
Integrated UPLC-MS, network pharmacology, and intestinal flora analysis to determine the treatment effect of Qiangzhi decoction on comorbid Tourette syndrome and RRTI
Highlight box
Key findings
• Qiangzhi decoction (QZD) provided comorbid Tourette syndrome (TS) and recurrent respiratory tract infection (RRTI) with multicomponent, multitarget therapy and regulated the balance of gut microbiota.
What is known and what is new?
• QZD can ameliorate the tic-like symptoms of TS and reduce recurrence of RRTI.
• QZD can restore the balance of gut microbiota in TS and RRTI model rats.
What is the implication, and what should change now?
• QZD could be a potential therapeutic drug for comorbid TS and RRTI, and the neurotransmitter and immunomodulatory effects need to be further confirmed.
Introduction
Tourette syndrome (TS) is a complex neurodevelopmental disorder characterized by the presence of multiple motor tics and 1 or more phonic tics that last more than 1 year (1). It is reported that its prevalence is between 0.6–1% in school-aged children, and there appears to be no geographical variability (2). The predominant cooccurring comorbid diseases of TS include obsessive-compulsive disorder (OCD), attention deficit hyperactivity disorder (ADHD), and depressive disorder, among others (3). TS is a heterogeneous disease with a multifactorial pathogenesis. The possible pathogenesis of TS is generally considered to be a combination of genetic, immunological, environmental, and psychological factors (4).
In the past 2 decades, the relationship between immune activation, infection, and TS have been widely studied (4-7). These studies focused on infections as a possible contributing factor in TS, particularly those caused by group A Streptococcus (GAS) and mycoplasma pneumonia (MP) (8,9). However, it is unlikely that GAS or MP infections would significantly affect neuropsychiatric symptoms years after their onset. It has been suggested that the immune response may play a triggering role in symptom recrudescence (10).
Recurrent respiratory tract infection (RRTI) is a common disease in childhood and refers to the frequency of upper or lower respiratory tract infections above a certain range in a year (11). Approximately 25% of children under 1 year old have RRTI (12,13), and the incidence shows an increasing tendency (14). Previous study has found that the immune function of children with RRTI was generally weaker than that of normal children, suggesting that abnormal immune function was an important cause of RRTI in children (15). Some studies have demonstrated increased frequency of respiratory infections in TS patients (16,17). A retrospective analysis of 273 children with tics showed that children with RRTI and TS had a higher recurrence rate of tics than children without RRTI, and that children with chronic tics and TS in particular were slower to improve, and RRTI was more likely to recur (17).
Qiangzhi decoction (QZD, patent no.ZL 201310000777.3) is a prescribed Chinese herbal decoction formula. QZD has been widely used for many years to treat TS, achieving remarkable outcomes. In clinical practice, children administered oral QZD showed improvement in tic symptoms along with improvement in RRTI (18). However, there is a lack of systematic and comprehensive research on the mechanism of QZD. Therefore, in this study, we constructed a multilevel network of “prescription-herbal medicine-component-disease-target” to explore the biological mechanism of QZD for TS and RRTI from the perspective of intestinal flora. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-23-936/rc).
Methods
Materials and reagents
Chromatographic grade of formic acid and methanol and mass spectrometry grade of acetonitrile were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Deionized water was purified by a Milli-Q system (Millipore, Bedford, MA, USA). Microsample total RNA Extraction Kit was purchased from Tiangen Biotech Co., Ltd. (Beijing, China) and TransScript One step gDNA removal and cDNA synthesis Supermix were purchased from Gold Biotechnology Co., Ltd. (Beijing, China).
Preparation of QZD
QZD consists of 7 kinds of traditional Chinese medicine (TCM) herbs (Table 1). All herbal plants were purchased from the Affiliated Hospital of Shandong Traditional Chinese Medicine University and in accordance with the standards of Chinese Pharmacopoeia (2020 edition), as confirmed by Professor Li Feng of Pharmacy College, Shandong Traditional Chinese Medicine University. The raw QZD materials were soaked for 1 hour in 10-fold (v/w) the amount of distilled water, followed by decoction for 2 hours. After collecting the filtrates, water was added to the residue (1:8, w/v), and the mixture was extracted with boiling water for another 1.5 hours. The filtrates were mixed and condensed to 0.99 g/mL, and the prepared QZD was stored at −80 ℃ and analyzed using ultrahigh-performance liquid chromatography coupled with quadrupole orbitrap mass spectrometry (UPLC-Q-Orbitrap-MS/MS).
Table 1
| Scientific name | Chinese name | Abbreviation | Used plant part | Daily dose of herb (g) | Composition (%) |
|---|---|---|---|---|---|
| Morinda officinalis HOW. | Bajitian | MO | Root | 9 | 9.09 |
| Aucklandia lappa Decne | Muxiang | AL | Root | 6 | 6.06 |
| Polygala tenuifolia Willd. | Yuanzhi | PT | Root | 9 | 9.09 |
| Poria cocos (Schw.) Wolf | Fuling | PC | sclerotium | 24 | 24.24 |
| Pinellia ternata (Thunb.) Breit. | Banxia | PB | Root | 9 | 9.09 |
| Atractylodes macrocephala Koidz. | Baizhu | AM | Rhizome | 18 | 18.18 |
| Dioscorea opposita Thunb. | Shanyao | DO | Rhizome | 24 | 24.24 |
UPLC-Q-orbitrap-MS/MS analysis
For UPLC-Q-Orbitrap-MS/MS analysis, 1,000 µL of 80% methanol was added to 200 µL of the QZD solution and vortexed for 10 minutes. The mixture was then centrifuged at 12,000 rpm for 10 minutes at 4 ℃, after which the supernatant was filtered and analyzed on the machine. The conditions for mass spectrometry were: ion source: electrospray ionization (ESI); scan mode: positive and negative ion switching scan; detection mode: full mass/data-dependent (dd)MS2; resolution: 70,000 (full mass), 17,500 (ddMS2); scan range: 100.0–1,500.0 m/z; spray voltage: 3.2 kV (positive); capillary temperature: 300 ℃; collision gas: high-purity argon (purity ≥99.999%); normalized collision energy (NCE): 30; sheath gas: nitrogen (purity ≥99.999%), 40 arb; auxiliary gas: nitrogen (purity ≥99.999%), 15 arb, 350 ℃; and data acquisition time: 30 minutes. The materials and conditions for chromatographic column were: AQ-C18, 150 × 2.1 mm, 1.8 µm (Welch Materials, Inc., West Haven, CT, USA); flow rate: 0.3 mL/min; aqueous phase: 0.1% formic acid/water solution; organic phase: methanol; eluent: methanol; column oven temperature: 35 ℃; autosampler temperature: 10.0 ℃; and autosampler injection volume: 5.00 µL (see Table 2 for the gradient elution procedure). SIEVE software version 2.1 (Thermo Fisher Scientific) was used for high-resolution liquid mass spectrometry data acquisition, and retention time correction, peak identification, peak extraction, peak integration, and peak alignment were determined. The data were then searched and compared against the mzCloud database.
Table 2
| Time (min) | Water phase ratio (%) | Organic phase ratio (%) |
|---|---|---|
| 1 | 98 | 2 |
| 5 | 80 | 20 |
| 10 | 50 | 50 |
| 15 | 20 | 80 |
| 20 | 5 | 95 |
| 27 | 5 | 95 |
| 28 | 98 | 2 |
| 30 | 98 | 2 |
Network pharmacology analysis
Construction of “chemical ingredient target”
Based on the experimental results of the UPLC-Q-Orbitrap-MS/MS analysis, PubChem (https://pubchem.ncbi.nlm.nih.gov/) was used to search for the structures of the active compounds of QZD. The obtained structural formula was uploaded to the SwissADME database (http://www.swissadme.ch/) for screening, with the screening parameter for gastrointestinal absorption set at “high”. The 2D structure formula of the compound was then imported into the SwissTargetPrediction database (http://swisstargetprediction.ch/) to predict the target sites, and the output was summarized. Next, the UniProt database (https://www.uniprot.org/) was searched, and the target was transformed into the corresponding standard gene name, which was then sorted out.
