Enhancing circadian rhythms—the circadian MEGA bundle as novel approach to treat critical illness
Introduction
Sunlight, and the day-night cycles it creates on Earth, underlies the evolutionary biochemistry of all living organisms (1-4). During the past two decades, research has begun to clarify the crucial role of the molecular circadian clock in human physiology, and conversely, the impact of circadian disruptions on disease development and severity (5). Research on circadian rhythms has particularly increased within the realm of critical care medicine, which addresses the care of patients admitted to the hospital with life-threatening organ dysfunction. These patients are often confined to the intensive care unit (ICU) for weeks, and their circadian rhythms are affected by the severity of their illness, the chaotic ICU environment, and therapeutic interventions which may occur at any time, day or night. Restoring circadian homeostasis has the potential to improve ICU clinical outcomes, but optimal therapeutic strategies have yet to be elucidated (6).
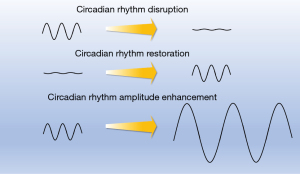
Research to date has centered on two important concepts: circadian disruption on the one hand, and circadian amplitude enhancement on the other (Figure 1). Circadian disruption refers to changes in the timing of biological rhythms at the cellular level (e.g., changes in expression of clock genes), the tissue/organ level (e.g., changes in the timing of melatonin release from the pineal gland), and/or the behavioral level (e.g., jet lag leading to changes in sleep timing and quality). These disruptions are known to occur due to changes in light signaling, although other causes are likely. Circadian disruptions may encompass phase shifts or changes in circadian amplitude; the exact mechanisms are not fully understood. Nevertheless, circadian disruption can have profound adverse metabolic consequences (7) and lead to diabetes (8), obesity (9), cardiovascular diseases (10), gastrointestinal disorders (11), impaired immune function (5) and depression (12).
Circadian amplitude enhancement refers to increasing the peak and the trough of a circadian cycle. Circadian amplitude enhancement has been associated with improved health, including reversal of a metabolic syndrome (13) and protection from myocardial ischemia (14). However, while research has not elucidated the mechanism behind circadian amplitude enhancement (15), strategies to enhance the circadian amplitude have been well established. Most of the time clinicians and researchers would not make the connection though. A classic example is timed exercise (16) or timed fastening (17). Surprisingly, many circadian amplitude enhancing strategies have never been tested in a clinical environment.
The global burden of critical illness and resulting need for ICU beds is anticipated to increase in coming decades (18), highlighting the importance of research to mitigate the dangers of the ICU environment and improve clinical outcomes for critically ill patients. Developing frameworks for care and therapeutic interventions through a circadian lens may provide a new angle for the future of critical care research.
Genetic underpinnings of circadian rhythms
A key feature of the circadian system is light synchronization (2,19), with sunlight as the dominant zeitgeber, or timekeeper. Zeitgebers synchronize the human circadian clock to the environment and maintain the 24-hour period. In fact, the human circadian clock is predominantly entrained by sun time rather than by social time (20). Sunlight activates retinal melanopsin receptors and signals the suprachiasmatic nuclei (SCN) via the retinohypothalamic tract. In the SCN, the signal is transmitted to the molecular clockwork (21). Without diurnal light signals, biological systems revert to an internal free-running clock, which is generally slightly longer than the 24-hour day and eventually fades in amplitude and periodicity over time (22). As anyone who has taken a long flight and then experienced the insomnia of jet lag will recognize, disruption of the circadian cycle can lead to changes in physiologic function.
Given that more than half of the protein-coding genome is rhythmic (23), circadian rhythms play an important role in almost all facets of human physiology (24-28) and accordingly, many pathophysiologic disease states also follow circadian cycles. For example, epidemiological data reveal that adverse cardiovascular events, including myocardial infarction (29), ventricular arrhythmias (30), and sudden cardiac death (31) all occur predominantly in the morning. Moreover, circadian disruption most likely contributes to disease progression and disease severity. While circadian disruption caused by, e.g., night shift work, can increase the incidence of cardiovascular events, original research indicated that night shift contributed to the disruption of circadian protein expression in breast tissue suggesting a contribution to cancer development. Indeed, it is now established that disruption of circadian rhythms plays a key role in tumorigenesis and facilitates the development of cancer (32-38) and that pharmacological modulators of the circadian clock could be potentially therapeutic (39). Here, circadian-based treatments could modulate the pharmacological ability of anti-cancer drugs towards improving therapeutic outcomes and be potentially incorporated into clinical trials for treatment optimization and improved patient survival. In fact, the circadian clock informs about the optimal timing and dosing of the drug, which is novel when compared to traditional pharmacotherapy. In addition, it has been found that a circadian rhythm-related genes-based scoring system can help to assess the prognosis of patients with lung adenocarcinoma (40). Another critical role of the circadian clock is to coordinate functions of the immune system (41-47). Thus, the physiology of immune cells, host-parasite interactions, inflammatory processes, or adaptive immune responses are time-of-day dependent (43).
Basic science research has contributed greatly to our current understanding of circadian rhythmicity and disease states, namely through the identification of a variety of genes, proteins, and transcription factors associated with circadian regulation. The circadian clock protein, period circadian regulator 2 (PER2), for example, is directly induced by light exposure (48). Murine models have shown that PER2 is not only involved in day-night cycles, but also endothelial function. Endothelial cells of Per2-knockout mice have decreased endothelial nitric oxide synthase activation, which has been associated with aortic endothelial dysfunction, impaired blood flow recovery, and increased risk of auto-amputation after hind limb ischemia in mice (49). Endothelial-specific Per2 knockout animals also showed increased vascular permeability and larger infarct sizes following myocardial ischemia (14). Some of these findings were attributed to impaired endothelial progenitor cell function as a result of the PER2 knockout (49). Murine models have further shown that the transcription factor CLOCK is one of the key components of circadian regulation within pacemaker neurons of the hypothalamic SCN. Interestingly, deletion of the CLOCK gene results in hyperphagia and obesity, leading to a metabolic syndrome of hyperleptinemia, hyperlipidemia, hepatic steatosis, hyperglycemia, and hypoinsulinemia (50).
