Metastatic signet ring cell carcinoma of unknown primary origin: a case report and review of the literature
Case presentation
A 73-year-old Caucasian male with a past medical history of benign prostatic hyperplasia presented to our hospital with a 4-day history of nausea, vomiting and abdominal pain that were associated with 30-pound weight loss and reduced appetite for 3 months. He works as a farmer and reports exposure to pesticides and has also previously worked for a printing press with reported exposure to asbestos. Family history was significant for colon cancer in his father and pancreatic cancer in his brother. Most recent colonoscopy was 2 years prior to presentation which was unremarkable.
Physical examination revealed normal vital signs as well as a distended abdomen with a positive shifting dullness and active bowel sounds. Complete blood cell count and comprehensive metabolic panel were unremarkable. Carcinoembryonic antigen, alpha fetoprotein and prostate-specific antigen were within normal limits. CA-125 was elevated at 59.3 U/mL (reference range, 0.0–34.0 U/mL) as was CA 19-9 191 (reference range, 0–35 U/mL). Abdominal X-ray revealed a distended stomach without evidence of bowel obstruction. Computed tomography of abdomen and pelvis with intravenous contrast (Figure 1) revealed a large amount of loculated fluid in the peritoneal cavity with associated omental thickening, multiple hypodense lesions in the spleen, as well as the accidental finding of bilateral pulmonary emboli for which the patient was started on therapeutic heparin.
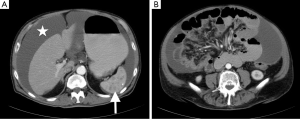
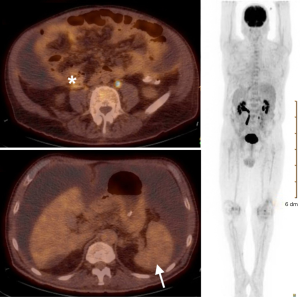
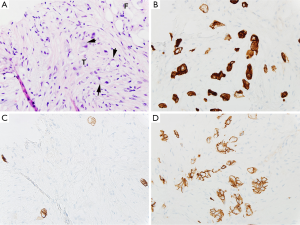
Computed tomography-guided paracentesis was performed and peritoneal fluid analysis revealed a normal white blood cell count (269/microliter, reference range <300/microliter) with a serum-ascitic albumin gradient of 0. Cytologic analysis with cell block revealed numerous clusters of malignant cells admixed with signet ring cells as well as scattered malignant glandular structures. Upper endoscopy revealed congestive gastropathy and erythematous duodenal mucosa. A colonoscopy was performed and was unremarkable. Random biopsies obtained during these procedures did not reveal any evidence of dysplasia or malignancy. He underwent a PET scan (Figure 2) which revealed mild diffuse uptake throughout the bowel which could represent diffuse omental and serosal implants, as well as non-FDG-avid hepatic cyst and splenic lesions. A CT-guided omental biopsy was later performed and revealed poorly differentiated invasive adenocarcinoma of signet ring cell type. The cells were positive for cytokeratin 7 (CK7) and E-cadherin, focally positive for cytokeratin 20 (CK20) (Figure 3), but were negative for thyroid transcription factor 1 (TTF-1), prostate-specific antigen, CDX2, synaptophysin and chromogranin.
Patient was not a surgical candidate and did not tolerate feedings in the hospital and was discharged with NGT and on TPN with a follow-up appointment to discuss management options. The patient eventually elected for hospice for which arrangements were made.
Discussion
Here we report a case of metastatic signet ring cell carcinoma (SRCC) for which the primary origin could not be discovered until now. In spite of the increasing sophistication in the diagnostic workup for malignancies, detailed investigations fail to reveal a primary site in about 3–5% of metastatic tumors (1). SRCC is a rare poorly differentiated aggressive subtype of adenocarcinoma that most commonly arises from the gastrointestinal tract (2). While 90% of SRCC tumors arise from the stomach, breast, or colon, almost every organ is a potential primary site (3). It is characterized by the histological appearance of signet ring cells in which the nucleus is pushed to the periphery by abundant intracytoplasmic mucin (4). It poses diagnostic and therapeutic difficulties as they tend to be aggressive and present at an advanced stage. Since the vast majority of SRCCs occur in the gastrointestinal tract (2), findings suspicious for peritoneal carcinomatosis or omental caking on CT imaging require prompt investigation that usually involves cytologic analysis of peritoneal fluid, imaging, colonoscopy and upper endoscopy (5).
We searched the PubMed database using the keywords “signet ring”, “unknown primary”, and “unknown origin”. Our search returned five case reports with a total of six patients that were published between 2002–2014 which are summarized in Table 1.

