Non-invasive ventilation in acute respiratory distress syndrome: helmet use saves lives?
Acute respiratory distress syndrome (ARDS) is a syndrome characterized by acute hypoxemic respiratory failure resulting from myriad of causes that injure the alveolar epithelium or the capillary endothelium or both (1,2). ARDS was first described by Ashbaugh et al. in 1967 (3). Over the years, there have been several changes in the definition of ARDS. The initial definition relied on the measurement of pulmonary capillary wedge pressure and did not include the application of positive end expiratory pressure (PEEP) as a criterion (4). The current widely accepted definition not only specifies the duration of the acuteness of presentation but also quantifies the severity of ARDS based on the degree of hypoxemia and includes PEEP in the definition (5). The management of ARDS is primarily based on a combination of supportive care and invasive mechanical ventilation. Apart from low tidal volume strategy and prone position ventilation, none of the other approaches have been shown to reduce mortality (6,7). The application of invasive mechanical ventilation is associated with several complications related to endotracheal intubation including ventilator-associated pneumonia (8). The current mortality rates across various centers varies between 30% and 40% (5,9). One strategy to avoid invasive mechanical ventilation is the use of non-invasive ventilation (NIV). NIV is the provision of positive airway pressure for mechanical ventilation without the need of an endotracheal airway (10,11). Positive airway pressure can be delivered either as continuous positive airway pressure (CPAP) or as bilevel positive airway pressure wherein the positive pressure is either same or different during inspiration and expiration, respectively (10,11). NIV can be administered either with the dedicated portable NIV ventilators or the intensive care unit (ICU) ventilators (12).
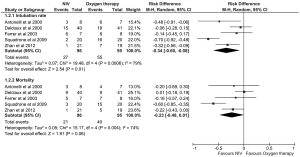
Ever since its inception in the early 21st century, NIV has been used for a variety of conditions causing respiratory failure, both acute and chronic. Currently, the conditions where NIV is the first line treatment include acute exacerbations of chronic obstructive pulmonary disease (COPD), acute cardiogenic pulmonary edema, acute respiratory failure in the immunocompromised and in weaning COPD patients off invasive ventilation (13-16). However, for other indications such as severe acute asthma or hypoxemic respiratory failure including ARDS, the use of NIV remains controversial (17-21). The physiological basis for the use of NIV in ARDS is due to the fact that it reduces dyspnea, unloads respiratory muscles, improves oxygenation, and hence may help in avoiding invasive mechanical ventilation (22,23). Despite the physiological rationale, there is lack of high quality data. A meta-analysis of three randomized controlled trials (RCTs) suggested that NIV in comparison to standard care did not reduce either the intubation rate or the mortality. However, the total number of patients was small and the authors concluded that further evidence is needed to ascertain the role of NIV in ARDS (24). A subsequent study that pooled the results of randomized and non-randomized studies (13 studies, 540 subjects) demonstrated that the use of NIV in ARDS was associated with an intubation rate of 48% suggesting that NIV could be beneficial in 50% of patients with ARDS, if properly chosen (22). A recent pooled analysis of 17 randomized trials of NIV in acute hypoxemic respiratory failure demonstrated superiority of NIV over standard treatment with oxygen supplementation (25). However, this review pooled the results of patients with acute hypoxemic respiratory failure of varied etiology (mucus plugging or atelectasis, cardiogenic pulmonary edema, pneumonia, pulmonary embolism, post-operative respiratory failure and others) rather than ARDS exclusively (25). Thus, the results of this analysis should be interpreted with caution. Few RCTs have investigated the role of NIV in patients with ARDS, and in some of these studies the outcomes for ARDS have been reported in the form of a subgroup analysis (Table 1). The results of these studies suggest that NIV can potentially decrease the intubation rates but not mortality, compared to oxygen therapy in the initial management of ARDS (Figure 1). However, there is significant clinical and statistical heterogeneity (I2=79%), which suggests that the effect of NIV in ARDS is likely to be extremely variable across patients.
