Recent advances on the mechanisms regulating cholangiocyte proliferation and the significance of the neuroendocrine regulation of cholangiocyte pathophysiology
Introduction
The liver (the largest organ of the human body) is considered as a gland capable of performing endocrine-metabolic and exocrine functions (1-3). The liver plays a key role in the homeostasis of the whole body metabolism. In particular, synthesizing many proteins and enzymes, the liver plays a primary role in the regulation of the energetic metabolism and it also contributes to the detoxification and elimination of a wide variety of both endogenous and exogenous molecules. In addition, the liver secretes bile that is necessary for the digestion of fats. The bile is then delivered to the second portion of the duodenum through intrahepatic bile ducts (4), whose lumen is lined by specialized epithelial cells: cholangiocytes (5,6) that modify the final composition of bile by a number of basal and hormone-regulated events (4,5,7,8).
Features of the biliary tree
The human biliary tree originates at the level of the biliary pole of hepatocytes that are responsible for the production of canalicular bile (9). Thereafter, the bile is secreted into the lumen of bile canalicular. The bile canalicular are narrow spaces of 0.5-2 µm delimited by simple introflections of the plasma membrane at the biliary poles of adjacent hepatocytes. The biliary pole of hepatocytes is characterized by numerous and short microvilli that protrude into the lumen and, it is isolated from the vascular pole by occluding junctions (10).
Near the biliary pole, the cytoskeleton of hepatocytes is characterized by many microfilaments predominantly formed by actin and myosin. These microfilaments that are located around the bile canalicular regulates the passage of bile along the canals themselves. The canals of Hering origin in proximity of portal spaces (10,11).
The point at which the bile canalicular continue into the canals of Hering is defined as the ductule-canalicular junction. At this level, intrahepatic bile ducts are partly lined by cholangiocytes and in part by hepatocytes. At this point of the biliary epithelium, partially undifferentiated cells have been identified: the hepatic progenitor cells (HPC) (12). HPC constitute a resident stem cell compartment in the liver and they are able to differentiate into both hepatocytes and cholangiocytes (13-15). The canals of Hering are, therefore, points of anatomical and physiological connection between the bile canalicular and interlobular bile ducts in the portal space. The canals of Hering are lined by a few (3 or 4) cubic-shaped cholangiocytes, which rest on a thin basement membrane (7,16). The canals of Hering continue into the interlobular ducts, lined by a continuous layer of cylindrical-shaped cholangiocytes (16). The interlobular ducts are characterized by a diameter of 15-100 µm and in the portal space they draw up alongside the ramifications of the hepatic artery and of the portal vein. The interlobular bile ducts then continue into ducts of progressively larger size to form two large intrahepatic ducts (right and left hepatic duct), which drain respectively the right and left hepatic lobe of the liver and, at the hilum, they give rise to the extrahepatic bile ducts (4,17,18).
According to diameter the human intrahepatic bile duct can be classified into: large ducts (>800 µm), and then gradually segmental ducts, zonal, septal, interlobular ducts (15-100 µm) and ductules (<15 µm) (16).
In rodents, the intrahepatic biliary ductal system is morphologically heterogeneous and is formed by small (<15 µm of diameter, lined by small cholangiocytes, 8 µm in size) and large (>15 µm of diameter, lined by large cholangiocytes, 15 µm in size) bile ducts (5,7).
Vascularization and innervation of the biliary tree
The biliary epithelium is nourished by terminal branches of the hepatic artery, which constitute a complex vascular system called the peribiliary plexus (PBP). The PBP, which extends up to the interlobular ducts flows into the hepatic sinusoids, either directly through lobular branches, which indirectly through branches of the portal vein or pre-lobular branches (17,19,20). In addition, numerous studies performed on rats have shown that small and large bile ducts have a different vascularization (21). In fact, the PBP is more extended in large bile ducts. The resulting difference of blood supply strongly influences the metabolic capabilities and functions of bile ducts of different size and of cholangiocytes that line their walls (17,22). The PBP is characterized by a bilaminar vascular network near the hilus of the liver and larger portal tracts, whereas it is formed by fewer connected vessels in the smaller portal tracts (23).
From a physiological point of view, the PBP is crucial in allowing the active reabsorption and therefore the modification of bile by cholangiocytes. In particular, it allows to reabsorb substances present in the bile and to bring them back to parenchymal cells (17). The canals of Hering and part of bile ductules are not directly vascularized (23), and this could in part justify the quiescent state under normal conditions of the HPC compartment located in this tract of the biliary tree (23).
The human intra- and extra-hepatic biliary epithelium is innervated by autonomic nerves that originate from the celiac plexus (sympathetic fibers) and the vagus nerve (parasympathetic fibers). These nerve fibers are organized to form the hepatic plexus divided into: anterior plexus around the hepatic artery and posterior plexus surrounding portal vein and bile duct (24).
Hepatic plexus (anterior and posterior) nerve fibers that originate through the hilum penetrate the liver parenchyma following the branches of the hepatic artery, the portal vein and the biliary tree. This innervation is also extended to hepatocytes and intrahepatic vascular structures (19,25).
In addition to classical neurotransmitters (e.g., adrenaline, noradrenaline and acetylcholine), the nerve fibers release in the liver numerous other neuropeptides such as neuropeptide Y (NPY), the calcitonin gene-related peptide (CGRP), somatostatin, vasoactive intestinal polypeptide (VIP), enkephalin and bombesin. Many of these neuropeptides have been shown to be able to stimulate or inhibit the functional activity of cholangiocytes (26,27).
Experimental models for the study of biliary tree and cholangiocytes
In both rat and human livers, cholangiocytes are heterogeneous in size (that can vary from 3 to 80 µm2) (7) and line bile ducts of different sizes (5,7). For example, intrahepatic bile ducts of small dimensions (small bile ducts: diameter less than 15 µm) (5) are lined by cholangiocytes of smaller size, whose diameter is about 8 µm (small cholangiocytes) (7). In contrast, bile ducts of greater size (large bile ducts: diameter greater than 15 µm) are lined by cholangiocytes more voluminous, whose diameter is around 15 µm (large cholangiocytes) (7,18). In addition to morphological heterogeneity, cholangiocytes display ultrastructural, antigenic, functional and proliferative heterogeneous responses (7,10,18,28). In fact, small cholangiocytes possess cuboidal shape and have a larger nucleus and scant cytoplasm (10). This aspect is typical of poorly differentiated cells in which it occurs an intense synthesis of RNA messenger but lower post-transcriptional activity. Conversely, large cholangiocytes have columnar shape, a small nucleus and abundant cytoplasm (7,18). Both small and large cholangiocytes express cytokeratin-7 and -19, alkaline phosphatase, whereas only large cholangiocytes the secretin receptor, cystic fibrosis transmembrane conductance regulator (CFTR) and chloride bicarbonate anion exchanger 2 (Cl-/HCO-3 AE2) and respond to secretin with enhanced secretory and proliferative activities (2,5,7,29-31).