While genetic data from mice are abundant, genetic studies on circadian rhythmicity and disease states in humans are less common. The existing literature confirms what has been shown in murine models, namely that circadian clock genes exert effects not just on behavior (e.g., sleep) but also key elements of homeostasis. For example, genetic studies revealed that polymorphisms in the PER2 gene are associated with an increased risk for type 2 diabetes and arterial hypertension (51,52). Genome-wide association studies have demonstrated that melatonin, a hormone used as a marker of the circadian phase, may be involved in blood glucose regulation (53). Table 1 summarizes known single nucleotide polymorphism in circadian rhythms genes and their association with diseases development in humans. Regardless, synchronization to a diurnal schedule is key to human homeostasis, and this is regulated through a complex set of genetic and molecular clocks. Indeed, if circadian rhythms are disrupted, e.g., via sleep deprivation, a diabetic phenotype (8), weight gain (9), changes in body composition, melatonin levels, and insulin resistance (71) can be found in healthy volunteers and most likely contributes to disease development in critical ill patients.
Table 1
| Gene | Association with human diseases | References |
|---|---|---|
| CLOCK | Energy intake, metabolic syndrome, obesity, overweight, BMI, type-2 diabetes, coronary heart disease related dyslipidemia, non-alcoholic fatty liver disease | (54-59) |
| BMAL1/ARNTL | Type 2 diabetes, hypertension, seasonal affective disorder, metabolic syndrome | (60,61) |
| CRY1 | Insulin resistance, type-2 diabetes | (62,63) |
| CRY2 | Type-2 diabetes, fasting glucose, glucose metabolism, HDL cholesterol | (64-67) |
| PER1 | Hepatocellular carcinoma | (68) |
| PER2 | Type-2 diabetes, high fasting blood glucose | (63,69) |
| NPAS2 | Hypertension | (69) |
| MTNR1B | Gestational diabetes mellitus, type-2 diabetes, fasting glucose, hemoglobin A1C, insulin resistance, birth weight, obesity-related traits | (64,70) |
SNP, single nucleotide polymorphism; BMI, body mass index; HDL, high-density lipoprotein.
Circadian amplitude enhancement
Circadian entrainment to diurnal light cycles is imperative to physiologic health, as described above. However, synchronization with external zeitgebers is not the only contributor to circadian health. Recent studies have shown that enhancing the amplitude of circadian cycles has significant effects on health and wellbeing (2,3,72-75). In contrast, dampening of the circadian amplitude is a part of the natural aging process with associated decline in organ function (76). Interventions that amplify circadian rhythms are still a nascent area of research but include light exposure and cyclical feeding amongst others.
Exposure to bright light amplifies PER2 expression in both mice and humans (14). Although this amplitude enhancement likely has multiple effects throughout the body, the literature thus far demonstrates decisively that bright light exposure and associated circadian amplitude enhancement mitigates cardiac injury after ischemia, protects from lung injury or completely resolves a metabolic syndrome in mice (13,14,77). This amplitude enhancement is dependent on the retina-melanopsin signaling pathway as it is abolished in blind mice (14,77). In contrast, cyclical feeding schedules also amplify circadian amplitude and improve physiologic function but do not rely on light signaling (78). For example, time-restricted feeding without reduction in caloric intake protected against excessive weight gain, adverse cardiac events, and improved sleep quality in mouse and fruit fly models (79-81). Indeed, human studies have confirmed that timed or restricted meals promote weight loss and improvement in energy metabolism (82).
Circadian disruption in critical illness
Multiorgan dysfunction defines most critical illness, and it is somewhat obvious that physiologic homeostasis and circadian rhythmicity is disrupted in this context. The hospital and ICU environment are also culprits for circadian disruption, given the constant ambient light and noise and around-the-clock nursing interventions. It is difficult to distinguish which causes more harm, the illness, or the intervention, but first we must demonstrate that circadian cycles are indeed disrupted in critical illness. Almost all circadian studies focusing on critical illness have occurred over the past two decades, and most use either actigraphy monitoring or melatonin levels as surrogates for circadian cycles; this may not be sufficient. Recent transcriptomic work has shown that gene expression patterns rapidly (within 24 hours) become abnormal during critical illness compared to healthy controls, but these patterns were not detectable in actigraphy or melatonin levels (83). Transcriptomic studies have demonstrated that critical illness is both a cause and a product of circadian misalignment. Interventions to restore and amplify circadian rhythms should be employed as early as possible in the disease course to avoid downstream complications and should target both the illness and the environment. Interventions in critical illness that have been mainly investigated to date include intense light therapy, melatonin, and sleep support.
Intense light therapy
Given our species’ dependence on sunlight, light cycling seems an obvious target to improve the ICU environment and patients’ circadian rhythmicity. Light must be at least 180 lux (i.e., the light level typically encountered in public stairwells) to entrain the human circadian system (84), and intense light (>10,000 lux, i.e., ambient sunlight) is most effective. For reference, direct sunlight ranges from 32,000 to 100,000 lux on Earth, indicating that most humans are light deprived in their modern environment.
There have been numerous studies of light cycling in the ICU environment to improve clinical outcomes. Many of these studies have focused on entraining circadian physiology to improve neurocognitive outcomes such as delirium. Outcomes have been mixed. While some studies have found that bright-light therapy reduced the incidence of postoperative delirium among ICU patients (85,86), others have not confirmed this association (87,88). The discordance could be explained by study design. Most studies of bright-light therapy in the ICU have been small and single-center; the study populations and primary outcomes are heterogeneous; the light therapy varies in both wavelength (i.e., blue component) and intensity (from 300 lux to >10,000 lux) and is applied in different time intervals and increments. Finally, no data exist which shows that light therapy is in fact effective in patients. In fact, a robust lab value which could be monitored to demonstrate effectiveness of light therapy has still to be established.
Neurocognitive outcomes also may not be the best metric of circadian physiology and other targets should be considered. Studies on healthy human subjects may help to identify appropriate clinical outcome metrics. In a prospective study, healthy human volunteers were exposed to 30 min of intense light or normal room light in the morning on 5 consecutive days and a blood and buccal tissue sample were taken after light therapy each day. Intense light therapy increased PER2 protein levels in both plasma and buccal tissue at both 9am and 9pm. The evening increase in PER2 levels was greater, which suggests that PER2 induction is possible hours after intense light therapy and that the circadian amplitude may have been increased (14). Plasma melatonin levels and triglycerides were also suppressed by intense light therapy in comparison to controls (14). A targeted metabolomics screen from these human plasma samples was performed to better characterize the effects of intense light on human metabolism. These studies found that intense light regulated intermediates of glycolysis and the Krebs cycle, indicating that light improved glucose metabolism (14). Given the known correlation between sleep quality and insulin sensitivity (75), light therapy may improve both sleep and glucose control. Future ICU research may utilize these clinical outcomes in addition to neurocognitive disorders. While bright-light therapy likely has some promise as a circadian restoration or amplification technique, the literature does not yet support its routine use in the ICU and remains insufficient to guide specific therapeutic interventions.