Acquisition of “Tourette syndrome” and “RRTI” targets
With “Tourette syndrome” and “recurrent respiratory tract infection” as the retrieval keywords, the related targets of these 2 diseases were found in the following sources: GeneCards database (https://www.genecards.org), DisGeNET database (https://www.disgenet.org), OMIM database (http://omim.org), PharmGkb (https://www.pharmgkb.org/), DrugBank database (https://go.drugbank.com/), and Therapeutic Target Database (http://db.idrblab.net/ttd/). After merging the results and removing duplications, the integrated targets of 6 databases were obtained.
Acquisition of “common targets”
The chemical composition-related targets in Section “Construction of “chemical ingredient target”” and disease targets in Section “Acquisition of “Tourette syndrome” and “RRTI”” targets were uploaded to draw a Venn diagram (https://bioinfogp.cnb.csic.es/tools/venny/) for analysis, and the overlapping genes were obtained as common targets.
Construction and analysis of the protein-protein interaction (PPI) network
The common targets were imported into the String database (https://cn.string-db.org/) to build its PPI using “Homo sapiens” as the selected species. Medium confidence was set to >0.4 to obtain the final intersection targets, and Cytoscape 3.7.2 software was then used to draw the PPI network.
Gene Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis
The target genes were imported into the Database for Annotation, Visualization, and Integrated Discovery (DAVID 6.8, https://david.ncifcrf.gov/), “Homo sapiens” was selected as the species, and KEGG-pathway, GOTERM_BP_DIRECT (BP), GOTERM_CC_DIRECT (CC), and GOTERM_MF_DIRECT (MF) were selected to perform GO and KEGG enrichment analysis of the overlapping genes. We obtained data for the biological processes (BPs), molecular functions (MFs), cellular components (CCs), and the involved pathways to draw GO histograms and KEGG enrichment bubble charts (http://www.bioinformatics.com.cn/).
Animal experiments
Animals
All tests were conducted in accordance with Chinese national laws and local guidelines. A total of 36 healthy male Sprague Dawley (SD) rats (age: 4–7 weeks, weight: 120–150 g) were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China; NO: SCXK 2016-0006) and maintained in plastic cages at 23±2 ℃ with free access to food and water. The rats were exposed to a 12-hour light/dark cycle with their environment maintained at a relative humidity of 30–40%. All animal experiment protocols were conducted in compliance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Ethical Committee of Shandong University of Traditional Chinese Medicine (No. YYLW20222000011). A protocol was prepared before the study without registration. All efforts were made to minimize animal suffering.
TS and RRTI model construction
The study was divided into 5 phases. From days 1–6, the rats underwent adaptive feeding and were provided food and water freely. From days 7–14, the rats were treated with 3,3-iminodipropionitrile (IDPN; Sigma-Aldrich, St. Louis, MO, USA) every 24 hours to induce a TS model (19). From days 15–42, the rats were administered different drugs depending on their treatment group. On days 21, 22, 23, and 27, cyclophosphamide was administered for immunosuppression. On day 42, lipopolysaccharide (LPS) was administered to induce acute lung infection.
The rats were randomly assigned to a control group (CON, n=6) or comorbid TS and RRTI model group (model group, n=30) by using the random number table method. The sample size was empirically estimated. In the model group, rats were injected with IDPN (300 mg∙kg-1) once daily for 7 days continuously. The control group was injected with an equal volume of 0.9% saline (15 mL∙kg-1) by intraperitoneal injection. After 7 days of treatment, the model was validated by assessing the rats for typical TS behavior, such as significant head twitching, putting forepaws around the mouth, biting, licking, shaking claws, body raising, or episodic utterance, for longer than 15 minutes in a 30-minute period. Counts were conducted once every 2 weeks by trained observers who were blinded to the group’s treatment. All TS model rats were validated in this batch as well as in our pilot observation using an identical protocol.
Subsequently, the rats in the TS model group with IDPN-induced TS were further assigned randomly to 5 groups: model group (model), tiapride hydrochloride and bacterial lysate capsules group (THT-FFS), QZD low-dose group (QZD-L), QZD moderate-dose group (QZD-M), and QZD high-dose group (QZD-H), with each group containing 6 rats. Once a day for 4 weeks, the CON and model group rats were administered normal saline by gastric perfusion at 10 mL/kg, while the QZD-L group received QZD at 2.5 g/kg, the QZD-M group at 5 g/kg, and the QZD-H group at 10 g/kg.
On days 21, 22, 23, and 28, the rats were intraperitoneally injected with CTX (40 mg/kg) for immunosuppression. On day 42, LPS was administered to induce acute lung infection by intraperitoneal injection. Four hours later, all rats were anaesthetized by intraperitoneal injection of 1% sodium pentobarbital (40 mg/kg).
Stool sample collection
Colonic stool samples from the rats were collected into sterile tubes and immediately frozen in liquid nitrogen for intestinal microbiota analysis.
Gut microbiota study
DNA extraction and polymerase chain reaction (PCR) amplification
Three stool samples were selected from each group. Total bacterial DNA was extracted using the Magnetic Soil and Stool DNA Kit (Qiagen, Valencia, California, USA) from fresh stool samples. Based on the manufacture’s instructions, the quantity and quality of the extracted DNA were measured using agarose gel electrophoresis. The V3–V4 region of 16S rRNA was amplified by PCR with forward primer CCTAYGGGRBGCASCAG and reverse primer GGACTACNNGGGTATCTAAT. The amplification system was prepared as follows: Phusion Master Mix (2×) (15 µL), Primer F (1 µM) (1 µL), Primers R (1 µM) (1 µL), gDNA (1 ng/µL) (10 µL), and ddH2O (added to 30 µL). The amplification conditions were initial denaturation at 98 ℃ for 1 minute, then 30 cycles consisting of denaturation at 98 ℃ for 10 seconds, annealing at 50 ℃ for 30 seconds, and extension at 72 ℃ for 30 seconds, with a final extension at 72 ℃ for 5 minutes. The PCR products were recovered and detected by 2% agarose gel electrophoresis to construct the MiSeq library.
High-throughput sequencing
For high-throughput sequencing, libraries were constructed using NEBNext Ultra DNA Library Prep Kit (Illumina, San Diego, CA, USA; E7370L) and tested and quantified by quantitative PCR using Agilent 5400.
Statistical analysis
Windows WPS Office version 11.1.0.13703 was used to summarize data. The topological data were analyzed by Cytoscape version 3.7.2, and pathway enrichment visualization analysis was derived from DAVID version 6.8. For the analysis of gut microbiota, the correlation between factors was calculated by Spearman’s rank sum correlation test, and redundancy analysis (RDA) was performed to analyze the correlation between environmental factors and the microbial community. The alpha level was set to P=0.05, and all P values were generated by two-sided tests.