A number of experimental models have improved our understand of the mechanisms that regulate cholangiocytes proliferation, apoptosis, secretion, transport, signal transduction and the corresponding dysfunctions that lead to the diseases of the biliary tract. Three types of cholangiocytes proliferation have been described (27). Type I or “typical” cholangiocyte proliferation: represents a hyperplastic reaction that involves an increase of the number of intrahepatic bile ducts, confined to the portal space. Cholangiocytes proliferating, form well-differentiated tubular structures that show a well-defined lumen (4,13). In rodents, “typical” cholangiocyte proliferation is observed in different experimental conditions such as extrahepatic bile duct ligation (BDL) (2,4,22), partial hepatectomy (32) and chronic treatment with L-proline (33). In addition, “typical” cholangiocytes proliferation is also observed after the acute administration of carbon tetrachloride (CCl4) (34) or chronic feeding α-naphthylisothiocyanate (ANIT) (35) or bile salts such as Taurocholate (36,37). In human liver, a typical cholangiocyte proliferation is observed during acute obstructive cholestasis and in the early stages of chronic cholestatic diseases. This type of proliferation appears to be due to the stretching of the existing bile ducts localized in the portal tracts (27,38).
Type II or “atypical” proliferation occurs in rodents after the administration of CCl4 (39) and it is present in humans after massive hepatic necrosis, alcoholic liver disease, focal nodular hyperplasia and chronic cholestatic diseases such as primary biliary cirrhosis (PBC) and PSC (27,40). This pathology is characterized by the proliferation of intrahepatic bile ducts not confined to portal areas but extended to the periportal area and in the adjacent liver parenchyma. This type of proliferation leads to the formation of irregular and tortuous ductular structures that do not possess a well-defined lumen and are associated with inflammation and infiltration of neutrophils; moreover, these neo-formed ducts are not functionally efficient. Unlike “typical” proliferation, in type II proliferation has been described the presence of transitional cells with phenotypic characteristics of both cholangiocytes and hepatocytes. This finding is in favor of the hypothesis that atypical proliferation is a phenomenon of metaplasia and may originate from the hepatic progenitor cell compartment rather than from the replication of pre-existing ducts (13,27).
Type III proliferation (“oval cell” proliferation) characterizes the early stages of carcinogenesis in rat liver and is caused by treatment with chemicals. This pathology is characterized by the formation of tubular irregular and disorganized structures with a not well defined lumen which protrude inside the hepatic lobule with alteration of the architecture of the entire parenchyma (27).
Oval cells, so called for their shape, represent a subpopulation of cells, located at levels of canals of Hering that are heterogeneous in the expression of proteins and cell surface markers (13,41-43). Oval cells show phenotypes of immature or neoplastic hepatocytes (e.g., expression of α-fetoprotein and albumin) and cholangiocytes (e.g., expression of cytokeratin 7 and 19) (41,44). Oval cells have the capability of differentiating into hepatocytes, cholangiocytes, enterocytes, and exocrine pancreatic cells (42,43).
BDL is the most used experimental model to study “typical” proliferation of cholangiocytes. This maneuver causes a rapid and marked proliferation of bile ducts leading to increased biliary mass (2,17). In the BDL rat model, the proliferation of cholangiocytes and the reorganization of the hepatic microvasculature occur according to a well-defined timetable. In fact, one week after BDL, the number of bile ducts increases, particularly at the edges of portal tracts. At this time-period, cholangiocytes appear to markedly proliferate. In this phase, the hepatic microcirculation is poorly changed (17,20,22,23). After two weeks, proliferation of bile ducts continues and bile ducts are characterized by a dilated lumen and lined by cholangiocytes resting on a basement membrane. At this stage, there is an active reorganization of the PBP that follows the proliferation of the bile ducts (20,45). After four weeks, the abundant proliferation of bile ducts is accompanied by septa of connective tissue located on the edge of the liver lobules. By means of vascular corrosion casts observed by scanning electron microscope (46) is possible to see a PBP that extends around the lobules and appears to be formed by homogeneous capillaries widely distributed that originate from arterioles arising from branches of the hepatic artery (20).
The evaluation of the molecular mechanisms that regulate the “typical” proliferation of cholangiocytes in BDL rats was made possible by the development of in vitro models such as the isolation and characterization of small and large cholangiocytes or intrahepatic bile duct units (IBDU) (2,5,7,30,37). The isolation of cholangiocytes from livers of normal or cholestatic rats is achieved through separation techniques based on immunoaffinity, i.e. through the use of antibodies expressed on the surface of all intrahepatic cholangiocytes (2,7). The isolation and study of IBDU of different sizes is essential for the direct assessment of the proliferative capacity of bile ducts. These units retain the morphological, phenotypic and functional characteristics of the intrahepatic bile ducts in situ (2,7). These tools have allowed us to define the morphologic and functional heterogeneity of intrahepatic bile ducts in normal and pathophysiological states. For example, after BDL large (but not small) cholangiocytes proliferate through the activation of cAMP-dependent signaling, leading to enhanced intrahepatic bile duct mass by activation of cAMP signaling (2,39,47). Also, cAMP induces cholangiocyte proliferation in association with increased activity of protein kinase A (PKA) and phosphorylation of Src-Tyrosine 139 and ERK1/2 (48). This signaling cascade is also activated by factors such as estrogens (49), nerve growth factor (50), and insulin-like growth factor-1 (IGF-1) (51). Conversely, serotonin decreases biliary hyperplasia in cholestatic rats by the inhibition of cAMP/PKA/Src/ERK1/2 signaling (52).
Regarding the function of small cholangiocytes, several studies have shown that the activation of D-myo-inositol 1,4,5-triphosphate (IP3)/Ca2+-dependent signaling regulates the function of these cells (53-56). Small mouse cholangiocytes proliferate after H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway (53). Further, histamine stimulates the proliferation of small and large cholangiocytes by activation of both IP3/Ca2+ and cAMP-dependent signaling mechanisms, respectively (56). For example, a study has shown that activation of alpha[1]-adrenergic receptors stimulate the growth of small mouse cholangiocytes via Ca2+-dependent activation of nuclear factor of activated T cells 2 and specificity protein 1 (54). A recent study has demonstrated the existence of a P2 signaling axis along the length of the biliary tree with the “upstream” small cholangiocytes releasing ATP, which can serve as a paracrine signaling molecule to “downstream” large cholangiocytes stimulating Ca2+-dependent secretion (55). Following functional damage of large cholangiocytes by apoptosis (e.g., after treatment acute administration of CCl4 and chronic treatment with gamma-aminobutyric acid (GABA), small cholangiocytes de novo proliferate and replenish the damaged biliary tree by amplification of Ca2+-dependent signaling and de novo acquisition of large cholangiocyte phenotypes (34,39,57).