Melatonin therapy
Melatonin has long been considered the gold standard to measure circadian rhythms in humans, e.g., the measurement of the dim light melatonin onset is key to diagnose circadian phase and related sleep disorders (89). As circadian rhythms are disrupted in critical illness, so is melatonin cycling (90). Meta-analyses of clinical trials have suggested that melatonin therapy improves sleep quality and reduces the incidence of delirium in ICU patients (91-94). Unfortunately, despite this evidence, the recent Pro-MEDIC trial of prophylactic melatonin to reduce delirium incidence did not support the routine early use of melatonin in critical illness (95). As with light therapy, differences in outcomes may be attributable to heterogeneity in study design; melatonin doses in these studies ranged from 3 to 30 mg nightly while some studies used the melatonin receptor-agonist ramelteon. Included patient populations were also heterogeneous. It is also possible that because delirium is a multifactorial endpoint, it may not be the best proxy for circadian disruption and/or restoration. The use of melatonin to improve circadian rhythms in the ICU is still in its infancy and ongoing research may improve with more standardization and the use of different outcomes (96,97).
Sleep support
Sleep is possibly a manifestation of circadian physiology (98). Critically ill patients are vulnerable to sleep disturbances including increased sleep latency, increased arousals, decreased rapid-eye-motion (REM) and decreased slow-wave sleep (99). Despite the established importance of sleep to overall health, it remains difficult to study sleep in critically ill patients. While new sleep scoring criteria are an intense area of research, many have yet to be validated (100,101). Nevertheless, sleep disturbances are associated with increased morbidity and mortality from critical illness (99).
While studies of sleep in the ICU have not focused on circadian physiology per se and a full review of sleep and sedation in critical illness and how they relate to circadian health is outside the scope of this review, interventions aimed at improving sleep are often employed to address circadian health in the ICU. Additionally, although we discuss the literature on melatonin therapy separately in this review, it is often included in sleep hygiene bundles. Non-pharmaceutical interventions to improve sleep include promoting daytime wakefulness, and the use of earplugs, eye masks, reductions in ICU noise, and reductions in nursing interventions during night hours. While one study found that sleep quality and duration was improved with the use of earplugs (102), another study reported no improvement in sleep architecture (103). More often, sleep hygiene interventions are applied together in the form of a bundle. In a relatively large prospective cohort study of medical and surgical ICU patients in the United Kingdom, Patel et al. demonstrated that a >90% compliance to an evidence-based sleep hygiene bundle improved patient sleep quality and reduced the incidence of delirium (104), which has been replicated several times (105-107). These data are valuable, but do not explicitly focus on the restoration or amplification of circadian rhythmicity. Efforts to connect sleep hygiene with circadian physiology may lead to broader applications of sleep hygiene for recovery from critical illness.
Cyclic nutrition support
Nutrition is a critical component of physiologic homeostasis and depends on circadian entrainment. Nutrition in the ICU is paramount, as many of these patients are frail or malnourished at baseline and prolonged immobility combined with poor oral intake exacerbates their poor nutritional status. Critical illness also compromises gastric motility and bowel absorption. As a result, many critically ill patients need nutritional support.
The timing of nutritional support in the ICU may preserve, restore, and/or even amplify circadian rhythms (108). Eating is regulated by the circadian clock in the SCN. Food intake must be synchronized with SCN-driven endocrine signals (109) in order to maintain metabolic rhythms in peripheral tissues and organs (110). Research on nighttime shift workers and volunteers has confirmed this, showing for example that nocturnal eating promotes glucose intolerance and hypertriglyceridemia (111-113). Nevertheless, ICU patients that require nutrition support generally receive tube feeds continuously, during the day and night. Clinical trials evaluating continuous versus cycled nutrition support in the ICU have understandably focused on caloric intake goals rather than circadian physiology. Intermittent feeding/fasting decreases insulin requirements but may have negligible effects on blood glucose levels, and the effects on gastric emptying and muscle metabolism/catabolism are inconclusive (108). Continuous feeding may be an iatrogenic circadian disruption and focused research on its association with circadian physiology is warranted.
Physical exercise
Physical exercise has been shown to entrain and amplify the circadian system in animals (114,115). Early mobilization is considered an essential component of ICU care (116), but this is not generally attributed to effects on circadian rhythmicity. While physical therapy during the ICU stay has been thoroughly studied, no studies exist on timed exercise to specifically promote circadian rhythmicity. Studies of intense light therapy using actigraphy monitors confirm that light therapy increases healthy volunteers’ day-time activity which also increases their circadian amplitude. Thus, physical exercise may have broader implications for circadian rhythms, and physical exercise during specific time periods should be prioritized for future ICU research with a focus on circadian rhythm restoration and amplification.
Other interventions
Temperature
Circadian rhythms are complex in both origin and downstream effects, and the development of novel therapies to restore and amplify these rhythms in critically ill patients will depend on focused research. Components of circadian health which to date have been almost completely overlooked in the critical care literature include temperature regulation and medication timing.
Temperature was one of the first variables identified to have a circadian rhythm in humans, in the 1800s (117). Core body temperature is integral to chemical processes and protein stability and is regulated by both the hypothalamus and the liver. The timing of daily temperature variation is integral to sleep and reveals the phase shift of circadian rhythms (118). Targeted temperature management became the focus of intense scrutiny to improve neurological outcomes after cardiac arrest research over the past decade (119), and yet broader applications in the ICU have not been broached.
Medications
Critically ill patients are almost universally exposed to polypharmacy, and complex medication administration patterns in an unpredictable, high-acuity environment are often subject to clinical workflow constraints. It is not well known that many widely used medications in the United States target circadian gene products (23) and as such the timing of administration, or chronotherapy, is critical. For example, studies on this topic in the mid-1990s showed that rhythmically delivered chemotherapy could reduce toxicity in colorectal cancer patients (120). However, this did not lead to changes in labeling by the U.S. Food and Drug Administration (FDA), treatment guidelines, or standards of practice and only four of the 50 currently most prescribed drugs have FDA-labeled time-of-day dosing recommendation (121). Moreover, most best-selling drugs in the United States target a circadian gene product (23). Thus, application of chronotherapy principles for the countless medications utilized in the ICU, and the effects on circadian health, remain unknown but may be clinically important.