Results
Analysis of chemical components in QZD by UHPLC-Q-Orbitrap-MS/MS
The major compounds in the QZD samples were identified by UHPLC-Q-Orbitrap-MS/MS analysis. The total negative and positive ion chromatograms of QZD (Figure 1) demonstrated the chemical composition of all compounds. For full scan experiments, the obtained raw data were preprocessed using SIEVE software, which identified components with mass spectrometric data on experimental and calculated m/z, error in parts per million (ppm), molecular formula, retention time (RT), and MS fragmentation pattern (matched within error tolerance of 25 ppm) with the proposed name of the compounds. As shown in Table 3, 96 chemical components were identified in QZD, including 18 carboxylic acids, 13 amino acids, 10 alkaloids, 8 fatty acids, 5 flavonoids, 4 terpenoids, 4 phenylpropanoids, 3 heterocyclic compounds, 3 aldehydes, and 2 amines, among others. The main chemical components were caprolactam, stearamide, 2-anisic acid, citric acid, trigonelline, betaine, formononetin, 5-hydroxymethyl-2-furaldehyde, 4',7-dihydroxyflavanone, and palmitic acid.

Table 3
| No. | RT (min) | Name | ESI | Formula | Molecular weight | Theoretical value (m/z) | Measured value (m/z) | Error (ppm) | Fragmention |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.093 | DL-Lysine | [M+H]+ | C6 H14 N2 O2 | 146.10563 | 147.10553 | 147.10588 | 2.3949 | 84.08140 |
| 2 | 1.149 | Ornithine | [M-H]- | C5 H12 N2 O2 | 132.0887 | 131.08988 | 131.08882 | −8.0680 | 113.07101 |
| 3 | 1.149 | DL-Arginine | [M-H]- | C6 H14 N4 O2 | 174.11078 | 173.11168 | 173.11350 | 10.5383 | 131.08145 |
| 4 | 1.154 | L-Histidine | [M-H]- | C6 H9 N3 O2 | 155.06846 | 154.06948 | 154.06919 | −1.8598 | 93.04445, 137.03453 |
| 5 | 1.232 | L-Cystathionine | [M+H]+ | C7 H14 N2 O4 S | 222.06735 | 223.06743 | 223.06658 | −3.8020 | 88.02207, 134.02727, 206.97220 |
| 6 | 1.285 | Threonine | [M-H]- | C4 H9 N O3 | 119.05697 | 118.05824 | 118.05890 | 5.5637 | 74.02332, 72.00767, 118.04977 |
| 7 | 1.302 | L-Aspartic acid | [M-H]- | C4 H7 N O4 | 133.03627 | 132.03751 | 132.03850 | 7.5153 | 88.03903, 115.00248 |
| 8 | 1.31 | L-Glutamic acid | [M-H]- | C5 H9 N O4 | 147.05202 | 146.05316 | 146.05424 | 7.4098 | 102.05475, 128.03418 |
| 9 | 1.404 | Proline | [M+H]+ | C5 H9 N O2 | 115.06363 | 116.06333 | 116.06390 | 4.9238 | 70.06601 |
| 10 | 1.411 | Betaine | [M+H]+ | C5 H11 N O2 | 117.07916 | 118.07898 | 118.07843 | −4.6460 | 59.07390, 58.06607 |
| 11 | 1.458 | Trigonelline | [M+H]+ | C7 H7 N O2 | 137.04769 | 138.04768 | 138.04696 | −5.2045 | 94.06572, 110.06045 |
| 12 | 1.502 | D-Raffinose | [M-H]- | C18 H32 O16 | 504.16906 | 503.16903 | 503.16665 | −4.7398 | 89.02305, 59.01242, 179.05545 |
| 13 | 1.64 | Gluconic acid | [M-H]- | C6 H12 O7 | 196.05758 | 195.05830 | 195.05720 | −5.6533 | 75.00732, 129.01816, 99.00741 |
| 14 | 1.688 | D-(-)-Quinic acid | [M-H]- | C7 H12 O6 | 192.0628 | 191.06339 | 191.06345 | 0.3240 | 85.0281 |
| 15 | 1.804 | L(-)-Pipecolinic acid | [M+H]+ | C6 H11 N O2 | 129.07923 | 130.07898 | 130.07796 | −7.8306 | 84.08147, 70.06596 |
| 16 | 1.846 | Glucose 1-phosphate | [M-H]- | C6 H13 O9 P | 260.02973 | 259.02972 | 259.02846 | −4.8604 | 78.95763, 96.96836 |
| 17 | 1.884 | Guanine | [M+NH4]+ | C5 H5 N5 O | 151.04947 | 152.04941 | 152.04795 | −9.6008 | 86.96390 |
| 18 | 1.963 | L-Tyrosine | [M+H]+ | C9 H11 N O3 | 181.07419 | 182.07389 | 182.07348 | −2.2695 | 91.05498, 136.07527, 123.04450, 80.92865 |
| 19 | 2.162 | Nicotinic acid | [M+H]+ | C6 H5 N O2 | 123.03231 | 124.03203 | 124.03259 | 4.5278 | − |
| 20 | 2.229 | 2-Anisic acid | [M+H]+ | C8 H8 O3 | 152.03176 | 153.04734 | 153.04810 | 4.9389 | 91.05470 |
| 21 | 2.23 | Nicotinamide | [M+H]+ | C6 H6 N2 O | 122.0483 | 123.04801 | 123.04757 | −3.5987 | 80.05016 |
| 22 | 2.387 | 3-Hydroxy-2-methylpyridine | [M+H]+ | C6 H7 N O | 109.05321 | 110.05276 | 110.05246 | −2.7609 | 82.06574,67.05506, 55.05497 |
| 23 | 2.665 | L-Pyroglutamic acid | [M+H]+ | C5 H7 N O3 | 129.04277 | 130.04259 | 130.04197 | −4.7914 | 84.04504, 56.05036 |
| 24 | 2.713 | L-Norleucine | [M+H]+ | C6 H13 N O2 | 131.09473 | 132.09463 | 132.09501 | 2.8869 | 86.09718, 60.73380 |
| 25 | 2.822 | 4-Oxoproline | [M-H]- | C5 H7 N O3 | 129.04149 | 128.04259 | 128.04316 | 4.4276 | 84.04392 |
| 26 | 3.057 | N-Benzylformamide | [M+NH4]+ | C8 H9 N O | 118.04211 | 136.06841 | 136.06896 | 4.0133 | 132.10222, 130.98218, 130.05025, 119.04963 |
| 27 | 3.058 | 4-Hydroxybenzaldehyde | [M-H]- | C7 H6 O2 | 122.0372 | 121.03678 | 121.03633 | −3.7132 | 93.03329 |
| 28 | 3.756 | Uracil | [M+H]+ | C4 H4 N2 O2 | 112.02771 | 113.02728 | 113.02706 | −1.9232 | 70.02959, 96.00846 |
| 29 | 3.771 | Uridine | [M-H]- | C9 H12 N2 O6 | 244.06959 | 243.06954 | 243.06824 | −5.3323 | 110.02351, 200.05606, 82.02844 |
| 30 | 4.526 | Citric acid | [M-H]- | C6 H8 O7 | 192.0262 | 191.0270026 | 191.02890 | 9.9327 | 111.0751, 87.00738, 85.02811 |
| 31 | 4.697 | Citraconic acid | [M-H]-1 | C5 H6 O4 | 130.02539 | 129.02661 | 129.02611 | −3.8649 | 85.02810 |
| 32 | 5.037 | 6,7-Dihydroxy-4-methylcoumarin | [M+H]+ | C10 H8 O4 | 192.04234 | 193.04226 | 193.04277 | 2.6485 | 91.05463, 119.04967, 65.03651, 57.27014 |
| 33 | 5.071 | Adenosine 3’5’-cyclic monophosphate | [M+H]+1 | C10 H12 N5 O6 P | 329.0526 | 330.05252 | 330.05281 | 0.8783 | 136.06195, 57.29844 |