One of the functions of cholangiocytes in humans is to modify the composition of bile. The bile produced by hepatocytes is particularly rich in bile salts, glutathione, lipids, proteins and other organic compounds and its flowing into bile ducts allows cholangiocytes to change its composition through mechanisms of reabsorption and secretion (58,59). Cholangiocytes in the adult liver are quiescent (34,39), since they express factors such as cyclin dependent kinase inhibitors p27, bcl2 and BclxL that are responsible for this mitotically dormant state (60). However, in pathological states cholangiocytes become mitotically activated both in experimental cholestasis (e.g., BDL and A feeding) (4,35) and in human cholangiopathies such as PBC and PSC classified as “vanishing bile duct syndrome” (58,59). The evolution of these cholangiopathies is characterized by an initial balance between cholangiocyte apoptosis with reduction of the number of bile ducts and compensatory cholangiocyte proliferation. By contrast, the end stage is characterized by the collapse of the proliferative capacity of cholangiocytes, resulting in the reduction of the number of bile ducts (vanishing bile duct syndrome). In these cases the compensated (non-ductopenic) stages of disease are characterized by a similar rate of apoptosis and proliferation, whereas in the terminal ductopenic stages apoptosis predominates favoring the evolution toward ductopenia. Therefore, in these pathologic conditions, the mechanism of repair based on the proliferation of residual cholangiocytes is not sufficient to avoid the evolution toward ductopenia. In this case then, stimulation of cholangiocyte proliferation could be a strategy for the management of human ductopenic pathologies affecting the intrahepatic biliary tree. On the other hand, reduction of cholangiocytes proliferation can be considered as the possible strategy for the management of pathologies characterized by uncontrolled proliferative conditions such as cholangiocarcinoma (61-63) and polycystic liver diseases (60,64,65). We know that progenitor cells are activated when injury is associated with impaired regeneration of the mature cell types involved (66,67). Several factors such as viruses and alcohol can cause acute and chronic human liver disease through damage and loss of hepatocytes and/or cholangiocytes. The degree of progenitor cell activation, and the number of intermediate hepatocytes is directly proportionate with the degree of inflammatory activity (67,68), and with the stage of the disease (67,69). This aspect indicates that the HPC compartment activation is linked with the evolution of the disease. At the ductopenic stage of cholangiopathies, the functionality of bile ducts is strongly altered due to the change in bile flow and subsequent biliary stasis (58,59). Therefore, the fine regulation of secretion and proliferation of cholangiocytes is critical for the normal and physiological function of the liver. These aspects are influenced and controlled by many factors, including bile acids, autonomic nervous system, neuroendocrine substances, growth factors and hormones (Figure 1).
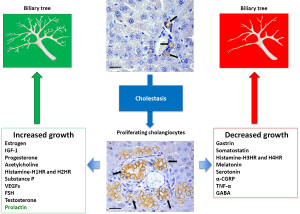
Regulation of cholangiocyte function and proliferation by hormones and neuropeptides
Sex hormones
Sex steroid hormones are able to influence the development and course of chronic liver diseases (70,71). It has been shown that cholangiocytes are estrogen-sensitive cells able to express both the estrogen receptors (ER-α and ER-β) in contrast to hepatocytes that express only the receptor ER-α (45). Our findings have shown that liver expresses estrogen and androgen receptors and experimentally both androgens and estrogens have been implicated in stimulating cell proliferation. Specifically, estrogens stimulate both in vitro and in vivo cholangiocyte proliferation and ERs may modulate cholangiocyte proliferation. In fact cholangiocyte proliferation, after 3 weeks of BDL, is associated with increased expression of both ER-α and ER-β (45,49,72). When tamoxifen (anti-estrogen) or ICI 182,780, a specific ER antagonist, were used to treat BDL rats, the intrahepatic bile duct mass markedly decreases in comparison with control BDL rats (45). These data were confirmed by ovariectomy of which effects reduce cholangiocyte proliferation in BDL (72). In vitro experiments showed that 17β-estradiol induces cholangiocytes proliferation by activation of the ER/Src/Shc/ERK1/2 pathway (49). In humans, it has been shown that at late stages of PBC (73) the biliary expression of ER is decreased and ER-α positivity in cholangiocytes is markedly lower than that of cholangiocytes in primary sclerosing cholangitis and alcoholic cirrhosis (73). It has been shown that the low expression of ER-α in late stages of PBC promotes the development of PBC toward ductopenia (73). The relevance of estrogens in sustaining biliary growth is also supported by the fact that in middle-aged women (mainly affected by PBC), estrogen and progesterone levels are decreased (74,75).
In patients with cholangiocarcinoma (CCA) the expression of ER-α is higher than in normal condition, whereas a similar expression of ER-β was observed (73,76). In ovary, prostate, colon, and breast cancers it has been shown that, neoplastic transformation and tumor progression is associated with upregulation of ER-α and downregulation of ER-β (77,78). These data confirm the hypothesis that ER-α promotes cell proliferation whereas the opposite is observed for ER-β (77-79).
Similarly, testosterone is important in sustaining biliary proliferation and ductal secretory activity in pathological conditions associated with functional damage of the biliary epithelium (80). Specifically, we demonstrated that: (I) cholangiocytes express testosterone receptors, 17β-hydroxysteroid-dehydrogenase3 (HSD3, the key enzyme regulating testosterone synthesis), and secrete testosterone; (II) castration influences the biliary growth in normal and BDL rats decreasing bile duct mass and secretin-stimulated cAMP levels, effects that were prevented by the exogenous administration of testosterone (80); These data suggest an autocrine role of testosterone in sustaining cholangiocyte proliferation during cholestasis. Parallel to these findings, other studies have shown the presence of functional ARs in liver cells, including hepatocytes and bile ducts from PBC patients (81). In particular, the expression of androgen receptors (ARs) was low in normal patients and increased in patients with PBC (81). The fact that testosterone prevents the loss of biliary growth and function supports the concept that androgens can be important for the management of ductopenic conditions associated with decreased testosterone levels as occurs in PBC (80). There is limited information regarding the role of progesterone in the regulation of biliary functions. We have recently demonstrated that: (I) progesterone stimulates proliferation of both male and female cholangiocytes (82); and (II) normal and BDL cholangiocytes expressed the biosynthetic pathway (i.e., steroidogenic acute regulatory protein or STAR, M3β-Hydroxysteroid dehydrogenase (M3β-HSD), and cytochrome P450 side-chain cleavage) for the synthesis of progesterone and secrete progesterone (82). These findings support the concept that endocrine, autocrine and paracrine mechanisms play an important role as compensatory mechanism for bile duct loss during cholangiopathies (73).