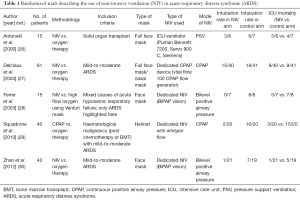
Full table
Several factors affect the outcome of NIV most important being the host factors including the underlying etiology of respiratory failure (COPD vs. others), the severity of respiratory failure (based on the degree of hypoxemia and the clinical features including the respiratory rate) and the underlying severity of the critical illness (based on the APACHE II score or other similar ICU scoring systems) (16,31,32). The performance of NIV may also depend upon device-related factors such as the gas source (compressed air or turbine pump), oxygen supply (high pressure or low pressure source), circuit (single or double limb) and the interface (type of mask) (10-12). The application of NIV requires an interface that acts as a connection between the patient and the ventilator for delivering the positive airway pressure (11). Whether an interface can influence outcome in ARDS is not known.
A recent study by Patel et al. is perhaps the first randomized trial that has investigated the role of interface in determining the outcomes during NIV in patients with ARDS (33). The authors hypothesized that the use of helmet would allow for delivery of higher airway pressures without air leak secondary to a better seal obtained with the helmet, thereby improving outcomes. This study was a single center trial in which consecutive patients with ARDS were randomized to receive NIV using either the face mask or the helmet. The study subjects were adults who fulfilled the Berlin criteria for ARDS and had required NIV for at least eight hours. All the study subjects had some underlying immunosuppression (solid malignancy, hematological malignancy, solid organ transplant and stem cell transplant). Patients in the facemask group were ventilated with a dedicated NIV machine (Philips Respironics V60) with a single limb circuit while those allocated to the helmet group were ventilated with an ICU ventilator (Engström Carestation, GE Healthcare) with a double limb circuit. The primary outcome was the proportion of study subjects who required endotracheal intubation. The secondary outcomes included the 28-day ventilator free days, ICU and hospital length of stay, ICU and 90-day mortality. Although the initial calculated study sample was 206, the study was stopped after an interim analysis and finally enrolled 83 subjects. Thirty-nine and 44 subjects were assigned to the conventional face mask and the helmet group, respectively. Surprisingly, the helmet group had a significantly lower intubation rate when compared with the face mask group (absolute difference, −43.5%; 95% CI, −62.4% to −24.3%, P<0.001). The difference in the intubation rates remained significant even after adjusting for the APACHE II score. Hospital and 90-day mortality and the ICU length of stay were also significantly lower in the helmet group. The use of helmet during NIV was associated with a significantly higher ventilator free-days. There was no difference in the mask associated complications such as skin ulceration.
There are several points to be considered before accepting the results of the study. The major strength of the trial was the inclusion of subjects with ARDS as per the Berlin definition. However, there were several limitations. One major weakness was that it compared two types of interface that are used during NIV without any group receiving high flow oxygen. Recently, high-flow oxygen (at a gas flow rate of 50 liters per minute) through nasal cannula (Optiflow, Fisher and Paykel Healthcare) was found to be similar in efficacy to NIV in preventing intubation rates in patients with non-hypercapnic acute respiratory failure (34). Also, the subjects in the study were all individuals with malignancy and post-transplant respiratory failure, unlike the etiologies with ARDS (pneumonia, sepsis, acute pancreatitis and others) that are seen in day-to-day practice. The authors apart from two different types of interfaces had also used two different types of NIV devices and whether the different machines had any influence on outcomes remains unknown. The dedicated NIV machines do not provide stable pressure support at higher pressures and hence may be inferior in conditions where higher pressure support is required (12,35-37). Also, the dedicated NIV machines with a single limb circuit require an expiratory port or swivel to avoid rebreathing. This may result in loss of the effective PEEP that is required to keep the collapsed alveoli open. The authors also hypothesized that use of face mask would be associated with higher leak around the mask but have not provided the amount of air leak in the two study groups. Although increased air-leak may theoretically be associated with higher NIV failure, the current NIV machines compensate for the air leaks. The trial was ended before the calculated number of subjects could be enrolled. Early conclusion of trials can exaggerate the magnitude of effect size due to multiplicity and hence the results of trial that end prematurely should be interpreted cautiously (38,39). Finally, it is a single-center trial and hence more data is required to confirm the findings of this study.