| 34 | 5.495 | L-Phenylalanine | [M+NH4]+ | C9 H11 N O2 | 165.07812 | 166.07898 | 166.07739 | −9.5653 | 120.08107, 103.05490 |
| 35 | 6.143 | 5-Hydroxymethyl-2-furaldehyde | [M+H]+ | C6 H6 O3 | 126.03204 | 127.03169 | 127.03132 | −2.9445 | 53.03959, 81.03414, 109.02886 |
| 36 | 6.54 | N1-Acetylspermine | [M+H]+ | C12 H28 N4 O | 244.22653 | 245.22631 | 245.22581 | −2.0452 | 100.07613, 58.06602, 112.11268 |
| 37 | 6.667 | Thymidine | [M-H]- | C10 H14 N2 O5 | 242.09038 | 241.09027 | 241.09189 | 6.7130 | 212.89168, 151.05023, 80.96397 |
| 38 | 6.708 | N6-Me-Adenosine | [M+H]+ | C11 H15 N5 O4 | 281.11269 | 282.11240 | 282.11287 | 1.6519 | 150.07730, 162.76067 |
| 39 | 6.814 | N,N-Dimethylaniline | [M+H]+ | C8 H11 N | 121.08941 | 122.08915 | 122.08960 | 3.6911 | 107.07320, 102.19013 |
| 40 | 7.498 | DL-Tryptophan | [M-H]- | C11 H12 N2 O2 | 204.0894 | 203.08988 | 203.08812 | −8.6544 | 116.04935, 74.02332, 142.06517, 159.09183 |
| 41 | 7.722 | Methyl 1-(hexopyranosyloxy)-5-hydroxy-7-(hydroxymethyl)-1,4a,5,7a-tetrahydrocyclopenta[c]pyran-4-carboxylate | [M-H]- | C17 H24 O11 | 404.13206 | 403.13186 | 403.13266 | 1.9805 | 127.03902, 241.07188, 101.02322 |
| 42 | 7.847 | Chlorogenic acid | [M-H]- | C16 H18 O9 | 354.09541 | 353.09508 | 353.09617 | 3.0809 | 191.05557, 85.02809 |
| 43 | 7.904 | Geniposidic acid | [M-H]- | C16 H22 O10 | 374.12145 | 373.12130 | 373.12417 | 7.7002 | 123.04398, 149.05981, 211.06079 |
| 44 | 8.105 | 5’-S-Methyl-5’-thioadenosine | [M+H]+ | C11 H15 N5 O3 S | 297.0898 | 298.08956 | 298.08800 | −5.2351 | 136.06192 |
| 45 | 8.15 | 3-Methylbenzophenone | [M+H]+ | C14 H12 O | 196.08501 | 197.08882 | 197.08947 | 3.3234 | 105.03400, 84.08159 |
| 46 | 8.529 | Caprolactam | [M+H]+ | C6 H11 N O | 113.08436 | 114.08406 | 114.08365 | −3.6288 | 79.05477, 69.07076 |
| 47 | 8.644 | Neochlorogenic acid | [M-H]- | C16 H18 O9 | 354.09541 | 353.09508 | 353.09417 | −2.5833 | 191.05553, 135.04407, 179.03436 |
| 48 | 8.661 | Shanzhiside methyl ester | [M-H]- | C17 H26 O11 | 406.15358 | 405.14751 | 405.14630 | −2.9906 | 243.08734, 101.02309, 127.03893 |
| 49 | 8.806 | Sibiricose A3 | [M-H]- | C19 H26 O13 | 462.1378 | 461.13734 | 461.13752 | 0.3884 | 137.02333, 461.13077, 93.03323 |
| 50 | 9.121 | 3-Methylglutaric acid | [M-H]- | C6 H10 O4 | 146.05693 | 145.05791 | 145.05859 | 4.6961 | 101.05950, 83.04886 |
| 51 | 9.27 | Salicylic acid | [M-H]- | C7 H6 O3 | 138.03053 | 137.03169 | 137.03225 | 4.0571 | 93.03320 |
| 52 | 9.414 | 5-Sulfosalicylic acid | [M-H]- | C7 H6 O6 S | 217.9882 | 216.98851 | 216.98793 | −2.6687 | 137.02335 |
| 53 | 9.42 | Asperulosidic acid | [M-H]- | C18 H24 O12 | 432.12703 | 431.12678 | 431.12975 | 6.8977 | 59.01240, 89.02304, 101.02308, 165.05487, 251.05620 |
| 54 | 9.658 | Acridine | [M+H]+ | C13 H9 N | 179.07372 | 180.07350 | 180.07299 | −2.8282 | − |
| 55 | 9.916 | Benzoic acid | [M-H]- | C7 H6 O2 | 122.03558 | 121.03678 | 121.03731 | 4.3835 | − |
| 56 | 10.135 | Isophthalic acid | [M-H]- | C8 H6 O4 | 166.02565 | 165.02661 | 165.02637 | −1.4463 | 121.02830 |
| 57 | 10.429 | Caffeic acid | [M-H]- | C9 H8 O4 | 180.04161 | 179.04226 | 179.04333 | 5.9833 | 135.04405, 179.03429 |
| 58 | 10.863 | Sibiricose A1 | [M-H]- | C23 H32 O15 | 548.17409 | 547.17412 | 547.17724 | 5.7015 | 205.05017, 223.06088, 164.04703, 265.07214, 59.01240 |
| 59 | 11.401 | Cycloolivil | [M-H]- | C20 H24 O7 | 376.1526 | 375.15220 | 375.15532 | 8.3084 | 179.07066, 122.03613, 195.06494, 191.07034 |
| 60 | 11.613 | 5,7-Dihydroxy-4-methylcoumarin | [M+H]+ | C10 H8 O4 | 192.04225 | 193.04226 | 193.04279 | 2.7521 | 73.49180 |
| 61 | 11.614 | 3-Coumaric acid | [M-H]- | C9 H8 O3 | 164.04642 | 163.04734 | 163.04804 | 4.2680 | 119.04901, 163.03915 |
| 62 | 12.043 | Suberic acid | [M-H]- | C8 H14 O4 | 174.08842 | 173.08921 | 173.08809 | −6.4644 | 111.08028, 83.04884 |
| 63 | 12.789 | 4-Hydroxyephedrine | [M+H]+ | C10 H15 N O2 | 181.09974 | 182.11028 | 182.11003 | −1.3658 | 122.09651, 55.93536, 63.92594 |
| 64 | 13.001 | 4,5-Dicaffeoylquinic acid | [M-H]- | C25 H24 O12 | 516.12727 | 515.12678 | 515.12500 | −3.4481 | 191.05556, 353.08801 |
| 65 | 13.779 | 4’,7-Dihydroxyflavanone | [M+H]+ | C15 H12 O4 | 256.07351 | 257.0735589 | 257.07269 | −3.3798 | 137.02342, 91.98053 |
| 66 | 14.198 | Azelaic acid | [M-H]- | C9 H16 O4 | 188.10412 | 187.10486 | 187.10585 | 5.2965 | 125.09601, 187.09694 |
| 67 | 14.422 | 4-Methoxycinnamic acid | [M+H]+ | C10 H10 O3 | 178.05249 | 179.06299 | 179.06178 | −6.7807 | 133.06508, 79.05508 |
| 68 | 15.213 | Prostaglandin K2 | [M-H]- | C20 H30 O5 | 350.19897 | 349.20932 | 349.20886 | −1.3289 | 81.95175 |
| 69 | 15.562 | Methyl palmitate | [M+H]+ | C17 H34 O2 | 269.28246 | 270.25588 | 270.25673 | 3.1440 | 57.07076, 70.06583, 93.49488 |
| 70 | 15.587 | 7-Hydroxycoumarine | [M+H]+ | C9 H6 O3 | 162.03165 | 163.03169 | 163.03294 | 7.6424 | 53.03942 |
| 71 | 15.626 | Monobutyl phthalate | [M-H]- | C12 H14 O4 | 222.08912 | 221.08921 | 221.08859 | −2.7994 | 71.04878, 121.02829, 59.03314 |
| 72 | 16.157 | Corchorifatty acid F | [M-H]- | C18 H32 O5 | 328.2252 | 327.22497 | 327.22790 | 8.9415 | 211.13359,171.10187,80.96378 |
| 73 | 16.255 | 2,3-Dinor prostaglandin E1 | [M-H]- | C18 H30 O5 | 326.19905 | 325.20932 | 325.20883 | −1.5192 | 289.18152, 135.08037 |