Cholangiocytes also express the long and short form of prolactin receptor (83). Also, chronic in vivo administration of prolactin to normal rats increases cholangiocyte proliferation and intrahepatic ductal mass (83). The proliferative effect of prolactin is associated with the activation of the IP3/Ca2+/PKC/Src/MAPK pathway and the phosphorylation of the JAK2/STAT5 signalling pathway (83). These findings demonstrate the ability of proliferating cholangiocytes to secrete prolactin, thus regulating their own proliferation by an autocrine loop.
Follicle stimulating hormone (FSH) is produced in the anterior pituitary gland of the brain (84). Several studies demonstrated that liver cirrhosis is associated with endocrine dysfunction, notably in the gonadal axis (85). In fact, gonadotropin deficiency occurs with liver damage and in some patients with hemochromatosis (86). We found that cholangiocytes expressed the FSH receptor (FSHR) and secreted FSH (86). Treatment of normal rats with FSH increases cholangiocyte growth via cAMP/ERK1/2/Elk-1 signalling mechanism, whereas administration of antide (a gonadotropin releasing hormone antagonist that blocks FSH secretion) or an anti-FSH antibody decreases cholangiocyte proliferation and ductal secretory responses (87).
Moreover, other hormones and growth factors cross talk together in normal and pathologic conditions. Indeed, the IGF1 has been shown to regulate cholangiocytes proliferation. In fact, the IGF1 is a circulating peptide (of which the liver is the main source of production) that acts locally as a growth factor with multiple endocrine, paracrine and autocrine functions (51,88,89). The IGF1 is synthesized by the liver under the control of pituitary GH (Growth Hormone) which is tied to specific receptors (GH-R), induces the synthesis and release of circulating IGF-1 that play a key role in postnatal growth of many organs. Therefore, in cholangiocytes there is a complex interaction between estrogen and IGF1, which together are involved in the modulation of proliferation, apoptosis and cell differentiation promoting the processes of tissue repair (51,90).
Secretin
Secretin is one of the main factors responsible of the regulation of biliary secretion (4,8). Specifically, in normal rodents, secretin stimulates ductal secretion in large cholangiocytes (the only cholangiocyte subpopulation that expresses secretin receptor) (2,5,7,30). The interaction of secretin with the specific receptor determines the activation of the enzyme, adenylyl cyclase, with a consequent increase in the synthesis of cAMP capable of acting on PKA. Phosphorylation of PKA leads to the opening of the chloride channel, CFTR, and subsequently the activation of the ion pump Cl-/HCO3- with secretion of HCO3- and, for osmotic gradient, H2O (28,31). In pathologic conditions of cholangiocyte hyperplasia there is enhanced secretin receptor expression (2,31,32) and secretin-stimulated cAMP PKA/CFTR/Cl-/HCO3- (2), leading to enhanced bicarbonate secretion in ductal bile (32). Instead in case of damage of large bile ducts there is decreased proliferation and down-regulation of secretin and secretin-stimulated pathway, with decreased bicarbonate secretion in bile (39,57). Secretin has also been shown to stimulate biliary proliferation both in vivo and in vitro models (29). On the contrary, secretin has been shown to inhibit cholangiocarcinoma growth both in vitro in cholangiocarcinoma cells and in vivo in athymic mice (91). The inhibitory effects of secretin on cholangiocarcinoma growth via dysregulation of the cAMP-dependent signaling mechanisms of the secretin receptor that coupled to Galpha(i) rather than Galpha(s) (91).
Gastrin
Gastrin, by interacting with its receptor cholecystokinin-B (CCK-B) located in the basolateral membrane of cholangiocytes, gastrin inhibits secretin-stimulated cAMP levels and bile secretion (4) by downregulation of cAMP signaling (92). Gastrin has also been shown to inhibit cholangiocyte hyperplasia in cholestatic rats and cholangiocarcinoma growth by Ca2+-dependent activation of protein kinase C-alpha (47,93).
Somatostatin
Rat and human cholangiocytes as well as cholangiocarcinoma cell lines express somatostatin receptor subtypes, SSTR2 (4,94). Somatostatin has been shown to inhibit secretin-induced ductal hypercholeresis and exocytosis by interacting with SSTR2 on cholangiocytes by downregulation of cAMP levels (94). Somatostatin by selectively interacting with SSTR2 receptors inhibits the proliferation of large cholangiocytes in BDL rats by decreasing intracellular cAMP levels (2). Somatostatin and its analogues also inhibit the growth of human cholangiocarcinoma cell lines expressing SSTR2 receptor subtypes (95).
Histamine
Recent studies showed that small and large cholangiocytes express H1-H4HRs. Histamine and the H1HR agonist increased small bile duct mass, whereas histamine and the H2HR agonist increased large bile duct mass. H1HR agonists stimulated IP3 levels, as well as PKCα phosphorylation and cholangiocytes proliferation, whereas H2HR agonists increased cAMP levels, as well as PKA phosphorylation and cholangiocytes proliferation (56). Agonists of H1HR stimulate small (but not large) cholangiocyte proliferation by activation of IP3/CaMK I/CREB signaling (53). Indeed, activation of H3HR induces inhibition of large biliary growth of BDL rats by down-regulation of the cAMP-dependent PKA/ERK1,2/Elk1 pathway (96).
It has been shown that histamine increases cholangiocarcinoma growth by an autocrine mechanism, and that the inhibition of H1HR and of histamine synthesis by blocking his precursor histidine decarboxylase (HDC) significantly decreases cholangiocarcinoma growth (97).
Melatonin
Melatonin is secreted from pineal gland as well as extrapineal tissues; it regulates cell mitosis by interacting with melatonin receptors (MT1 and MT2) by modulating cAMP and clock genes expression. In fact, in recent studies we have shown that cholangiocytes express MT1 and MT2 receptors, and clock genes such as Circadian Locomotor Output Cycles Kaput (CLOCK), Aryl hydrocarbon receptor nuclear translocator-like (ARNTL or BMAL1), cryptochrome 1 (CRY1), and Period circadian protein 1 (PER1) that were all up-regulated following BDL. Both in vivo and in vitro, melatonin decreased cholangiocytes proliferation in cholestatic rats by reducing the expression of clock genes by downregulation of cAMP levels and PKA phosphorylation in cholangiocytes (98). In the liver, melatonin has been shown to reduce oxidative damage and hepatic proliferation, and to stimulate apoptosis of hepatocytes in rats undergoing partial hepatectomy (99). Other studies have shown that melatonin ameliorates liver fibrosis (100), and reduces BDL-induced systemic oxidative stress in cholestatic rats (101).