Novel therapeutic concept in critical illness: the circadian MEGA bundle
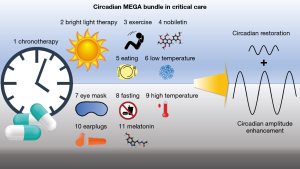
Maintaining circadian entrainment is critical for overall health, but beyond maintenance, therapeutic circadian amplitude enhancement has emerged as a potential protective mechanism in different settings (13,79) and is currently under intense investigation (15,122,123) (Figures 1,2). Circadian amplitude enhancement can be achieved by timed light therapy, timed exercise, timed feeding or giving circadian amplitude enhancing molecules at the appropriate time as shown by numerous studies in animals (13-15,77,124-126). Whether timed temperature changes or timed administration of drugs could have similar effects is currently unknown. However, this seems very likely given what is known about circadian entrainment. In any case, existing data do support the potential benefit of a “MEGA bundle” strategy for the ICU where all the above-mentioned circadian rhythm targeting parameters are coordinated. Published data also supports the addition of a pharmacologic adjunct to the bundle that can reset, synchronize, and increase the circadian amplitude. Several of these circadian amplitude-enhancing small molecules were identified in studies utilizing a high throughput screen (127). One of the natural compounds identified was the flavonoid nobiletin, which robustly enhances the circadian amplitude (13). Since then nobiletin has been shown to protect against the metabolic syndrome (13), midazolam induced delirium (122), and ischemia/reperfusion injury of the heart (128,129) liver (130) and kidneys (131) in mice. Human studies are rare but have demonstrated that nobiletin-containing food is beneficial for improving memory dysfunction in healthy elderly subjects (132) and can protect isolated human islets against hypoxia and oxidative stress-induced apoptosis (133). Moreover, a growing body of laboratory studies and randomized trials support cardiometabolic benefits of flavonoid-rich foods such as cocoa, tea, and berries. Flavonoid-rich cocoa produces small but measurable benefits on blood pressure (BP), endothelial function, insulin resistance, and blood lipids (134-136). How nobiletin or flavonoids more generally affect circadian rhythms in humans, however, remains incompletely understood. As shown in Figure 2, based on current evidence, we propose not only to administer bright light during the day, initiate timed feeding, timed physical and occupational therapy, timed interventions, and timed medication administration, but also to protocolize control of ambient temperatures and to administer drugs that could enhance the circadian amplitude.
Conclusions
Circadian entrainment and amplitude enhancement are integral to patients’ overall health and well-being, and likely even more important during response to and recovery from critical illness. It is imperative that future mechanistic research continue to confirm that circadian re-alignment and amplification are achieved by a given intervention before we can move to more robust studies of clinical endpoints. In the meantime, despite uncertainty regarding specific therapeutic best practice, many basic interventions to promote circadian concordance are relatively simple, inexpensive, and unlikely to cause significant harm. Many circadian health strategies overlap, and we propose the use of a mega-bundle for ICU circadian health, to include intense light therapy each morning, cyclic nutrition support, timed physical therapy, nighttime melatonin administration, morning administration of circadian rhythm amplitude enhancers, cyclic temperature control and a nocturnal sleep hygiene bundle. This will require multidisciplinary collaboration and institutional support, but the global public health implications might be immense. Although more study is needed, we strongly believe that restoration and amplification of circadian rhythms will result in improved outcomes for critically ill patients.
Acknowledgments
Funding: Research reported in this publication was supported by the National Heart, Lung, and Blood Institute and National Institute of Aging of the National Institutes of Health under Award Number R56HL156955 and R03AG078956. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Translational Medicine for the series “Highlights in Anesthesia and Critical Care Medicine”. The article has undergone external peer review.
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5127/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5127/coif). The series “Highlights in Anesthesia and Critical Care Medicine” was commissioned by the editorial office without any funding or sponsorship. TE served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Translational Medicine from November 2021 to October 2023. BS served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bartman CM, Eckle T. Circadian-Hypoxia Link and its Potential for Treatment of Cardiovascular Disease. Curr Pharm Des 2019;25:1075-90. [Crossref] [PubMed]
- Brainard J, Gobel M, Scott B, et al. Health implications of disrupted circadian rhythms and the potential for daylight as therapy. Anesthesiology 2015;122:1170-5. [Crossref] [PubMed]
- Brainard J, Gobel M, Bartels K, et al. Circadian rhythms in anesthesia and critical care medicine: potential importance of circadian disruptions. Semin Cardiothorac Vasc Anesth 2015;19:49-60. [Crossref] [PubMed]
- Bonney S, Hughes K, Harter PN, et al. Cardiac period 2 in myocardial ischemia: clinical implications of a light dependent protein. Int J Biochem Cell Biol 2013;45:667-71. [Crossref] [PubMed]
- Fishbein AB, Knutson KL, Zee PC. Circadian disruption and human health. J Clin Invest 2021;131:e148286. [Crossref] [PubMed]
- Jobanputra AM, Scharf MT, Androulakis IP, et al. Circadian Disruption in Critical Illness. Front Neurol 2020;11:820. [Crossref] [PubMed]