| 74 | 16.598 | Genistein | [M-H]- | C15 H10 O5 | 270.05272 | 269.05282 | 269.05362 | 2.9607 | 251.03505, 223.03871 |
| 75 | 16.677 | Dodecanedioic acid | [M-H]- | C12 H22 O4 | 230.15158 | 229.15181 | 229.15430 | 10.8697 | 211.13364, 167.14334 |
| 76 | 16.803 | Formononetin | [M-H]- | C16 H12 O4 | 268.07357 | 267.07356 | 267.07228 | −4.7884 | 269.07867 |
| 77 | 17.316 | 3-Hydroxyflavone | [M-H]- | C15 H10 O3 | 238.06282 | 237.06299 | 237.06354 | 2.3024 | 209.0603 |
| 78 | 17.468 | (+/-)9,10-dihydroxy-12Z-octadecenoic acid | [M-H]- | C18 H34 O4 | 318.24617 | 317.27082 | 317.26932 | −4.7170 | 79.95593 |
| 79 | 18.443 | 7-Methyl-3-methylene-6-(3-oxobutyl)-3,3a,4,7,8,8a-hexahydro-2H-cyclohepta[b]furan-2-one | [M+H]+ | C15 H20 O3 | 248.13045 | 249.14124 | 249.14289 | 6.6047 | 157.36812 |
| 80 | 18.521 | Asiatic acid | [M-H]- | C30 H48 O5 | 488.35078 | 487.35017 | 487.35329 | 6.3925 | 469.33405 |
| 81 | 18.701 | Rubiadin | [M-H]- | C15 H10 O4 | 254.05801 | 253.05791 | 253.05674 | −4.6187 | 225.05545 |
| 82 | 18.749 | Dibutyl phthalate | [M+H]+ | C16 H22 O4 | 278.15165 | 279.15181 | 279.15300 | 4.2659 | 149.02344, 57.07087 |
| 83 | 19.334 | 2-(8-Hydroxy-4a,8-dimethyldecahydro-2-naphthalenyl)acrylic acid | [M-H]- | C15 H24 O3 | 234.16182 | 251.17254 | 251.17334 | 3.1667 | 80.96381 |
| 84 | 19.335 | 7-Methylxanthine | [M-H]- | C6 H6 N4 O2 | 166.04733 | 165.04908 | 165.04945 | 2.2693 | 149.00861 |
| 85 | 19.547 | Hexadecanedioic acid | [M-H]- | C16 H30 O4 | 286.21478 | 285.21441 | 285.21561 | 4.2094 | 223.20648, 267.19675 |
| 86 | 19.648 | (+/-)12(13)-DiHOME | [M-H]- | C18 H34 O4 | 314.23537 | 313.24571 | 313.24809 | 7.5993 | 195.13866, 113.09566, 59.01227 |
| 87 | 21.401 | Sedanolide | [M+H]+ | C12 H18 O2 | 194.12 | 195.13068 | 195.12930 | −7.0712 | 67.84259 |
| 88 | 21.679 | Palmitoyl ethanolamide | [M+H]+ | C18 H37 N O2 | 299.2826 | 300.28243 | 300.28389 | 4.8640 | 62.06094 |
| 89 | 21.716 | α-Linolenic acid | [M-H]- | C18 H30 O2 | 278.22499 | 277.22499 | 277.22771 | 9.8115 | 209.11641 |
| 90 | 21.745 | Choline | [M+H]+ | C5 H13 N O | 103.10014 | 104.10754 | 104.10756 | 0.2010 | 60.08171, 58.06609 |
| 91 | 21.766 | Hexadecanamide | [M+H]+ | C16 H33 N O | 255.25607 | 256.25621 | 256.25537 | −3.2962 | 57.07074 |
| 92 | 22.134 | Ursolic acid | [M-H]- | C30 H48 O3 | 456.36082 | 455.36035 | 455.36355 | 7.0375 | 112.98459 |
| 93 | 22.142 | Jasmonic acid | [M-H]- | C12 H18 O3 | 210.12518 | 209.12559 | 209.12490 | −3.3206 | 178.91049, 163.11214 |
| 94 | 22.867 | Stearamide | [M+H]+ | C18 H37 N O | 283.28721 | 284.28751 | 284.28480 | −9.5495 | 57.07078 |
| 95 | 22.893 | Palmitic acid | [M-H]- | C16 H32 O2 | 256.24028 | 255.24023 | 255.24200 | 6.9336 | 94.31187 |
| 96 | 24.314 | Stearic acid | [M-H]- | C18 H36 O2 | 284.27186 | 283.27153 | 283.27259 | 3.7406 | – |
RT, retention time; ESI, electrospray ionization; QZD, Qiangzhi decoction; UPLC-Q-orbitrap-MS/MS, ultrahigh-performance liquid chromatography coupled ith quadrupole orbitrap mass spectrometry.
Mechanism of QZD in treating TS and RRTI based on network pharmacology
Target prediction and screening
As shown in the results of Section “Analysis of Chemical Component in QZD by UHPLC-Q-Orbitrap-MS/MS”, 96 chemical components were identified by UHPLC-Q-Orbitrap-MS/MS. In addition, 584 targets were obtained for the chemical components of QZD, as shown in Figure 2A.

Using “Tourette syndrome” as the keywords, 177 disease targets were found in DisGeNet, 1,139 in GenCards, 181 in OMIM, 145 in PharmGkb, and 4 in TTD. Using “Recurrence respiratory tract infection” as the keywords, 318 disease targets were found in DisGeNet, 7,826 in GenCards, 73 in OMIM, and 613 in PharmGkb. The targets were then transformed by the UniProt database, and 1,501 TS targets and 8,039 RRTI targets were obtained after removing duplicates. Finally, 848 intersection targets of the 2 diseases were obtained.
As shown in Figure 2B, Venn diagram analysis was performed to intersect the disease targets and drug targets, and 102 drug-disease intersection gene targets were obtained.
Construction and analysis of the PPI Network
The targets that acted with active components were submitted to the STRING database to build a PPI network, and protein interaction data with a score > 0.4 was selected (Figure 2C). We obtained a total of 102 nodes and 786 edges. The size and lightness of the color of the node indicated the node degree.
Finally, the 20 top targets with the highest nodal degree values were obtained, which were SLC6A4, GRM5, SLC6A3, GRIN2B, CNR1, IL6, CTNNB1, TNF, COMT, NTRK2, DLG4, DRD2, GRM2, IL1B, HTR1A, HTR2A, SLC6A2, CHRNA4, HTR3A, and GRIN1 (Figure 2D).
GO enrichment and KEGG pathway analyses
Figure 2C shows the targets of QZD in the treatment of TS and RRTI, reflecting the multiple therapeutic effects of QZD. With P<0.01 and false discovery rate (FDR) <0.01 as the screening criteria, GO analysis showed that the 102 targets of QZD in the treatment of TS and RRTI involved 1,045 BPs, 109 CCs, and 133 MFs.
The BPs of the targets included synaptic and transsynaptic signaling, chemical synaptic transmission, regulation of ion transport, regulation of membrane potential, and regulation of secretion (Figure 3A).