In CCA there is dysregulation of aralkylamine N-acetyltransferase (AANAT)/N-Acetylserotonin-O-methyltransferase (ASMT) (key enzymes regulating melatonin secretion) → melatonin → melatonin receptor axis, which inhibited melatonin secretion and subsequently enhanced CCA growth (102).
Growth factors and cytokines
A number of studies have shown that epidermal growth factor (EGF), hepatocyte growth factor (HGF), IGF1, interleukina-6 (IL-6), interleukin-1α, tumor necrosis factor α (TNF-α) and Vascular Endothelial Growth Factor (VEGF) stimulate both in vitro and in vivo cholangiocyte proliferation (27,51,103). In particular about VEGF it has been shown that cholangiocytes secrete VEGF and express VEGFR-2 and VEGFR-3, all of which are amplified in BDL cholangiocytes. VEGF induces cholangiocyte proliferation by activation of IP3/[Ca2+]I/protein kinase C alpha and phosphorylation of Src/ERK1/2 and it mediates the adaptive proliferative response of cholangiocytes to cholestasis via autocrine and paracrine mechanisms (22).
Neuropeptides
Several studies have shown that cholinergic, adrenergic and dopaminergic nerve fibers modulate cholangiocytes functions (104). It has been recently shown that cholangiocyte proliferation is positively modulated by parasympathetic and sympathetic innervation but negatively modulated by the serotoninergic system. Acetylcholine by acting on M3 subtype receptor expressed by cholangiocytes, promote proliferative and secretory activities of cholangiocytes (3). In support of these data it has been shown that vagotomy induces the disappearance of M3 acetylcholine receptor in BDL but not in normal rats, reducing cholangiocyte proliferation, and activating apoptotic cell death together with decreased cAMP intracellular levels (3). Maintenance of cAMP levels by forskolin prevented the vagotomy-induced biliary damage (3).
Adrenergic innervation also regulates hepatic proliferation. It has been shown that proliferating cholangiocytes in BDL rats express α1 and α2 adrenergic receptors (19). Adrenergic innervation affects cholangiocytes proliferation by modulating intracellular levels of cAMP, as demonstrated by the chemical sympathetic denervation of the liver by 6-hydroxydopamine (6-OHDA). In fact the treatment with 6-OHDA increased cholangiocyte apoptosis and decreased cholangiocyte proliferation in association with decreased cAMP levels, and reduced phosphorylation of PKA, ERK1/2, and AKT (19). The changes induced by 6-OHDA in cholangiocyte proliferation were prevented by dobutamine a β1 adrenergic receptor agonist, and clenbuterol a β2 adrenergic receptor agonist (19). 6-OHDA induced effects on biliary hyperplasia were also prevented by taurocholic acid feeding (105), a finding that suggests that bile acids may influence the proliferation of bile ducts. In fact, cholangiocytes in BDL rat are very sensitive to variations in composition of bile salts. It has been shown that while litocholate, taurocholate and taurolitocholate favor the proliferation of cholangiocytes, ursodeoxycholate and tauroursodeoxycholate inhibit proliferation (106). Cholangiocytes also express D2 dopamine receptor, whose activation by quinelorane induces inhibition of secretin-stimulated choleresis by increased PKC-gamma expression and decrease of PKA activity (107).
Recently, we have shown that cholangiocytes express the serotonin 1A and 1B receptors (52). Their activation inhibits the growth of the intrahepatic biliary tree in the BDL model, by activation of the IP3/Ca2+/PKC signaling pathway. Cholangiocytes are able to secrete serotonin, neutralization of which enhances cholangiocyte proliferation in the course of cholestasis (52).
Also, sensory innervation may regulate the hyperplasia of the biliary epithelium in cholestatic states (108). In fact high levels of the sensory neuropeptide α-CGRP promote biliary hyperplasia in the BDL cholestatic model. In vitro, both α and β-CGRP increased biliary proliferation by the activation of cAMP-dependent PKA (108).
A recent study has also established that cholangiocytes express the neurokinin-1 receptor (NK-1R) which expression is up-regulated following BDL. In vitro, Substance P, a member of the tachykinin peptide family, increases cAMP levels, proliferation, and PKA phosphorylation of cholangiocytes (109).
Also, we demonstrated that GABA has heterogeneous effects on the apoptotic, proliferative, and secretory functions of small and large cholangiocytes in BDL rats. In fact we showed that chronic administration of GABA to BDL rats: induces apoptosis, reduces proliferation and decreases Ca2+-dependent adenylyl cyclase 8 (AC8) expression and secretin-stimulated choleresis in large cholangiocytes. Instead after GABA administration: small cholangiocytes de novo proliferate leading to an increased number of small ducts; show membrane translocation and phosphorylation of Ca2+-dependent PKCβII; and de novo express SR, CFTR, Cl-/HCO3- and AC8, and secrete water and electrolytes in response to secretin. During damage of large cholangiocytes, small ducts replenish the intrahepatic biliary tree by amplification of both Ca2+-dependent signaling and the acquisition of large cholangiocyte phenotypes (57).
Conclusions
In conclusion, as widely demonstrated, during chronic liver diseases, cholangiocytes are not quiescent but strongly activated cells constituting a neuroendocrine compartment, which is regulated by several and different factors including hormones, neuropeptides, cytokines, and growth factors. This neuroendocrine compartment produces and secretes several factors through which it regulates its own function and proliferation and also those of cells, which constitute the surrounding environment. Understand how to manage the neuroendocrine compartment and the several factors that this compartment is able to produce and secrete can be very important for the comprehension of progression of cholestatic liver diseases.
Acknowledgements
E. Gaudio was supported by research project grant from the University “Sapienza” of Rome and FIRB grant #RBAP10Z7FS_001 and by PRIN grant # 2009X84L84_001.
Disclosure: The authors declare no conflict of interest.
References
- Munshi MK, Priester S, Gaudio E, et al. Regulation of biliary proliferation by neuroendocrine factors: implications for the pathogenesis of cholestatic liver diseases. Am J Pathol 2011;178:472-84. [PubMed]
- Alpini G, Glaser SS, Ueno Y, et al. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol 1998;274:G767-75. [PubMed]
- LeSagE G, Alvaro D, Benedetti A, et al. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology 1999;117:191-9. [PubMed]
- Alpini G, Lenzi R, Sarkozi L, et al. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest 1988;81:569-78. [PubMed]
- Alpini G, Glaser S, Robertson W, et al. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol 1997;272:G1064-74. [PubMed]
- Alpini G, Prall RT, LaRusso NF. The pathobiology of biliary epithelia. In: Arias IM, Boyer JL, Chisari FV, et al. eds. The Liver; Biology & Pathobiology, 4th Ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2001:421-35.
