, et al. Targeted over-expression of endothelin-1 in astrocytes leads to more severe brain damage and vasospasm after subarachnoid hemorrhage. BMC Neurosci 2013;14:131. [Crossref] [PubMed]
- Cao L, Xu CB, Zhang Y, et al. Secondhand smoke exposure induces Raf/ERK/MAPK-mediated upregulation of cerebrovascular endothelin ETA receptors. BMC Neurosci 2011;12:109. [Crossref] [PubMed]
- Hino A, Tokuyama Y, Kobayashi M, et al. Increased expression of endothelin B receptor mRNA following subarachnoid hemorrhage in monkeys. J Cereb Blood Flow Metab 1996;16:688-97. [Crossref] [PubMed]
- Zuccarello M, Boccaletti R, Romano A, et al. Endothelin B receptor antagonists attenuate subarachnoid hemorrhage-induced cerebral vasospasm. Stroke 1998;29:1924-9. [Crossref] [PubMed]
- Ayata C, Lauritzen M. Spreading Depression, Spreading Depolarizations, and the Cerebral Vasculature. Physiol Rev 2015;95:953-93. [Crossref] [PubMed]
- Lauritzen M, Dreier JP, Fabricius M, et al. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab 2011;31:17-35. [Crossref] [PubMed]
- Gorji A. Spreading depression: a review of the clinical relevance. Brain Res Brain Res Rev 2001;38:33-60. [Crossref] [PubMed]
- Major S, Huo S, Lemale CL, et al. Direct electrophysiological evidence that spreading depolarization-induced spreading depression is the pathophysiological correlate of the migraine aura and a review of the spreading depolarization continuum of acute neuronal mass injury. Geroscience 2020;42:57-80. [Crossref] [PubMed]
- Dreier JP, Major S, Pannek HW, et al. Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex. Brain 2012;135:259-75. [Crossref] [PubMed]
- Winkler MK, Dengler N, Hecht N, et al. Oxygen availability and spreading depolarizations provide complementary prognostic information in neuromonitoring of aneurysmal subarachnoid hemorrhage patients. J Cereb Blood Flow Metab 2017;37:1841-56. [Crossref] [PubMed]
- Lublinsky S, Major S, Kola V, et al. Early blood-brain barrier dysfunction predicts neurological outcome following aneurysmal subarachnoid hemorrhage. EBioMedicine 2019;43:460-72. [Crossref] [PubMed]
- Gursoy-Ozdemir Y, Qiu J, Matsuoka N, et al. Cortical spreading depression activates and upregulates MMP-9. J Clin Invest 2004;113:1447-55. [Crossref] [PubMed]
- Sadeghian H, Lacoste B, Qin T, et al. Spreading depolarizations trigger caveolin-1-dependent endothelial transcytosis. Ann Neurol 2018;84:409-23. [Crossref] [PubMed]
- Lemale CL, Lückl J, Horst V, et al. Migraine Aura, Transient Ischemic Attacks, Stroke, and Dying of the Brain Share the Same Key Pathophysiological Process in Neurons Driven by Gibbs-Donnan Forces, Namely Spreading Depolarization. Front Cell Neurosci 2022;16:837650. [Crossref] [PubMed]
- Chuquet J, Hollender L, Nimchinsky EA. High-resolution in vivo imaging of the neurovascular unit during spreading depression. J Neurosci 2007;27:4036-44. [Crossref] [PubMed]
- Peters O, Schipke CG, Hashimoto Y, et al. Different mechanisms promote astrocyte Ca2+ waves and spreading depression in the mouse neocortex. J Neurosci 2003;23:9888-96. [Crossref] [PubMed]
- Dreier JP, Körner K, Ebert N, et al. Nitric oxide scavenging by hemoglobin or nitric oxide synthase inhibition by N-nitro-L-arginine induces cortical spreading ischemia when K+ is increased in the subarachnoid space. J Cereb Blood Flow Metab 1998;18:978-90. [Crossref] [PubMed]
- Fung C. Inhaled Nitric Oxide Treatment for Aneurysmal SAH Patients With Delayed Cerebral Ischemia. Front Neurol 2022;13:817072. [Crossref] [PubMed]
- Furchgott RF, Martin W, Cherry PD. Blockade of endothelium-dependent vasodilation by hemoglobin: a possible factor in vasospasm associated with hemorrhage. Adv Prostaglandin Thromboxane Leukot Res 1985;15:499-502. [PubMed]
- Pluta RM, Hansen-Schwartz J, Dreier J, et al. Cerebral vasospasm following subarachnoid hemorrhage: time for a new world of thought. Neurol Res 2009;31:151-8. [Crossref] [PubMed]
- Sabri M, Ai J, Knight B, et al. Uncoupling of endothelial nitric oxide synthase after experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab 2011;31:190-9. [Crossref] [PubMed]
- Iadecola C, Zhang F. Permissive and obligatory roles of NO in cerebrovascular responses to hypercapnia and acetylcholine. Am J Physiol 1996;271:R990-1001. [PubMed]
- Petzold GC, Haack S, von Bohlen Und Halbach O, et al. Nitric oxide modulates spreading depolarization threshold in the human and rodent cortex. Stroke 2008;39:1292-9. [Crossref] [PubMed]
- Dreier JP, Woitzik J, Fabricius M, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain 2006;129:3224-37. [Crossref] [PubMed]
- Dreier JP, Major S, Manning A, et al. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain 2009;132:1866-81. [Crossref] [PubMed]
- Lückl J, Lemale CL, Kola V, et al. The negative ultraslow potential, electrophysiological correlate of infarction in the human cortex. Brain 2018;141:1734-52. [Crossref] [PubMed]
- Theis M, Jauch R, Zhuo L, et al. Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J Neurosci 2003;23:766-76. [Crossref] [PubMed]
- Largo C, Cuevas P, Herreras O. Is glia disfunction the initial cause of neuronal death in ischemic penumbra? Neurol Res 1996;18:445-8. [Crossref] [PubMed]
- Kimelberg HK. Astrocytic swelling in cerebral ischemia as a possible cause of injury and target for therapy. Glia 2005;50:389-97. [Crossref] [PubMed]
- Menyhárt Á, Frank R, Farkas AE, et al. Malignant astrocyte swelling and impaired glutamate clearance drive the expansion of injurious spreading depolarization foci. J Cereb Blood Flow Metab 2022;42:584-99. [Crossref] [PubMed]
- Ghaemi A, Alizadeh L, Babaei S, et al. Astrocyte-mediated inflammation in cortical spreading depression. Cephalalgia 2018;38:626-38. [Crossref] [PubMed]
- Hübel N, Hosseini-Zare MS, Žiburkus J, et al. The role of glutamate in neuronal ion homeostasis: A case study of spreading depolarization. PLoS Comput Biol 2017;13:e1005804. [Crossref] [PubMed]
- Basarsky TA, Duffy SN, Andrew RD, et al. Imaging spreading depression and associated intracellular calcium waves in brain slices. J Neurosci 1998;18:7189-99. [Crossref] [PubMed]
- Wu DC, Chen RY, Cheng TC, et al. Spreading Depression Promotes Astrocytic Calcium Oscillations and Enhances Gliotransmission to Hippocampal Neurons. Cereb Cortex 2018;28:3204-16. [Crossref] [PubMed]