The CCs included dendrites, synaptic membrane (including presynaptic and postsynaptic membrane), axons, receptor complex, and neuronal cell body (Figure 3B).
The MFs included neurotransmitter receptor activity, G protein-coupled amine receptor activity, serotonin receptor activity, amine binding, postsynaptic neurotransmitter receptor activity, and transmitter-gated ion channel activity (Figure 3C).
We used DAVID software to obtain 126 KEGG pathways, and 96 pathways had a P value <0.01. As shown in Figure 3D, the top 20 signal KEGG pathways included neuroactive ligand-receptor interaction, calcium signaling, neurodegeneration, cAMP signaling, glutamatergic synapse, serotonergic synapse, dopaminergic synapse, and PI3K-Akt signaling.
QZD regulated the gut microbiota to remodel gut homeostasis
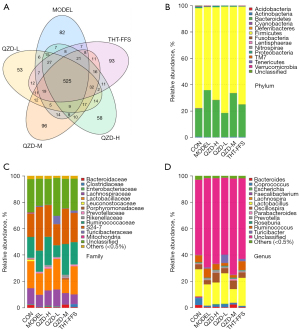
Three fecal samples from each group were prepared for sequencing to obtain the operational taxonomic units (OTUs). In total, 1173 OTUs were identified at a 97% similarity threshold, 525 of which were detected in all 6 groups (Figure 4A). At the phylum level, the relative abundance of Firmicutes in the model group was downregulated, while that of Bacteroidetes was upregulated (Figure 4B). At the family level, the relative abundance of Enterobacteriaceae and Lachnospiraceae of the model group were significantly lower, whereas that of Bacteroidetes family S24-7 OTUs was higher (Figure 4C). In the QZD-H, QZD-M, and THT-FFS groups, the relative abundance of these bacteria could be restored to the same level as these of CON group. At the genus level, compared with the CON group, the relative abundance of Coprococcus and Escherichia in the model group was downregulated (Figure 4D). After administration of QZD, the proportions of these 2 genera were increased.
Analysis of alpha diversity indicated species abundance and diversity within a single sample. As shown in Figure 5A, the model group’s Chao1 index was reduced significantly (P<0.01) compared with the CON group, while that of the QZD-H and THT-FFS groups was increased compared with the model group. In addition, the level of Shannon of the model group was lower in microbial communities compared with that of the CON group. Meanwhile, microbial communities showed an increasing trend in the QZD-H and THT-FFS groups (Figure 5B,5C), suggesting the gut microbial structural of model rats had been changed, and high-dose QZD treatment increased the diversity of the gut microbiota in TS and RRTI model rats.

Analysis of beta diversity indicated the similarity of microbial composition between groups. A heatmap based on Bray-Curtis is shown in Figure 5D. Compared with the CON group, the species composition in the model group was quite different, while the community structure in the QZD-L group was similar.
Linear discriminant analysis effect size (LEfSe) was performed to analyze the differences in community structure between groups to identify differential flora. As shown in Figure 6A-6D, compared with the CON group, Muribaculaceae, Intestinimonas, and Prevotellaceae were significantly overrepresented in the model group, while in the QZD groups, Negativicutes, Acidaminococcaceae, Flintibacter, Phascolarctobacterium, and Eubacteriaceae were enriched.

Discussion
Recent studies have reported that infectious agents and the subsequent cerebral immune imbalance appear to be crucial pathological factors in TS (6,20). Recurrent respiratory tract infections are very common in the early years of life, causing significant burden to individuals, families, and society. The coronavirus disease of 2019 (COVID-19) epidemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), continues to spread around the world, and reinfection remains a major challenge in combating COVID-19. Recurrent respiratory infections that may result should be taken seriously. In addition, throughout the epidemic, a surge in tic disorders and TS has been reported (21). In light of the simultaneous increase in TS and RRTI, and the clinical relevance, more attention should be given to their cotreatment.
Although TS and RRTI are different diseases, as a fundamental principle of traditional TCM—“treating different diseases with the same treatment”—different diseases with the same pathological basis can follow the same treatment pattern. In accordance with the basic theory of TCM and the Zhiyi Dialectical Theory, the functions of QZD include comforting and strengthening the mind, cultivating the Shen (spirit), and replenishing Zhi (wisdom). Previous study has shown that QZD can significantly reduce tic disorders while also reducing RRTI episodes (18).
The UHPLC-Q-Orbitrap-MS/MS analysis of QZD showed 96 chemical components and 584 drug targets, and 102 intersecting genes were obtained after intersection with disease targets.
The Drug-Herbs-Ingredients-Targets Network demonstrated that most of the compounds display a unique one-to-many pharmacological characteristic, meaning that one compound can act on multiple targets, thereby creating a synergistic effect of multiple therapeutic interventions mechanisms in QZD treatment. In this study, alpha-linolenic acid (Q89) was found to have more targets and is an essential fatty acid with hypolipidemic, anti-inflammatory, anti-tumor, and immune-enhancing effects (22,23); 2-Anisic acid (Q20) was found to possess anti-platelet aggregation effects (24); Jasmonic acid (Q93) has been shown to inhibit pathogenic bacteria, induce the MAP kinase cascade pathway, calcium channels, and interact with numerous signaling molecules (25,26).
Different active ingredients are associated with different numbers of target genes, and the same target can be activated by various types of active ingredients. This suggests that there is a synergistic effect between multiple ingredients in the formula, which may produce synergistic effects through their combined effect on one another.
SLC6A4, one member of the solute carrier family, maps to chromosome 17q11.2 (27). The serotonin transporter (SERT/SLC6A4) is an important drug target in the treatment of multiple disorders, including depression, anxiety, autism, TS, and peripherally-based disorders of cardiovascular and gastrointestinal systems.
Interleukin-6 (IL-6) has both proinflammatory and anti-inflammatory activities (28,29) and contributes to the transition from acute inflammation to chronic inflammation (30). IL-6 is a multidirectional cytokine that has important roles in both the immune system and the nervous system, affecting mood, sleep, and neuroimmune regulation, among others. It has been recognized that IL-6 affects immunity in lung diseases and can also influence the mechanical function of the lung through potential effects on smooth muscle (31).
In this study, the results of GO function and KEGG pathway enrichment analysis found cyclic adenosine 3’5’ monophosphate (cAMP), serotonergic, and dopaminergic pathways were associated with QZD in TS and RRTI treatment. As an important second messenger, cAMP is involved in many different cellular activities, acting through cAMP-dependent effectors such as protein kinase A (PKA) and exchange proteins directly activated by cAMP (32). Yang et al. (33) found that the cAMP-PKA pathway was involved in the SARS-CoV-2 infection process and induced the activation of the downstream transcription factor cAMP response element-binding protein (CREB). In studies of neuropsychiatric disorders such as TS, abnormalities in second messengers are often found. In postmortem brain samples with TS, Singer et al. (34) found mean levels of cAMP reduced in the frontal, temporal, and occipital cortices, and in the putamen. In addition to its key role in human health and medicine, the cAMP signaling pathway is also closely linked to the virulence of fungal plant pathogens, animal pathogens, and Plasmodium (35). Liao et al. (36) applied Lactobacillus plantarum PS128 in the treatment of twitch-like behavior in TS rats, showing that it could reduce cAMP levels in model rats and modulate the phosphorylation of DARPP-32 and thus regulate the intestinal flora.