- Alpini G, Roberts S, Kuntz SM, et al. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology 1996;110:1636-43. [PubMed]
- Kanno N, LeSage G, Glaser S, et al. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol 2001;281:G612-25. [PubMed]
- Nathanson MH, Boyer JL. Mechanisms and regulation of bile secretion. Hepatology 1991;14:551-66. [PubMed]
- Benedetti A, Bassotti C, Rapino K, et al. A morphometric study of the epithelium lining the rat intrahepatic biliary tree. J Hepatol 1996;24:335-42. [PubMed]
- Carpino F, Gaudio E, Marinozzi G, et al. A scanning and transmission electron microscopic study of experimental extrahepatic cholestasis in the rat. J Submicrosc Cytol 1981;13:581-98. [PubMed]
- Spee B, Carpino G, Schotanus BA, et al. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut 2010;59:247-57. [PubMed]
- Roskams TA, Theise ND, Balabaud C, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology 2004;39:1739-45. [PubMed]
- Gaudio E, Carpino G, Cardinale V, et al. New insights into liver stem cells. Dig Liver Dis 2009;41:455-62. [PubMed]
- Turner R, Lozoya O, Wang Y, et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology 2011;53:1035-45. [PubMed]
- Carruthers JS, Steiner JW. Studies on the fine structure of proliferated bile ductules. I. Changes of cytoarchitecture of biliary epithelial cells. Can Med Assoc J 1961;85:1223-36. [PubMed]
- Gaudio E, Franchitto A, Pannarale L, et al. Cholangiocytes and blood supply. World J Gastroenterol 2006;12:3546-52. [PubMed]
- Kanno N, LeSage G, Glaser S, et al. Functional heterogeneity of the intrahepatic biliary epithelium. Hepatology 2000;31:555-61. [PubMed]
- Glaser S, Alvaro D, Francis H, et al. Adrenergic receptor agonists prevent bile duct injury induced by adrenergic denervation by increased cAMP levels and activation of Akt. Am J Physiol Gastrointest Liver Physiol 2006;290:G813-26. [PubMed]
- Gaudio E, Pannarale L, Franchitto A, et al. Hepatic microcirculation as a morpho-functional basis for the metabolic zonation in normal and pathological rat liver. Ital J Anat Embryol 1995;100:419-28. [PubMed]
- Gaudio E, Pannarale L, Onori P, et al. A scanning electron microscopic study of liver microcirculation disarrangement in experimental rat cirrhosis. Hepatology 1993;17:477-85. [PubMed]
- Gaudio E, Barbaro B, Alvaro D, et al. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology 2006;130:1270-82. [PubMed]
- Gaudio E, Onori P, Pannarale L, et al. Hepatic microcirculation and peribiliary plexus in experimental biliary cirrhosis: a morphological study. Gastroenterology 1996;111:1118-24. [PubMed]
- Reilly FD, McCuskey PA, McCuskey RS. Intrahepatic distribution of nerves in the rat. Anat Rec 1978;191:55-67. [PubMed]
- Glaser S, Francis H, Demorrow S, et al. Heterogeneity of the intrahepatic biliary epithelium. World J Gastroenterol 2006;12:3523-36. [PubMed]
- Glaser SS, Gaudio E, Miller T, et al. Cholangiocyte proliferation and liver fibrosis. Expert Rev Mol Med 2009;11:e7. [PubMed]
- Alvaro D, Mancino MG, Glaser S, et al. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology 2007;132:415-31. [PubMed]
- Glaser SS, Gaudio E, Rao A, et al. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest 2009;89:456-69. [PubMed]
- Glaser S, Lam IP, Franchitto A, et al. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology 2010;52:204-14. [PubMed]
- Alpini G, Ulrich C, Roberts S, et al. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol 1997;272:G289-97. [PubMed]
- Alpini G, Ulrich CD 2nd, Phillips JO, et al. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. Am J Physiol 1994;266:G922-8. [PubMed]
- Lesage G, Glaser SS, Gubba S, et al. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology 1996;111:1633-44. [PubMed]
- Vacanti JP, Folkman J. Bile duct enlargement by infusion of L-proline: potential significance in biliary atresia. J Pediatr Surg 1979;14:814-8. [PubMed]
- LeSage GD, Benedetti A, Glaser S, et al. Acute carbon tetrachloride feeding selectively damages large, but not small, cholangiocytes from normal rat liver. Hepatology 1999;29:307-19. [PubMed]
- Lesage G, Glaser S, Ueno Y, et al. Regression of cholangiocyte proliferation after cessation of ANIT feeding is coupled with increased apoptosis. Am J Physiol Gastrointest Liver Physiol 2001;281:G182-90. [PubMed]
- Alpini G, Glaser SS, Rodgers R, et al. Functional expression of the apical Na+-dependent bile acid transporter in large but not small rat cholangiocytes. Gastroenterology 1997;113:1734-40. [PubMed]
- Alpini G, Glaser SS, Ueno Y, et al. Bile acid feeding induces cholangiocyte proliferation and secretion: evidence for bile acid-regulated ductal secretion. Gastroenterology 1999;116:179-86. [PubMed]
- Slott PA, Liu MH, Tavoloni N. Origin, pattern, and mechanism of bile duct proliferation following biliary obstruction in the rat. Gastroenterology 1990;99:466-77. [PubMed]
- LeSage GD, Glaser SS, Marucci L, et al. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol 1999;276:G1289-301. [PubMed]
- Desmet VJ. Current problems in diagnosis of biliary disease and cholestasis. Semin Liver Dis 1986;6:233-45. [PubMed]
- Lowes KN, Brennan BA, Yeoh GC, et al. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol 1999;154:537-41. [PubMed]
- Ren P, Silberg DG, Sirica AE. Expression of an intestine-specific transcription factor (CDX1) in intestinal metaplasia and in subsequently developed intestinal type of cholangiocarcinoma in rat liver. Am J Pathol 2000;156:621-7. [PubMed]
- Farber E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Cancer Res 1956;16:142-8. [PubMed]
- Roskams T, Cassiman D, De Vos R, et al. Neuroregulation of the neuroendocrine compartment of the liver. Anat Rec A Discov Mol Cell Evol Biol 2004;280:910-23. [PubMed]
- Alvaro D, Alpini G, Onori P, et al. Estrogens stimulate proliferation of intrahepatic biliary epithelium in rats. Gastroenterology 2000;119:1681-91. [PubMed]
- Pannarale L, Onori P, Ripani M, et al. Precapillary patterns and perivascular cells in the retinal microvasculature. A scanning electron microscope study. J Anat 1996;188:693-703. [PubMed]