Full table
Since identification of the primary site of cancer usually dictates the treatment and expected prognosis, the inability to identify a primary site of cancer poses many challenges. Immunohistochemical studies can be useful in suggesting an origin and therefore may guide investigations and management options. The pattern of results of CK20 and CK7 immunohistochemical staining may be particularly helpful in suggesting a primary site. CK20 is a low molecular weight cytokeratin that is normally expressed in the gastrointestinal epithelium, urothelium, and in Merkel cells (6). CK7 is usually expressed by tumors of the lung, ovary, endometrium, and breast, but not in tumors of the lower gastrointestinal tract. In a study by Tot that included 93 autopsy cases of adenocarcinoma of known primary site, a CK20-positive and CK7-negative pattern correctly predicted a colorectal primary in 17 of 21 cases (81 percent) (7). Moreover, positive immunohistochemical staining for CDX-2 was predictive of colorectal carcinoma. In the same study, a CK20-positive and CK7-positive pattern predicted a cancer of pancreaticobiliary origin in 11 of 14 cases (79 percent). In our reported case, samples stained positive for CK7 and focally positive for CK20. E-cadherin is often not expressed in gastric and breast SRCCs (1). In addition, testing for estrogen and progesterone receptors (PRs) expression in metastatic SRCC of unknown primary can be useful as these receptors can be potential targets for anti-hormonal agents (1). Moreover, molecular tumor profiling (MTP) can help predict the tissue of origin in patients with cancer of unknown primary. A large prospective trial by Hainsworth et al. found that patients who received MTP-directed therapy had a favorable median survival time compared to patients treated with empiric cancer of unknown primary regimens (8).
Immunohistochemical studies and molecular profiling can be useful in predicting the response of a tumor of unknown primary origin to a certain chemotherapeutic regimen. Patients with peritoneal carcinomatosis which stain positive for CK20 and CDX-2 respond well to chemotherapy regimens developed for patients with metastatic colorectal carcinoma (9). Kusakari et al. suggested that treatment with S-1 may be promising for selected patients with mediastinal SRCC of unknown primary origin expressing low levels of thymidylate synthase (10). Out of the six reported patients with metastatic SRCC of unknown primary origin in Table 1, four patients received chemotherapeutic agents. The first patient received 5-fluorouracil (5-FU)/folinic acid (11) and the second patient was treated with S-1 (a novel derivative of 5-FU) plus cisplatin/irinotecan (10). The third and fourth patients received hormonal therapy for positive immunostaining for estrogen and PRs (1). Two patients with SRCC of unknown primary origin received supportive care only and survived for 3 weeks and 3 months (4,5), respectively. Treatment was associated with a longer median survival (30 months) when compared to the two patients who received conservative management (median survival of about 2 months).
The prognosis of the SRCCs seems to be worse than tumors without an SRCC component for tumors with known primary origin (1). Not enough data related to prognostication is available for SRCC of unknown primary origin in our review of the literature. The median survival for the cases found on our literature review is 20 months. Patients who received some form of therapy had better survival rates than patients who received conservative management. Standards of care are yet to be established in those patients. Therefore, enrollment in clinical trials, in addition to providing supportive care, is encouraged when appropriate. The patient reported in this case was alive 3 months after the diagnosis of SRCC with unknown primary origin was made.
Metastatic SRCC of unknown primary origin is a rare poorly differentiated aggressive subtype of adenocarcinoma that poses diagnostic and therapeutic difficulties as it tends to present at an advanced stage and is associated with a poor prognosis. This work emphasizes the importance of detailed histopathological, immunohistochemical, and molecular analyses which can help predict an origin site and help predict investigations and management options.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Gregoire C, Muller G, Machiels JP, et al. Metastatic signet-ring cell carcinoma of unknown primary origin. Acta Clin Belg 2014;69:135-8. [Crossref] [PubMed]
- Hou Y, Li Y, Shen D, et al. Primary peritoneal serous carcinoma with signet ring cells. Gynecol Oncol Case Rep 2011;1:4-5. [Crossref] [PubMed]
- Chu PG, Weiss LM. Immunohistochemical characterization of signet-ring cell carcinomas of the stomach, breast, and colon. Am J Clin Pathol 2004;121:884-92. [Crossref] [PubMed]
- Shin SY, Park H, Chae SW, et al. Microangiopathic hemolytic anemia as the first manifestation of metastatic signet ring cell carcinoma of unknown origin: a case report and review of literature. Korean J Lab Med 2011;31:157-61. [Crossref] [PubMed]
- O'Kane D, Dean K, Nightingale R, et al. Metastatic signet ring cell carcinoma of unknown primary source. BMJ Case Rep 2014;2014.
- Su AI, Welsh JB, Sapinoso LM, et al. Molecular classification of human carcinomas by use of gene expression signatures. Cancer Res 2001;61:7388-93. [PubMed]
- Tot T. Adenocarcinomas metastatic to the liver: the value of cytokeratins 20 and 7 in the search for unknown primary tumors. Cancer 1999;85:171-7. [Crossref] [PubMed]
- Hainsworth JD, Rubin MS, Spigel DR, et al. Molecular gene expression profiling to predict the tissue of origin and direct site-specific therapy in patients with carcinoma of unknown primary site: a prospective trial of the Sarah Cannon research institute. J Clin Oncol 2013;31:217-23. [Crossref] [PubMed]
- Varadhachary GR, Raber MN, Matamoros A, et al. Carcinoma of unknown primary with a colon-cancer profile-changing paradigm and emerging definitions. Lancet Oncol 2008;9:596-9. [Crossref] [PubMed]
- Kusakari C, Soda H, Nakamura Y, et al. Mediastinal signet-ring cell carcinoma of unknown primary: long-term survival by treatment with S-1, a novel derivative of 5-fluorouracil. Lung Cancer 2007;56:139-41. [Crossref] [PubMed]
- Heidemann J, Gockel HR, Winde G, et al. Signet-ring cell carcinoma of unknown primary location. Metastatic to lower back musculature - remission following FU/FA chemotherapy. Z Gastroenterol 2002;40:33-6. [Crossref] [PubMed]