Currently, what is the role of NIV in ARDS? The answer to this question is like finding the holy grail. A fair indication based on the current level of evidence would be to judiciously institute NIV using the ICU ventilator in subjects with mild-to-moderate ARDS (Table 2). The patients should be closely monitored for improvement in the physiological parameters (respiratory rate, heart rate, oxygen status). Apart from the severity of the underlying disease, failure in improvement of PaO2/FiO2 ratio after one hour of NIV use should prompt endotracheal intubation (22,40). Future studies should use a uniform definition of ARDS, have a comparator arm of standard care with high-flow oxygen, and should preferably use the same equipment in both the study arms.

Full table
Acknowledgements
None.
Footnote
Provenance: This is a Guest Commentary commissioned by Section Editor Zhi Mao, MD (Department of Critical Care Medicine, Chinese People’s Liberation Army General Hospital, Beijing, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 2012;122:2731-40. [Crossref] [PubMed]
- Agarwal R, Aggarwal AN, Gupta D, et al. Etiology and outcomes of pulmonary and extrapulmonary acute lung injury/ARDS in a respiratory ICU in North India. Chest 2006;130:724-9. [Crossref] [PubMed]
- Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet 1967;2:319-23. [Crossref] [PubMed]
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-24. [Crossref] [PubMed]
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159-68. [Crossref] [PubMed]
- Tortora G, Ciardiello F. Protein kinase A as target for novel integrated strategies of cancer therapy. Ann N Y Acad Sci 2002;968:139-47. [Crossref] [PubMed]
- Agarwal R, Srinivasan A, Aggarwal AN, et al. Adaptive support ventilation for complete ventilatory support in acute respiratory distress syndrome: a pilot, randomized controlled trial. Respirology 2013;18:1108-15. [PubMed]
- British Thoracic Society Standards of Care Committee. Non-invasive ventilation in acute respiratory failure. Thorax 2002;57:192-211. [Crossref] [PubMed]
- Keenan SP, Sinuff T, Burns KE, et al. Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ 2011;183:E195-214. [Crossref] [PubMed]
- Scala R, Naldi M. Ventilators for noninvasive ventilation to treat acute respiratory failure. Respir Care 2008;53:1054-80. [PubMed]
- AlYami MA, AlAhmari MD, Alotaibi H, et al. Evaluation of efficacy of non-invasive ventilation in Non-COPD and non-trauma patients with acute hypoxemic respiratory failure: A systematic review and meta-analysis. Ann Thorac Med 2015;10:16-24. [PubMed]
- Agarwal R, Aggarwal AN, Gupta D, et al. Role of noninvasive positive-pressure ventilation in postextubation respiratory failure: a meta-analysis. Respir Care 2007;52:1472-9. [PubMed]
- Agarwal R, Srinivas R. Noninvasive ventilation in acute heart failure. Am J Med 2007;120:e19; author reply e21.