- Glaser S, Benedetti A, Marucci L, et al. Gastrin inhibits cholangiocyte growth in bile duct-ligated rats by interaction with cholecystokinin-B/Gastrin receptors via D-myo-inositol 1,4,5-triphosphate-, Ca(2+)-, and protein kinase C alpha-dependent mechanisms. Hepatology 2000;32:17-25. [PubMed]
- Francis H, Glaser S, Ueno Y, et al. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol 2004;41:528-37. [PubMed]
- Alvaro D, Onori P, Metalli VD, et al. Intracellular pathways mediating estrogen-induced cholangiocyte proliferation in the rat. Hepatology 2002;36:297-304. [PubMed]
- Gigliozzi A, Alpini G, Baroni GS, et al. Nerve growth factor modulates the proliferative capacity of the intrahepatic biliary epithelium in experimental cholestasis. Gastroenterology 2004;127:1198-209. [PubMed]
- Alvaro D, Metalli VD, Alpini G, et al. The intrahepatic biliary epithelium is a target of the growth hormone/insulin-like growth factor 1 axis. J Hepatol 2005;43:875-83. [PubMed]
- Marzioni M, Glaser S, Francis H, et al. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology 2005;128:121-37. [PubMed]
- Francis H, Glaser S, Demorrow S, et al. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol 2008;295:C499-513. [PubMed]
- Alpini G, Franchitto A, Demorrow S, et al. Activation of alpha(1) -adrenergic receptors stimulate the growth of small mouse cholangiocytes via calcium-dependent activation of nuclear factor of activated T cells 2 and specificity protein 1. Hepatology 2011;53:628-39. [PubMed]
- Woo K, Sathe M, Kresge C, et al. Adenosine triphosphate release and purinergic (P2) receptor-mediated secretion in small and large mouse cholangiocytes. Hepatology 2010;52:1819-28. [PubMed]
- Francis HL, Demorrow S, Franchitto A, et al. Histamine stimulates the proliferation of small and large cholangiocytes by activation of both IP3/Ca2+ and cAMP-dependent signaling mechanisms. Lab Invest 2012;92:282-94. [PubMed]
- Mancinelli R, Franchitto A, Gaudio E, et al. After damage of large bile ducts by gamma-aminobutyric acid, small ducts replenish the biliary tree by amplification of calcium-dependent signaling and de novo acquisition of large cholangiocyte phenotypes. Am J Pathol 2010;176:1790-800. [PubMed]
- Desmet VJ, van Eyken P, Roskams T. Histopathology of vanishing bile duct diseases. Adv Clin Path 1998;2:87-99. [PubMed]
- Desmet VJ. Histopathology of chronic cholestasis and adult ductopenic syndrome. Clin Liver Dis 1998;2:249-64, viii. [PubMed]
- Harnois DM, Que FG, Celli A, et al. Bcl-2 is overexpressed and alters the threshold for apoptosis in a cholangiocarcinoma cell line. Hepatology 1997;26:884-90. [PubMed]
- Gatto M, Bragazzi MC, Semeraro R, et al. Cholangiocarcinoma: update and future perspectives. Dig Liver Dis 2010;42:253-60. [PubMed]
- Onori P, DeMorrow S, Gaudio E, et al. Caffeic acid phenethyl ester decreases cholangiocarcinoma growth by inhibition of NF-kappaB and induction of apoptosis. Int J Cancer 2009;125:565-76. [PubMed]
- DeMorrow S, Francis H, Gaudio E, et al. The endocannabinoid anandamide inhibits cholangiocarcinoma growth via activation of the noncanonical Wnt signaling pathway. Am J Physiol Gastrointest Liver Physiol 2008;295:G1150-8. [PubMed]
- Alvaro D, Gigliozzi A, Attili AF. Regulation and deregulation of cholangiocyte proliferation. J Hepatol 2000;33:333-40. [PubMed]
- Alvaro D, Onori P, Alpini G, et al. Morphological and functional features of hepatic cyst epithelium in autosomal dominant polycystic kidney disease. Am J Pathol 2008;172:321-32. [PubMed]
- Sirica AE, Cihla HP. Isolation and partial characterizations of oval and hyperplastic bile ductular cell-enriched populations from the livers of carcinogen and noncarcinogen-treated rats. Cancer Res 1984;44:3454-66. [PubMed]
- Yang S, Koteish A, Lin H, et al. Oval cells compensate for damage and replicative senescence of mature hepatocytes in mice with fatty liver disease. Hepatology 2004;39:403-11. [PubMed]
- Xiao JC, Ruck P, Adam A, et al. Small epithelial cells in human liver cirrhosis exhibit features of hepatic stem-like cells: immunohistochemical, electron microscopic and immunoelectron microscopic findings. Histopathology 2003;42:141-9. [PubMed]
- Roskams T, De Vos R, Van Eyken P, et al. Hepatic OV-6 expression in human liver disease and rat experiments: evidence for hepatic progenitor cells in man. J Hepatol 1998;29:455-63. [PubMed]
- Eagon PK, Porter LE, Francavilla A, et al. Estrogen and androgen receptors in liver: their role in liver disease and regeneration. Semin Liver Dis 1985;5:59-69. [PubMed]
- Alvaro D, Angelico M, Cantafora A, et al. Improvement of estradiol 17 beta-D-glucuronide cholestasis by intravenous administration of dimethylethanolamine in the rat. Hepatology 1991;13:1158-72. [PubMed]
- Alvaro D, Alpini G, Onori P, et al. Effect of ovariectomy on the proliferative capacity of intrahepatic rat cholangiocytes. Gastroenterology 2002;123:336-44. [PubMed]
- Alvaro D, Invernizzi P, Onori P, et al. Estrogen receptors in cholangiocytes and the progression of primary biliary cirrhosis. J Hepatol 2004;41:905-12. [PubMed]
- Beckmann MW, Jap D, Djahansouzi S, et al. Hormone replacement therapy after treatment of breast cancer: effects on postmenopausal symptoms, bone mineral density and recurrence rates. Oncology 2001;60:199-206. [PubMed]
- Reddy A, Prince M, James OF, et al. Tamoxifen: a novel treatment for primary biliary cirrhosis? Liver Int 2004;24:194-7. [PubMed]
- Alvaro D, Barbaro B, Franchitto A, et al. Estrogens and insulin-like growth factor 1 modulate neoplastic cell growth in human cholangiocarcinoma. Am J Pathol 2006;169:877-88. [PubMed]
- Bardin A, Hoffmann P, Boulle N, et al. Involvement of estrogen receptor beta in ovarian carcinogenesis. Cancer Res 2004;64:5861-9. [PubMed]
- Lau KM, LaSpina M, Long J, et al. Expression of estrogen receptor (ER)-alpha and ER-beta in normal and malignant prostatic epithelial cells: regulation by methylation and involvement in growth regulation. Cancer Res 2000;60:3175-82. [PubMed]