Dysfunction of the dopaminergic (DA) pathway is associated with tic symptoms in TS (37), which is mainly related to excessive DA innervation (34,38), highly sensitive DA receptors, and presynaptic DA abnormality. Dopamine also plays a major immunomodulatory role, with studies showing dopamine receptors(6,38,39) could be expressed on T cells, B cells, natural killer (NK) cells, and macrophages (39,40). Another study showed that dopamine could be synthesized and stored in follicular helper T cells and released upon cognate interaction with B cells (41). In a study of SARS-CoV-2 infected patients, dopamine biosynthetic enzyme L-Dopa decarboxylase mRNA expression was found to be negatively correlated with SARS-CoV-2 RNA levels, and viral infection downregulated the biosynthetic portion of the dopamine pathway regardless of comorbidities (42).
Serotonin [5-hydroxytryptamine (5-HT)], a fundamental neurotransmitter of the central nervous system, is synthesized from tryptophan (43). 5-HT is produced in the raphe nuclei of the brainstem and is responsible for regulating mood, stress response, sleep, and cognition (8,44). In addition, 5-HT is an important signaling molecule of the peripheral nervous system, especially in the gastrointestinal tract. In some patients with TS, 5-hydroxyindoleacetic acid, a major metabolite of serotonin, has decreased secretion in cerebrospinal fluid, peripheral blood, and 24-hour urine (45). Recent study showed that 5-HT regulates DA neurons through several 5-HT receptors in the brain. Most of the effects of 5-HT on dopamine neurons are indirect, rather than direct effects on DA nerve terminals (46). Peripheral serotonin has been shown to have critical roles in the regulation of both innate and adaptive immune responses (47,48). Recent study found serum serotonin levels might be considered a predictor of outcome in SARS-CoV-2 infection (49).
QZD treatment of TD combined with RRTI has shown to have a positive association with various biological processes and signaling pathways, including trans-synaptic signaling, behavioral changes, chemical synaptic transmission, cellular response to nitrogen compounds, regulation of secretion, and other pathophysiological processes. These clinical benefits are believed to result from the synergy of multiple targets on complex biological processes. The enrichment of different targets in the same biological process further supports the existence of synergistic actions between multiple targets.
The gut microbiome is a unique symbiotic system within the human body. The “microbiome-gut-brain axis” regulates immune balance, metabolites, and the nervous system through dynamic bidirectional communication. This bidirectional communication can be realized through the interaction of multiple nervous systems, including the central nervous system, the autonomic nervous system, and the enteric nervous system (50). In this regard, gut microbiota can influence host behavior, mood, and cognition by affecting the alteration of neurotransmitters (51). In our study, the intestinal flora of rats in the model group changed significantly compared with that of the CON group. A study of pediatric patients aged 4–8 years with acute onset neuropsychiatric syndrome (PANS) showed changes in abundance of the phylum Bacteroidetes and Firmicutes compared to a healthy control group (52). A large number of bacteria can manufacture or secrete neurochemicals. For example, Lactobacillus and Bifidobacterium can secrete gamma-aminobutyric acid (GABA) (53), and Escherichia can produce norepinephrine. It has been found that in addition to butyrate production, Coprococcus may also be associated with 3,4-dihydroxyphenylacetic acid (DOPAC) production, which is thought to be a dopamine metabolite (54). Dysbiosis of the gut microbiota also affects lung immunity, which in turn affects lung health and respiratory disease. Recent study has found that children with RRTI have significantly fewer numbers of Bifidobacteria and Lactobacilli and more Escherichia in their intestines compared to healthy children (55).
Conclusions
In this study, we analyzed the drug composition of QZD, conducted network pharmacology for comorbid TS and RRTI, and performed intestinal flora analysis, with our results revealing a multicomponent, multitarget, and multipathway synergistic treatment for comorbid TS and RRTI with QZD. Our study provides new insights into the role of QZD in improving comorbid TS and RRTI and identifies potential targets for drug development. However, further studies are needed to confirm this hypothesis.
Acknowledgments
We thank the Experimental Center, Shandong University of Traditional Chinese Medicine for technical support and assistance.
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81774249), and Introduce Innovative Teams of 2021 “High School 20 Items” Project (No. 2020GXRC013).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-936/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-936/dss
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-936/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-936/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiment protocols were conducted in compliance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Ethical Committee of Shandong University of Traditional Chinese Medicine (No. YYLW20222000011).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brander G, Isomura K, Chang Z, et al. Association of Tourette Syndrome and Chronic Tic Disorder With Metabolic and Cardiovascular Disorders. JAMA Neurol 2019;76:454-61. [Crossref] [PubMed]
- Scharf JM, Miller LL, Gauvin CA, et al. Population prevalence of Tourette syndrome: a systematic review and meta-analysis. Mov Disord 2015;30:221-8. [Crossref] [PubMed]
- Robertson MM, Cavanna AE, Eapen V. Gilles de la Tourette syndrome and disruptive behavior disorders: prevalence, associations, and explanation of the relationships. J Neuropsychiatry Clin Neurosci 2015;27:33-41. [Crossref] [PubMed]
- Jiang J, Chen M, Huang H, et al. The Aetiology of Tourette Syndrome and Chronic Tic Disorder in Children and Adolescents: A Comprehensive Systematic Review of Case-Control Studies. Brain Sci 2022;12:1202. [Crossref] [PubMed]
- Tsetsos F, Yu D, Sul JH, et al. Synaptic processes and immune-related pathways implicated in Tourette syndrome. Transl Psychiatry 2021;11:56. [Crossref] [PubMed]
- Krause DL, Müller N. The Relationship between Tourette's Syndrome and Infections. Open Neurol J 2012;6:124-8. [Crossref] [PubMed]
- Dale RC. Tics and Tourette: a clinical, pathophysiological and etiological review. Curr Opin Pediatr 2017;29:665-73. [Crossref] [PubMed]
- Michely J, Eldar E, Martin IM, et al. A mechanistic account of serotonin's impact on mood. Nat Commun 2020;11:2335. [Crossref] [PubMed]
- Bellanti JA. Recurrent respiratory tract infections in paediatric patients. Drugs 1997;54:1-4. [Crossref] [PubMed]
- Eftimiadi C, Eftimiadi G, Vinai P. Staphylococcus aureus Colonization Modulates Tic Expression and the Host Immune Response in a Girl with Tourette Syndrome. Front Psychiatry 2016;7:31. [Crossref] [PubMed]
- Subspecialty Group of Respiratory Diseases. Editorial Board, Chinese Journal of Pediatrics. Clinical concept and management of recurrent respiratory tract infections in children (revised). Zhonghua Er Ke Za Zhi 2008;46:108-10. [PubMed]
- Chiappini E, Santamaria F, Marseglia GL, et al. Prevention of recurrent respiratory infections: Inter-society Consensus. Ital J Pediatr 2021;47:211. [Crossref] [PubMed]
- de Benedictis FM, Bush A. Recurrent lower respiratory tract infections in children. BMJ 2018;362:k2698. [Crossref] [PubMed]
- Cuppari C, Colavita L, Miraglia Del Giudice M, et al. Recurrent respiratory infections between immunity and atopy. Pediatr Allergy Immunol 2020;31:19-21. [Crossref] [PubMed]
- Cui QK, Li H, Li Z, et al. Study on the mechanism of the Modified Ginseng-Schisandra Decoction (MGSD) in the treatment of recurrent respiratory tract infection (RRTI) based on network pharmacology. Transl Pediatr 2021;10:1701-11. [Crossref] [PubMed]
- Kawikova I, Grady BP, Tobiasova Z, et al. Children with Tourette's syndrome may suffer immunoglobulin A dysgammaglobulinemia: preliminary report. Biol Psychiatry 2010;67:679-83. [Crossref] [PubMed]
- Xin Z, Min W, Ni J, et al. A retrospective analysis on clinical effects of recurrent respiratory infection on “Qufeng Zhidong Decoction”in treating tic disorders. Shanghai Journal of Traditional Chinese Medicine 2015;49:56-8.