- Agarwal R, Gupta R, Aggarwal AN, et al. Noninvasive positive pressure ventilation in acute respiratory failure due to COPD vs. other causes: effectiveness and predictors of failure in a respiratory ICU in North India. Int J Chron Obstruct Pulmon Dis 2008;3:737-43. [PubMed]
- Agarwal R, Aggarwal AN, Gupta D. Non-invasive ventilation in acute asthma. Int J Tuberc Lung Dis 2006;10:1182-3. [PubMed]
- Agarwal R, Nath A, Gupta D. Noninvasive ventilation in Plasmodium vivax related ALI/ARDS. Intern Med 2007;46:2007-11. [Crossref] [PubMed]
- Agarwal R, Handa A, Aggarwal AN, et al. Outcomes of noninvasive ventilation in acute hypoxemic respiratory failure in a respiratory intensive care unit in north India. Respir Care 2009;54:1679-87. [PubMed]
- Gupta D, Nath A, Agarwal R, et al. A prospective randomized controlled trial on the efficacy of noninvasive ventilation in severe acute asthma. Respir Care 2010;55:536-43. [PubMed]
- Sehgal IS, Dhooria S, Aggarwal AN, et al. Noninvasive ventilation in acute respiratory distress syndrome: Primum non nocere. J Crit Care 2016;32:226. [Crossref] [PubMed]
- Agarwal R, Aggarwal AN, Gupta D. Role of noninvasive ventilation in acute lung injury/acute respiratory distress syndrome: a proportion meta-analysis. Respir Care 2010;55:1653-60. [PubMed]
- L'Her E, Deye N, Lellouche F, et al. Physiologic effects of noninvasive ventilation during acute lung injury. Am J Respir Crit Care Med 2005;172:1112-8. [Crossref] [PubMed]
- Agarwal R, Reddy C, Aggarwal AN, et al. Is there a role for noninvasive ventilation in acute respiratory distress syndrome? A meta-analysis. Respir Med 2006;100:2235-8. [Crossref] [PubMed]
- Xu X, Yuan B, Liang Q, et al. Noninvasive ventilation for acute lung injury a meta-analysis of randomized controlled trials. Heart Lung 2016;45:249-57. [Crossref] [PubMed]
- Antonelli M, Conti G, Bufi M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA 2000;283:235-41. [Crossref] [PubMed]
- Delclaux C, L'Her E, Alberti C, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: A randomized controlled trial. JAMA 2000;284:2352-60. [Crossref] [PubMed]
- Ferrer M, Esquinas A, Leon M, et al. Noninvasive ventilation in severe hypoxemic respiratory failure: a randomized clinical trial. Am J Respir Crit Care Med 2003;168:1438-44. [Crossref] [PubMed]
- Squadrone V, Massaia M, Bruno B, et al. Early CPAP prevents evolution of acute lung injury in patients with hematologic malignancy. Intensive Care Med 2010;36:1666-74. [Crossref] [PubMed]
- Zhan Q, Sun B, Liang L, Yan X, et al. Early use of noninvasive positive pressure ventilation for acute lung injury: a multicenter randomized controlled trial. Crit Care Med 2012;40:455-60. [Crossref] [PubMed]
- Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med 2007;35:18-25. [Crossref] [PubMed]
- Sharma S, Agarwal R, Aggarwal AN, et al. A survey of noninvasive ventilation practices in a respiratory ICU of North India. Respir Care 2012;57:1145-53. [Crossref] [PubMed]
- Patel BK, Wolfe KS, Pohlman AS, et al. Effect of Noninvasive Ventilation Delivered by Helmet vs Face Mask on the Rate of Endotracheal Intubation in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2016;315:2435-41. [Crossref] [PubMed]
- Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015;372:2185-96. [Crossref] [PubMed]
- Scala R. Bi-level home ventilators for non invasive positive pressure ventilation. Monaldi Arch Chest Dis 2004;61:213-21. [Crossref] [PubMed]
- Richard JC, Carlucci A, Breton L, et al. Bench testing of pressure support ventilation with three different generations of ventilators. Intensive Care Med 2002;28:1049-57. [Crossref] [PubMed]
- Tassaux D, Strasser S, Fonseca S, et al. Comparative bench study of triggering, pressurization, and cycling between the home ventilator VPAP II and three ICU ventilators. Intensive Care Med 2002;28:1254-61. [Crossref] [PubMed]
- Schulz KF, Grimes DA. Multiplicity in randomised trials II: subgroup and interim analyses. Lancet 2005;365:1657-61. [Crossref] [PubMed]
- Meade MO. Pro/con clinical debate: It is acceptable to stop large multicentre randomized controlled trials at interim analysis for futility. Con: the hazards of stopping for futility. Crit Care 2005;9:34-6; discussion 34-6. [PubMed]
- Sehgal IS, Chaudhuri S, Dhooria S, et al. A study on the role of noninvasive ventilation in mild-to-moderate acute respiratory distress syndrome. Indian J Crit Care Med 2015;19:593-9. [Crossref] [PubMed]