- Marzioni M, Torrice A, Saccomanno S, et al. An oestrogen receptor β-selective agonist exerts anti-neoplastic effects in experimental intrahepatic cholangiocarcinoma. Dig Liver Dis 2012;44:134-42. [PubMed]
- Yang F, Priester S, Onori P, et al. Castration inhibits biliary proliferation induced by bile duct obstruction: novel role for the autocrine trophic effect of testosterone. Am J Physiol Gastrointest Liver Physiol 2011;301:G981-91. [PubMed]
- Hinchliffe SA, Woods S, Gray S, et al. Cellular distribution of androgen receptors in the liver. J Clin Pathol 1996;49:418-20. [PubMed]
- Glaser S, DeMorrow S, Francis H, et al. Progesterone stimulates the proliferation of female and male cholangiocytes via autocrine/paracrine mechanisms. Am J Physiol Gastrointest Liver Physiol 2008;295:G124-G136. [PubMed]
- Taffetani S, Glaser S, Francis H, et al. Prolactin stimulates the proliferation of normal female cholangiocytes by differential regulation of Ca2+-dependent PKC isoforms. BMC Physiol 2007;7:6. [PubMed]
- Ulloa-Aguirre A, Uribe A, Zariñán T, et al. Role of the intracellular domains of the human FSH receptor in G(alphaS) protein coupling and receptor expression. Mol Cell Endocrinol 2007;260-262:153-62. [PubMed]
- Gonzales PH, Rhoden CR, Luz C, et al. Male gonadal function, prolactin secretion and lactotroph population in an experimental model of cirrhosis. Braz J Med Biol Res 2007;40:1383-8. [PubMed]
- Diamond T, Stiel D, Posen S. Osteoporosis in hemochromatosis: iron excess, gonadal deficiency, or other factors? Ann Intern Med 1989;110:430-6. [PubMed]
- Mancinelli R, Onori P, Gaudio E, et al. Follicle-stimulating hormone increases cholangiocyte proliferation by an autocrine mechanism via cAMP-dependent phosphorylation of ERK1/2 and Elk-1. Am J Physiol Gastrointest Liver Physiol 2009;297:G11-26. [PubMed]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 1995;16:3-34. [PubMed]
- Alvaro D, Macarri G, Mancino MG, et al. Serum and biliary insulin-like growth factor I and vascular endothelial growth factor in determining the cause of obstructive cholestasis. Ann Intern Med 2007;147:451-9. [PubMed]
- Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res 2001;49:568-81. [PubMed]
- Onori P, Wise C, Gaudio E, et al. Secretin inhibits cholangiocarcinoma growth via dysregulation of the cAMP-dependent signaling mechanisms of secretin receptor. Int J Cancer 2010;127:43-54. [PubMed]
- Glaser SS, Rodgers RE, Phinizy JL, et al. Gastrin inhibits secretin-induced ductal secretion by interaction with specific receptors on rat cholangiocytes. Am J Physiol 1997;273:G1061-70. [PubMed]
- Kanno N, Glaser S, Chowdhury U, et al. Gastrin inhibits cholangiocarcinoma growth through increased apoptosis by activation of Ca2+-dependent protein kinase C-alpha. J Hepatol 2001;34:284-91. [PubMed]
- Tietz PS, Alpini G, Pham LD, et al. Somatostatin inhibits secretin-induced ductal hypercholeresis and exocytosis by cholangiocytes. Am J Physiol 1995;269:G110-8. [PubMed]
- Tan CK, Podila PV, Taylor JE, et al. Human cholangiocarcinomas express somatostatin receptors and respond to somatostatin with growth inhibition. Gastroenterology 1995;108:1908-16. [PubMed]
- Francis H, Franchitto A, Ueno Y, et al. H3 histamine receptor agonist inhibits biliary growth of BDL rats by downregulation of the cAMP-dependent PKA/ERK1/2/ELK-1 pathway. Lab Invest 2007;87:473-87. [PubMed]
- Francis H, DeMorrow S, Venter J, et al. Inhibition of histidine decarboxylase ablates the autocrine tumorigenic effects of histamine in human cholangiocarcinoma. Gut 2012;61:753-64. [PubMed]
- Renzi A, Glaser S, Demorrow S, et al. Melatonin inhibits cholangiocyte hyperplasia in cholestatic rats by interaction with MT1 but not MT2 melatonin receptors. Am J Physiol Gastrointest Liver Physiol 2011;301:G634-43. [PubMed]
- Kirimlioglu H, Ecevit A, Yilmaz S, et al. Effect of resveratrol and melatonin on oxidative stress enzymes, regeneration, and hepatocyte ultrastructure in rats subjected to 70% partial hepatectomy. Transplant Proc 2008;40:285-9. [PubMed]
- Tahan G, Akin H, Aydogan F, et al. Melatonin ameliorates liver fibrosis induced by bile-duct ligation in rats. Can J Surg 2010;53:313-8. [PubMed]
- Esrefoglu M, Gül M, Emre MH, et al. Protective effect of low dose of melatonin against cholestatic oxidative stress after common bile duct ligation in rats. World J Gastroenterol 2005;11:1951-6. [PubMed]
- Han Y, Demorrow S, Invernizzi P, et al. Melatonin exerts by an autocrine loop antiproliferative effects in cholangiocarcinoma: its synthesis is reduced favoring cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol 2011;301:G623-33. [PubMed]
- Joplin R, Hishida T, Tsubouchi H, et al. Human intrahepatic biliary epithelial cells proliferate in vitro in response to human hepatocyte growth factor. J Clin Invest 1992;90:1284-9. [PubMed]
- Alvaro D, Alpini G, Jezequel AM, et al. Role and mechanisms of action of acetylcholine in the regulation of rat cholangiocyte secretory functions. J Clin Invest 1997;100:1349-62. [PubMed]
- Marzioni M, Ueno Y, Glaser S, et al. Cytoprotective effects of taurocholic acid feeding on the biliary tree after adrenergic denervation of the liver. Liver Int 2007;27:558-68. [PubMed]
- Alpini G, Glaser S, Robertson W, et al. Bile acids stimulate proliferative and secretory events in large but not small cholangiocytes. Am J Physiol 1997;273:G518-29. [PubMed]
- Glaser S, Alvaro D, Roskams T, et al. Dopaminergic inhibition of secretin-stimulated choleresis by increased PKC-gamma expression and decrease of PKA activity. Am J Physiol Gastrointest Liver Physiol 2003;284:G683-94. [PubMed]
- Glaser SS, Ueno Y, DeMorrow S, et al. Knockout of alpha-calcitonin gene-related peptide reduces cholangiocyte proliferation in bile duct ligated mice. Lab Invest 2007;87:914-26. [PubMed]
- Glaser S, Gaudio E, Renzi A, et al. Knockout of the neurokinin-1 receptor reduces cholangiocyte proliferation in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol 2011;301:G297-305. [PubMed]