- Ji X, Yan Z. Clinical Study on Qiangzhi Zufang Treating Tic Disorder with recurrent respiratory Tract Infection. Acta Chinese Medicine and Pharmacology 2021;49:72-6.
- Li Y, Yan Z, Jin Z, et al. Different Doses of IDPN on Tourette Syndrome Rats. Chinese Archives of Traditional Chinese Medicine 2016;34:2442-4.
- Parker-Athill EC, Ehrhart J, Tan J, et al. Cytokine correlations in youth with tic disorders. J Child Adolesc Psychopharmacol 2015;25:86-92. [Crossref] [PubMed]
- Horner O, Hedderly T, Malik O. The changing landscape of childhood tic disorders following COVID-19. Paediatr Child Health (Oxford) 2022;32:363-7. [Crossref] [PubMed]
- Shang X, Yang X, Ding Z. The Intestinal Absorption Characteristics of Compound Safflower Seed Oil were Investigated by Everted Gut Sac Method. Modern Food Science and Technology 2023;39:1-7.
- Tingting M, Guo H, Linfei J, et al. Extraction and Microencapsulation of Eucommia Seed Oil. Academic Periodical of Farm Products Processing 2012; 10: 81-85+89.
- Xueying Z, Li L, Kai Y, et al. Clinical observation of Gualou Xiebai Granule in the treatment of PCI postoperative restenosis in soft plaque of coronary artery with syndrome of phlegm-dampness blocking 2017;51:41-4.
- Dathe W, Rönsch H, Preiss A, et al. Endogenous plant hormones of the broad bean, Vicia faba L. (-)-jasmonic acid, a plant growth inhibitor in pericarp. Planta. 1981;153:530-5. [Crossref] [PubMed]
- Ruan J, Zhou Y, Zhou M, et al. Jasmonic Acid Signaling Pathway in Plants. Int J Mol Sci 2019;20:2479. [Crossref] [PubMed]
- Ponleitner M, Szöllősi D, El-Kasaby A, et al. Thermal Unfolding of the Human Serotonin Transporter: Differential Effect by Stabilizing and Destabilizing Mutations and Cholesterol on Thermodynamic and Kinetic Stability. Mol Pharmacol 2022;101:95-105. [Crossref] [PubMed]
- Tao Y, Xu P, Zhu W, et al. Changes of Cytokines in Children With Tic Disorder. Front Neurol 2021;12:800189. [Crossref] [PubMed]
- Marcos-Pérez D, Sánchez-Flores M, Proietti S, et al. Association of inflammatory mediators with frailty status in older adults: results from a systematic review and meta-analysis. Geroscience 2020;42:1451-73. [Crossref] [PubMed]
- Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther 2006;8:S3. [Crossref] [PubMed]
- Rincon M, Irvin CG. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci 2012;8:1281-90. [Crossref] [PubMed]
- Efetova M, Petereit L, Rosiewicz K, et al. Separate roles of PKA and EPAC in renal function unraveled by the optogenetic control of cAMP levels in vivo. J Cell Sci 2013;126:778-88. [PubMed]
- Yang Q, Tang J, Cao J, et al. SARS-CoV-2 infection activates CREB/CBP in cellular cyclic AMP-dependent pathways. J Med Virol 2023;95:e28383. [Crossref] [PubMed]
- Singer HS, Hahn IH, Moran TH. Abnormal dopamine uptake sites in postmortem striatum from patients with Tourette's syndrome. Ann Neurol 1991;30:558-62. [Crossref] [PubMed]
- Borchers A, Pieler T. Programming pluripotent precursor cells derived from Xenopus embryos to generate specific tissues and organs. Genes (Basel) 2010;1:413-26. [Crossref] [PubMed]
- Liao JF, Cheng YF, Li SW, et al. Lactobacillus plantarum PS128 ameliorates 2,5-Dimethoxy-4-iodoamphetamine-induced tic-like behaviors via its influences on the microbiota-gut-brain-axis. Brain Res Bull 2019;153:59-73. [Crossref] [PubMed]
- Buse J, Schoenefeld K, Münchau A, et al. Neuromodulation in Tourette syndrome: dopamine and beyond. Neurosci Biobehav Rev 2013;37:1069-84. [Crossref] [PubMed]
- Minzer K, Lee O, Hong JJ, et al. Increased prefrontal D2 protein in Tourette syndrome: a postmortem analysis of frontal cortex and striatum. J Neurol Sci 2004;219:55-61. [Crossref] [PubMed]
- Levite M. Dopamine and T cells: dopamine receptors and potent effects on T cells, dopamine production in T cells, and abnormalities in the dopaminergic system in T cells in autoimmune, neurological and psychiatric diseases. Acta Physiol (Oxf) 2016;216:42-89. [Crossref] [PubMed]
- Arce-Sillas A, Sevilla-Reyes E, Álvarez-Luquín DD, et al. Expression of Dopamine Receptors in Immune Regulatory Cells. Neuroimmunomodulation 2019;26:159-66. [Crossref] [PubMed]
- Papa I, Saliba D, Ponzoni M, et al. T(FH)-derived dopamine accelerates productive synapses in germinal centres. Nature 2017;547:318-23. [Crossref] [PubMed]
- Mpekoulis G, Kalliampakou KI, Milona RS, et al. Significance of Catecholamine Biosynthetic/Metabolic Pathway in SARS-CoV-2 Infection and COVID-19 Severity. Cells 2022;12:12. [Crossref] [PubMed]
- Ye D, Xu H, Tang Q, et al. The role of 5-HT metabolism in cancer. Biochim Biophys Acta Rev Cancer 2021;1876:188618. [Crossref] [PubMed]
- Kanova M, Kohout P. Serotonin-Its Synthesis and Roles in the Healthy and the Critically Ill. Int J Mol Sci 2021;22:4837. [Crossref] [PubMed]
- Paschou P, Fernandez TV, Sharp F, et al. Genetic susceptibility and neurotransmitters in Tourette syndrome. Int Rev Neurobiol 2013;112:155-77. [Crossref] [PubMed]
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther 2007;113:296-320. [Crossref] [PubMed]
- Shajib MS, Khan WI. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol (Oxf) 2015;213:561-74. [Crossref] [PubMed]
- Lin L, Hu K. Serotonin is a multifaceted player in the immune response. Front Biosci (Landmark Ed) 2021;26:253-4. [Crossref] [PubMed]
- Soria-Castro R, Meneses-Preza YG, Rodríguez-López GM, et al. Severe COVID-19 is marked by dysregulated serum levels of carboxypeptidase A3 and serotonin. J Leukoc Biol 2021;110:425-31. [Crossref] [PubMed]
- Liang S, Wu X, Hu X, Wang T, Jin F. Recognizing Depression from the Microbiota-Gut-Brain Axis. Int J Mol Sci 2018;19:1592. [Crossref] [PubMed]
- Mittal R, Debs LH, Patel AP, et al. Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J Cell Physiol 2017;232:2359-72. [Crossref] [PubMed]
- Quagliariello A, Del Chierico F, Russo A, et al. Gut Microbiota Profiling and Gut-Brain Crosstalk in Children Affected by Pediatric Acute-Onset Neuropsychiatric Syndrome and Pediatric Autoimmune Neuropsychiatric Disorders Associated With Streptococcal Infections. Front Microbiol 2018;9:675. [Crossref] [PubMed]
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry 2013;74:720-6. [Crossref] [PubMed]
- Valles-Colomer M, Falony G, Darzi Y, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol 2019;4:623-32. [Crossref] [PubMed]
- Li KL, Wang BZ, Li ZP, et al. Alterations of intestinal flora and the effects of probiotics in children with recurrent respiratory tract infection. World J Pediatr 2019;15:255-61. [Crossref] [PubMed]