- Sletten TL, Cappuccio FP, Davidson AJ, et al. Health consequences of circadian disruption. Sleep 2020;43:zsz194. [Crossref] [PubMed]
- Van Cauter E, Blackman JD, Roland D, et al. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest 1991;88:934-42. [Crossref] [PubMed]
- Krueger PM, Reither EN, Peppard PE, et al. Cumulative exposure to short sleep and body mass outcomes: a prospective study. J Sleep Res 2015;24:629-38. [Crossref] [PubMed]
- Chellappa SL, Vujovic N, Williams JS, et al. Impact of Circadian Disruption on Cardiovascular Function and Disease. Trends Endocrinol Metab 2019;30:767-79. [Crossref] [PubMed]
- Voigt RM, Forsyth CB, Keshavarzian A. Circadian rhythms: a regulator of gastrointestinal health and dysfunction. Expert Rev Gastroenterol Hepatol 2019;13:411-24. [Crossref] [PubMed]
- Logan RW, McClung CA. Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci 2019;20:49-65. [Crossref] [PubMed]
- He B, Nohara K, Park N, et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab 2016;23:610-21. [Crossref] [PubMed]
- Oyama Y, Bartman CM, Bonney S, et al. Intense Light-Mediated Circadian Cardioprotection via Transcriptional Reprogramming of the Endothelium. Cell Rep 2019;28:1471-1484.e11. [Crossref] [PubMed]
- Gloston GF, Yoo SH, Chen ZJ. Clock-Enhancing Small Molecules and Potential Applications in Chronic Diseases and Aging. Front Neurol 2017;8:100. [Crossref] [PubMed]
- Gabriel BM, Zierath JR. Circadian rhythms and exercise - re-setting the clock in metabolic disease. Nat Rev Endocrinol 2019;15:197-206. [Crossref] [PubMed]
- Adafer R, Messaadi W, Meddahi M, et al. Food Timing, Circadian Rhythm and Chrononutrition: A Systematic Review of Time-Restricted Eating's Effects on Human Health. Nutrients 2020;12:3770. [Crossref] [PubMed]
- Halpern NA, Goldman DA, Tan KS, et al. Trends in Critical Care Beds and Use Among Population Groups and Medicare and Medicaid Beneficiaries in the United States: 2000-2010. Crit Care Med 2016;44:1490-9. [Crossref] [PubMed]
- Jakubcakova V, Oster H, Tamanini F, et al. Light entrainment of the mammalian circadian clock by a PRKCA-dependent posttranslational mechanism. Neuron 2007;54:831-43. [Crossref] [PubMed]
- Roenneberg T, Kumar CJ, Merrow M. The human circadian clock entrains to sun time. Curr Biol 2007;17:R44-5. [Crossref] [PubMed]
- Takahashi JS, Hong HK, Ko CH, et al. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet 2008;9:764-75. [Crossref] [PubMed]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature 2002;418:935-41. [Crossref] [PubMed]
- Zhang R, Lahens NF, Ballance HI, et al. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A 2014;111:16219-24. [Crossref] [PubMed]
- Weber MA, Drayer JI, Nakamura DK, et al. The circadian blood pressure pattern in ambulatory normal subjects. Am J Cardiol 1984;54:115-9. [Crossref] [PubMed]
- Degaute JP, van de Borne P, Linkowski P, et al. Quantitative analysis of the 24-hour blood pressure and heart rate patterns in young men. Hypertension 1991;18:199-210. [Crossref] [PubMed]
- Angleton P, Chandler WL, Schmer G. Diurnal variation of tissue-type plasminogen activator and its rapid inhibitor (PAI-1). Circulation 1989;79:101-6. [Crossref] [PubMed]
- Otto ME, Svatikova A, Barretto RB, et al. Early morning attenuation of endothelial function in healthy humans. Circulation 2004;109:2507-10. [Crossref] [PubMed]
- Turton MB, Deegan T. Circadian variations of plasma catecholamine, cortisol and immunoreactive insulin concentrations in supine subjects. Clin Chim Acta 1974;55:389-97. [Crossref] [PubMed]
- Muller JE, Stone PH, Turi ZG, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 1985;313:1315-22. [Crossref] [PubMed]
- Twidale N, Taylor S, Heddle WF, et al. Morning increase in the time of onset of sustained ventricular tachycardia. Am J Cardiol 1989;64:1204-6. [Crossref] [PubMed]
- Willich SN, Goldberg RJ, Maclure M, et al. Increased onset of sudden cardiac death in the first three hours after awakening. Am J Cardiol 1992;70:65-8. [Crossref] [PubMed]
- Lee Y. Roles of circadian clocks in cancer pathogenesis and treatment. Exp Mol Med 2021;53:1529-38. [Crossref] [PubMed]
- Sulli G, Lam MTY, Panda S. Interplay between Circadian Clock and Cancer: New Frontiers for Cancer Treatment. Trends Cancer 2019;5:475-94. [Crossref] [PubMed]
- Kinouchi K, Sassone-Corsi P. Metabolic rivalry: circadian homeostasis and tumorigenesis. Nat Rev Cancer 2020;20:645-61. [Crossref] [PubMed]
- Puppala A, Rankawat S, Ray S. Circadian Timekeeping in Anticancer Therapeutics: An Emerging Vista of Chronopharmacology Research. Curr Drug Metab 2021;22:998-1008. [Crossref] [PubMed]
- Ercolani L, Ferrari A, De Mei C, et al. Circadian clock: Time for novel anticancer strategies? Pharmacol Res 2015;100:288-95. [Crossref] [PubMed]
- Soták M, Sumová A, Pácha J. Cross-talk between the circadian clock and the cell cycle in cancer. Ann Med 2014;46:221-32. [Crossref] [PubMed]
- Kaur P, Mohamed NE, Archer M, et al. Impact of Circadian Rhythms on the Development and Clinical Management of Genitourinary Cancers. Front Oncol 2022;12:759153. [Crossref] [PubMed]
- Antoch MP, Kondratov RV. Pharmacological modulators of the circadian clock as potential therapeutic drugs: focus on genotoxic/anticancer therapy. Handb Exp Pharmacol 2013;289-309. [Crossref] [PubMed]
- Zhang F, Zhang Q, Jiang T, et al. Characteristics of circadian rhythm-related genes and establishment of a prognostic scoring system for lung adenocarcinoma with experimental verification: a bioinformatics analysis. J Thorac Dis 2022;14:3934-54. [Crossref] [PubMed]
- Wang C, Lutes LK, Barnoud C, et al. The circadian immune system. Sci Immunol 2022;7:eabm2465. [Crossref] [PubMed]
- Fagiani F, Di Marino D, Romagnoli A, et al. Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transduct Target Ther 2022;7:41. [Crossref] [PubMed]
- Orozco-Solis R, Aguilar-Arnal L. Circadian Regulation of Immunity Through Epigenetic Mechanisms. Front Cell Infect Microbiol 2020;10:96. [Crossref] [PubMed]
- Ballesta A, Innominato PF, Dallmann R, et al. Systems Chronotherapeutics. Pharmacol Rev 2017;69:161-99. [Crossref] [PubMed]
- Wang T, Wang Z, Yang P, et al. PER1 prevents excessive innate immune response during endotoxin-induced liver injury through regulation of macrophage recruitment in mice. Cell Death Dis 2016;7:e2176. [Crossref] [PubMed]
- Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol 2013;13:190-8. [Crossref] [PubMed]
- Li CX, Liang DD, Xie GH, et al. Altered melatonin secretion and circadian gene expression with increased proinflammatory cytokine expression in early-stage sepsis patients. Mol Med Rep 2013;7:1117-22. [Crossref] [PubMed]
- Eckle T, Hartmann K, Bonney S, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med 2012;18:774-82. [Crossref] [PubMed]
- Wang CY, Wen MS, Wang HW, et al. Increased vascular senescence and impaired endothelial progenitor cell function mediated by mutation of circadian gene Per2. Circulation 2008;118:2166-73. [Crossref] [PubMed]
- Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005;308:1043-5. [Crossref] [PubMed]
- Hudec M, Dankova P, Solc R, et al. Epigenetic Regulation of Circadian Rhythm and Its Possible Role in Diabetes Mellitus. Int J Mol Sci 2020;21:3005. [Crossref] [PubMed]
- Spiegel K, Knutson K, Leproult R, et al. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol (1985) 2005;99:2008-19. [Crossref] [PubMed]
- Lyssenko V, Nagorny CL, Erdos MR, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet 2009;41:82-8. [Crossref] [PubMed]
- Garaulet M, Lee YC, Shen J, et al. Genetic variants in human CLOCK associate with total energy intake and cytokine sleep factors in overweight subjects (GOLDN population). Eur J Hum Genet 2010;18:364-9. [Crossref] [PubMed]
- Bandín C, Martinez-Nicolas A, Ordovás JM, et al. Differences in circadian rhythmicity in CLOCK 3111T/C genetic variants in moderate obese women as assessed by thermometry, actimetry and body position. Int J Obes (Lond) 2013;37:1044-50. [Crossref] [PubMed]
- Corella D, Asensio EM, Coltell O, et al. CLOCK gene variation is associated with incidence of type-2 diabetes and cardiovascular diseases in type-2 diabetic subjects: dietary modulation in the PREDIMED randomized trial. Cardiovasc Diabetol 2016;15:4. [Crossref] [PubMed]
- Garaulet M, Corbalán MD, Madrid JA, et al. CLOCK gene is implicated in weight reduction in obese patients participating in a dietary programme based on the Mediterranean diet. Int J Obes (Lond) 2010;34:516-23. [Crossref] [PubMed]
- Gomez-Delgado F, Garcia-Rios A, Alcala-Diaz JF, et al. Chronic consumption of a low-fat diet improves cardiometabolic risk factors according to the CLOCK gene in patients with coronary heart disease. Mol Nutr Food Res 2015;59:2556-64. [Crossref] [PubMed]
- Sookoian S, Gianotti TF, Schuman M, et al. Gene prioritization based on biological plausibility over genome wide association studies renders new loci associated with type 2 diabetes. Genet Med 2009;11:338-43. [Crossref] [PubMed]
- Partonen T, Treutlein J, Alpman A, et al. Three circadian clock genes Per2, Arntl, and Npas2 contribute to winter depression. Ann Med 2007;39:229-38. [Crossref] [PubMed]
- Woon PY, Kaisaki PJ, Bragança J, et al. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci U S A 2007;104:14412-7. [Crossref] [PubMed]
- Dashti HS, Smith CE, Lee YC, et al. CRY1 circadian gene variant interacts with carbohydrate intake for insulin resistance in two independent populations: Mediterranean and North American. Chronobiol Int 2014;31:660-7. [Crossref] [PubMed]
- Kelly MA, Rees SD, Hydrie MZ, et al. Circadian gene variants and susceptibility to type 2 diabetes: a pilot study. PLoS One 2012;7:e32670. [Crossref] [PubMed]
- Barker A, Sharp SJ, Timpson NJ, et al. Association of genetic Loci with glucose levels in childhood and adolescence: a meta-analysis of over 6,000 children. Diabetes 2011;60:1805-12. [Crossref] [PubMed]
- Dupuis J, Langenberg C, Prokopenko I, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 2010;42:105-16. [Crossref] [PubMed]
- Renström F, Koivula RW, Varga TV, et al. Season-dependent associations of circadian rhythm-regulating loci (CRY1, CRY2 and MTNR1B) and glucose homeostasis: the GLACIER Study. Diabetologia 2015;58:997-1005. [Crossref] [PubMed]
- Manning AK, Hivert MF, Scott RA, et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet 2012;44:659-69. [Crossref] [PubMed]
- Zhang Z, Ma F, Zhou F, et al. Functional polymorphisms of circadian negative feedback regulation genes are associated with clinical outcome in hepatocellular carcinoma patients receiving radical resection. Med Oncol 2014;31:179. [Crossref] [PubMed]
- Englund A, Kovanen L, Saarikoski ST, et al. NPAS2 and PER2 are linked to risk factors of the metabolic syndrome. J Circadian Rhythms 2009;7:5. [Crossref] [PubMed]
- Kovac U, Jasper EA, Smith CJ, et al. The Association of Polymorphisms in Circadian Clock and Lipid Metabolism Genes With 2(nd) Trimester Lipid Levels and Preterm Birth. Front Genet 2019;10:540. [Crossref] [PubMed]
- Meng R, Cao Y, Kong Y, et al. Effects of circadian rhythm disorder on body composition in women aged 31-40 years. Ann Palliat Med 2021;10:340-9. [Crossref] [PubMed]
- Ritchie HK, Stothard ER, Wright KP. Entrainment of the Human Circadian Clock to the Light-Dark Cycle and its Impact on Patients in the ICU and Nursing Home Settings. Curr Pharm Des 2015;21:3438-42. [Crossref] [PubMed]
- Arble DM, Ramsey KM, Bass J, et al. Circadian disruption and metabolic disease: findings from animal models. Best Pract Res Clin Endocrinol Metab 2010;24:785-800. [Crossref] [PubMed]
- Foster RG, Peirson SN, Wulff K, et al. Sleep and circadian rhythm disruption in social jetlag and mental illness. Prog Mol Biol Transl Sci 2013;119:325-46. [Crossref] [PubMed]
- Depner CM, Stothard ER, Wright KP Jr. Metabolic consequences of sleep and circadian disorders. Curr Diab Rep 2014;14:507. [Crossref] [PubMed]
- Schroeder AM, Colwell CS. How to fix a broken clock. Trends Pharmacol Sci 2013;34:605-19. [Crossref] [PubMed]
- Oyama Y, Shuff SR, Burns N, et al. Intense light-elicited alveolar type 2-specific circadian PER2 protects from bacterial lung injury via BPIFB1. Am J Physiol Lung Cell Mol Physiol 2022;322:L647-61. [Crossref] [PubMed]
- Greenhill C. Timing of feeding for longevity in mice. Nat Rev Endocrinol 2022;18:457. [Crossref] [PubMed]
- Hatori M, Vollmers C, Zarrinpar A, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 2012;15:848-60. [Crossref] [PubMed]
- Noyan H, El-Mounayri O, Isserlin R, et al. Cardioprotective Signature of Short-Term Caloric Restriction. PLoS One 2015;10:e0130658. [Crossref] [PubMed]
- Melkani GC, Panda S. Time-restricted feeding for prevention and treatment of cardiometabolic disorders. J Physiol 2017;595:3691-700. [Crossref] [PubMed]
- Allison KC, Hopkins CM, Ruggieri M, et al. Prolonged, Controlled Daytime versus Delayed Eating Impacts Weight and Metabolism. Curr Biol 2021;31:650-657.e3. [Crossref] [PubMed]
- Maas MB, Iwanaszko M, Lizza BD, et al. Circadian Gene Expression Rhythms During Critical Illness. Crit Care Med 2020;48:e1294-9. [Crossref] [PubMed]
- Lewy AJ, Wehr TA, Goodwin FK, et al. Light suppresses melatonin secretion in humans. Science 1980;210:1267-9. [Crossref] [PubMed]
- Potharajaroen S, Tangwongchai S, Tayjasanant T, et al. Bright light and oxygen therapies decrease delirium risk in critically ill surgical patients by targeting sleep and acid-base disturbances. Psychiatry Res 2018;261:21-7. [Crossref] [PubMed]
- Taguchi T, Yano M, Kido Y. Influence of bright light therapy on postoperative patients: a pilot study. Intensive Crit Care Nurs 2007;23:289-97. [Crossref] [PubMed]
- Zhang KS, Pelleg T, Hussain S, et al. Prospective Randomized Controlled Pilot Study of High-Intensity Lightbox Phototherapy to Prevent ICU-Acquired Delirium Incidence. Cureus 2021;13:e14246. [Crossref] [PubMed]
- Simons KS, Laheij RJ, van den Boogaard M, et al. Dynamic light application therapy to reduce the incidence and duration of delirium in intensive-care patients: a randomised controlled trial. Lancet Respir Med 2016;4:194-202. [Crossref] [PubMed]
- Keijzer H, Smits MG, Duffy JF, et al. Why the dim light melatonin onset (DLMO) should be measured before treatment of patients with circadian rhythm sleep disorders. Sleep Med Rev 2014;18:333-9. [Crossref] [PubMed]
- Oxlund J, Knudsen T, Strøm T, et al. Serum melatonin concentration in critically ill patients randomized to sedation or non-sedation. Ann Intensive Care 2021;11:40. [Crossref] [PubMed]
- Zhang Q, Gao F, Zhang S, et al. Prophylactic use of exogenous melatonin and melatonin receptor agonists to improve sleep and delirium in the intensive care units: a systematic review and meta-analysis of randomized controlled trials. Sleep Breath 2019;23:1059-70. [Crossref] [PubMed]
- Khaing K, Nair BR. Melatonin for delirium prevention in hospitalized patients: A systematic review and meta-analysis. J Psychiatr Res 2021;133:181-90. [Crossref] [PubMed]
- You W, Fan XY, Lei C, et al. Melatonin intervention to prevent delirium in hospitalized patients: A meta-analysis. World J Clin Cases 2022;10:3773-86. [Crossref] [PubMed]
- Han Y, Tian Y, Wu J, et al. Melatonin and Its Analogs for Prevention of Post-cardiac Surgery Delirium: A Systematic Review and Meta-Analysis. Front Cardiovasc Med 2022;9:888211. [Crossref] [PubMed]
- Wibrow B, Martinez FE, Myers E, et al. Prophylactic melatonin for delirium in intensive care (Pro-MEDIC): a randomized controlled trial. Intensive Care Med 2022;48:414-25. [Crossref] [PubMed]
- Prendergast NT, Tiberio PJ, Girard TD. Treatment of Delirium During Critical Illness. Annu Rev Med 2022;73:407-21. [Crossref] [PubMed]
- Madrid-Navarro CJ, Sanchez-Galvez R, Martinez-Nicolas A, et al. Disruption of Circadian Rhythms and Delirium, Sleep Impairment and Sepsis in Critically ill Patients. Potential Therapeutic Implications for Increased Light-Dark Contrast and Melatonin Therapy in an ICU Environment. Curr Pharm Des 2015;21:3453-68. [Crossref] [PubMed]
- Scott BK. Disruption of Circadian Rhythms and Sleep in Critical Illness and its Impact on the Development of Delirium. Curr Pharm Des 2015;21:3443-52. [Crossref] [PubMed]
- Boyko Y, Toft P, Ørding H, et al. Atypical sleep in critically ill patients on mechanical ventilation is associated with increased mortality. Sleep Breath 2019;23:379-88. [Crossref] [PubMed]
- Watson PL, Pandharipande P, Gehlbach BK, et al. Atypical sleep in ventilated patients: empirical electroencephalography findings and the path toward revised ICU sleep scoring criteria. Crit Care Med 2013;41:1958-67. [Crossref] [PubMed]
- Pisani MA, Friese RS, Gehlbach BK, et al. Sleep in the intensive care unit. Am J Respir Crit Care Med 2015;191:731-8. [Crossref] [PubMed]
- Scotto CJ, McClusky C, Spillan S, et al. Earplugs improve patients' subjective experience of sleep in critical care. Nurs Crit Care 2009;14:180-4. [Crossref] [PubMed]
- Gabor JY, Cooper AB, Crombach SA, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med 2003;167:708-15. [Crossref] [PubMed]
- Patel J, Baldwin J, Bunting P, et al. The effect of a multicomponent multidisciplinary bundle of interventions on sleep and delirium in medical and surgical intensive care patients. Anaesthesia 2014;69:540-9. [Crossref] [PubMed]
- Tonna JE, Dalton A, Presson AP, et al. The Effect of a Quality Improvement Intervention on Sleep and Delirium in Critically Ill Patients in a Surgical ICU. Chest 2021;160:899-908. [Crossref] [PubMed]
- Huang YC, Chang YC, Wu CY, et al. Applying a Sleep Care Bundle to Improve Quality of Sleep in Patients in the Intensive Care Unit. Hu Li Za Zhi 2020;67:98-105. [PubMed]
- Topcu N, Tosun Z. Efforts to improve sleep quality in a medical intensive care unit: effect of a protocol of non-pharmacological interventions. Sleep Breath 2022;26:803-10. [Crossref] [PubMed]
- Kouw IWK, Heilbronn LK, van Zanten ARH. Intermittent feeding and circadian rhythm in critical illness. Curr Opin Crit Care 2022;28:381-8. [Crossref] [PubMed]
- Báez-Ruiz A, Guerrero-Vargas NN, Cázarez-Márquez F, et al. Food in synchrony with melatonin and corticosterone relieves constant light disturbed metabolism. J Endocrinol 2017;235:167-78. [Crossref] [PubMed]
- Greenwell BJ, Trott AJ, Beytebiere JR, et al. Rhythmic Food Intake Drives Rhythmic Gene Expression More Potently than the Hepatic Circadian Clock in Mice. Cell Rep 2019;27:649-657.e5. [Crossref] [PubMed]
- Chellappa SL, Qian J, Vujovic N, et al. Daytime eating prevents internal circadian misalignment and glucose intolerance in night work. Sci Adv 2021;7:eabg9910. [Crossref] [PubMed]
- Chaix A, Manoogian ENC, Melkani GC, et al. Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Annu Rev Nutr 2019;39:291-315. [Crossref] [PubMed]
- Dashti HS, Gómez-Abellán P, Qian J, et al. Late eating is associated with cardiometabolic risk traits, obesogenic behaviors, and impaired weight loss. Am J Clin Nutr 2021;113:154-61. [Crossref] [PubMed]
- Sasaki H, Hattori Y, Ikeda Y, et al. Forced rather than voluntary exercise entrains peripheral clocks via a corticosterone/noradrenaline increase in PER2::LUC mice. Sci Rep 2016;6:27607. [Crossref] [PubMed]
- Schroeder AM, Truong D, Loh DH, et al. Voluntary scheduled exercise alters diurnal rhythms of behaviour, physiology and gene expression in wild-type and vasoactive intestinal peptide-deficient mice. J Physiol 2012;590:6213-26. [Crossref] [PubMed]
- Davidson JE, Harvey MA, Bemis-Dougherty A, et al. Implementation of the Pain, Agitation, and Delirium Clinical Practice Guidelines and promoting patient mobility to prevent post-intensive care syndrome. Crit Care Med 2013;41:S136-45. [Crossref] [PubMed]
- Davy J. XIV. On the temperature of man. Philosophical Transactions of the Royal Society of London 1845;135:319-33. [Crossref]
- Harding EC, Franks NP, Wisden W. The Temperature Dependence of Sleep. Front Neurosci 2019;13:336. [Crossref] [PubMed]
- Granfeldt A, Holmberg MJ, Nolan JP, et al. Targeted temperature management in adult cardiac arrest: Systematic review and meta-analysis. Resuscitation 2021;167:160-72. [Crossref] [PubMed]
- Lévi FA, Zidani R, Vannetzel JM, et al. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. J Natl Cancer Inst 1994;86:1608-17. [Crossref] [PubMed]
- Ruben MD, Smith DF, FitzGerald GA, et al. Dosing time matters. Science 2019;365:547-9. [Crossref] [PubMed]
- Gile J, Scott B, Eckle T. The Period 2 Enhancer Nobiletin as Novel Therapy in Murine Models of Circadian Disruption Resembling Delirium. Crit Care Med 2018;46:e600-8. [Crossref] [PubMed]
- Wang H, van Spyk E, Liu Q, et al. Time-Restricted Feeding Shifts the Skin Circadian Clock and Alters UVB-Induced DNA Damage. Cell Rep 2017;20:1061-72. [Crossref] [PubMed]
- Oyama Y, Bartman CM, Gile J, et al. Circadian MicroRNAs in Cardioprotection. Curr Pharm Des 2017;23:3723-30. [Crossref] [PubMed]
- Shuff S, Oyama Y, Walker L, et al. Circadian Angiopoietin-Like-4 as a Novel Therapy in Cardiovascular Disease. Trends Mol Med 2021;27:627-9. [Crossref] [PubMed]
- Oyama Y, Walker LA, Eckle T. Targeting circadian PER2 as therapy in myocardial ischemia and reperfusion injury. Chronobiol Int 2021;38:1262-73. [Crossref] [PubMed]
- Chen Z, Yoo SH, Park YS, et al. Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc Natl Acad Sci U S A 2012;109:101-6. [Crossref] [PubMed]
- Oyama Y, Bartman CM, Gile J, et al. The Circadian PER2 Enhancer Nobiletin Reverses the Deleterious Effects of Midazolam in Myocardial Ischemia and Reperfusion Injury. Curr Pharm Des 2018;24:3376-83. [Crossref] [PubMed]
- Zhang BF, Jiang H, Chen J, et al. Nobiletin ameliorates myocardial ischemia and reperfusion injury by attenuating endoplasmic reticulum stress-associated apoptosis through regulation of the PI3K/AKT signal pathway. Int Immunopharmacol 2019;73:98-107. [Crossref] [PubMed]
- Dusabimana T, Kim SR, Kim HJ, et al. Nobiletin ameliorates hepatic ischemia and reperfusion injury through the activation of SIRT-1/FOXO3a-mediated autophagy and mitochondrial biogenesis. Exp Mol Med 2019;51:1-16. [Crossref] [PubMed]
- Güvenç M, Cellat M, Uyar A, et al. Nobiletin Protects from Renal Ischemia-Reperfusion Injury in Rats by Suppressing Inflammatory Cytokines and Regulating iNOS-eNOS Expressions. Inflammation 2020;43:336-46. [Crossref] [PubMed]
- Yamada S, Shirai M, Ono K, et al. Beneficial effects of a nobiletin-rich formulated supplement of Sikwasa (C. depressa) peel on cognitive function in elderly Japanese subjects; A multicenter, randomized, double-blind, placebo-controlled study. Food Sci Nutr 2021;9:6844-53. [Crossref] [PubMed]
- Keshtkar S, Kaviani M, Jabbarpour Z, et al. Protective effect of nobiletin on isolated human islets survival and function against hypoxia and oxidative stress-induced apoptosis. Sci Rep 2019;9:11701. [Crossref] [PubMed]
- Corti R, Flammer AJ, Hollenberg NK, et al. Cocoa and cardiovascular health. Circulation 2009;119:1433-41. [Crossref] [PubMed]
- Shrime MG, Bauer SR, McDonald AC, et al. Flavonoid-rich cocoa consumption affects multiple cardiovascular risk factors in a meta-analysis of short-term studies. J Nutr 2011;141:1982-8. [Crossref] [PubMed]
- Hooper L, Kay C, Abdelhamid A, et al. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr 2012;95:740-51. [Crossref] [PubMed]