
Giant pulmonary sclerosing pneumocytoma with potentially malignant biological behavior: a case report and literature review
Highlight box
Key findings
• We encountered a case of pulmonary sclerosing pneumocytoma (PSP) of massive size and a relatively high proliferation rate, for which a rigorous diagnostic process was needed. In this report, we summarize the clinical, imaging, and pathologic features of PSP to provide recommendations for its management.
What is known and what is new?
• PSP is a rare pulmonary benign tumor, generally isolated, and has a diameter less than 3 cm.
• PSP lacks specificity in clinical and radiological characteristics.
• PSP has complex pathological manifestations.
• PSP shows potentially malignant biological behavior.
• Fine-needle aspiration for PSP can be useful but limited in diagnosis and differential diagnosis.
• Surgical treatment is the preferred treatment for PSP.
What is the implication, and what should change now?
• The diagnosis and treatment of PSP require a concerted effort among clinicians, radiologists, and pathologists. The currently accepted potential malignant features of PSP should be reviewed and updated.
Introduction
Pulmonary sclerosing pneumocytoma (PSP), a rare pulmonary benign tumor, was originally described by Liebow and Hubbell as a tumor of vascular origin with obvious sclerosis (1) and thus named “sclerosing hemangioma”. With the development of pathology and immunohistochemical techniques, it was eventually confirmed to originate from primitive respiratory epithelial cells (type II alveolar cells) and to consist of 4 structures (solid, sclerotic, papillary, and hemangiomatoid) in histopathology. In 2015, the World Health Organization officially changed its name to “sclerosing alveolar cell tumor” and classifies it as an adenoma subtype (2). However, it has been recently found that some patients with PSP develop lymphatic or distant metastasis. Qilu Hospital of Shandong University admitted a patient with a giant PSP 9.5 cm in diameter that had potentially malignant biological behavior, which is extremely rare. To increase clinician awareness, we have summarized the clinical, imaging, and pathologic features of PSP; provided recommendations for management; and reviewed the potential malignant features of PSP. We present this article in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4049/rc).
Case presentation
General information
A 46-year-old female presented with dry cough and dyspnea for 17 consecutive days. She reported no hemoptysis, chest pain, or fever. Chest computed tomography (CT) from a local hospital revealed a huge space-occupying lesion on the right side of the mediastinum. For further diagnosis and treatment, she was transferred to the department of Pulmonary and Critical Care Medicine, Qilu Hospital of Shandong University. The timeline in Figure 1 shows the historical and recent care information of the patient. She was a nonsmoker, and there was no notable disorder in her family history. On physical examination, the patient was alert but not in acute distress. Her vital measurements were as follows: pulse, 64 beats per minute; respiratory rate, 18 breaths per minute; blood pressure, 104/64 mmHg (1 mmHg =0.133 kPa); and temperature, 36.3 ℃. No superficial lymphadenopathy was palpable. Her heart rate was regular and without a murmur. The right upper lung percussion was solid, breath sounds were weakened, and the remainder of the lungs were clear. The neurologic and abdomen examinations were unremarkable. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.

Imaging examination
Chest CT scans revealed a large soft-tissue mass shadow 8.9 cm × 7.1 cm in size with multiple spot-like calcification foci in the right lung. The adjacent lung tissue was compressed, and the internal density was not uniform (Figure 1A). Contrast-enhanced CT showed multiple vascular shadows in the arterial phase, delayed enhancement in the surrounding area, and a clear boundary (Figure 1B,1C). The mediastinum was centered, not enlarged, and without swollen lymph nodes. Bilateral fibrous foci with slight inflammation were observed in the right lung.
Laboratory and other examinations
The white-cell count was 4.71×109 per liter, with 67.9% neutrophils and 26.8% lymphocytes; the hemoglobin was 109.0 g per liter; and the platelet count was 408×109 per liter. The albumin level was 37.6 g per liter, the erythrocyte sedimentation rate was 78.00 mm per hour, and the neuron-specific enolase level was 20.90 ng per milliliter (normal range, 0 to 16.3). Bronchoscopy showed external bronchial constriction in the middle lobe of the right lung (RML) but no obstruction in the remaining bronchus. The patient subsequently underwent endobronchial ultrasound-guided transbronchial lung biopsy (EBUS-TBLB) which revealed a handful of lymphocytes and epithelia, but this did not help in making a definitive diagnosis. Later, a CT-guided lung puncture biopsy was performed as was a head and abdomen contrast-enhanced CT and whole-body scan to assess her overall condition. Fortunately, no signs of distant metastasis were found.
Diagnosis and treatment
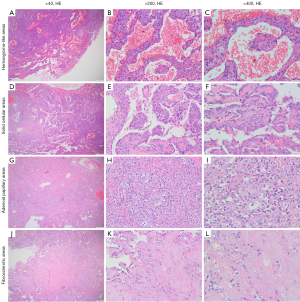
The pathology findings of CT-guided lung puncture biopsy were consistent with those of PSP. After pre-operative pathological evaluation, the patient underwent right lung mass resection and mediastinal hilar lymph node dissection via video-assisted thoracoscopy (VATS). Intraoperatively, there was a poorly circumscribed tumor 9.5 cm × 8 cm × 5 cm in size observed microscopically at the junction of the upper and middle lobes of the right lung. It was nodular and soft to the touch with a grayish yellow or white cut surface. Multiple small lymph nodes were also found in the mediastinal hilum. Postoperative pathological hematoxylin and eosin (HE) staining showed that the tumor was composed of surface cuboidal-like cells and round interstitial cells with typical histological structure, which consisted of 4 histomorphologies including hemangioma-like areas, solid cellular areas, adenoid papillary areas, and fibrosclerotic areas. Dilated blood cell pools were found in the hemangioma-like areas, and tumor cells were scattered throughout the vascular spaces (Figure 2A-2C). The solid cellular areas showed a dense cellular distribution and mainly consisted of round interstitial cells (Figure 2D-2F). The adenoid papillary areas were composed of branching papillae covered by surface cuboidal-like cells, with interstitial cells and focal sclerosis scattered in the axial structure (Figure 2G-2I). Additionally, there was marked proliferation of fibrous tissue with hyalinization in the fibrosclerotic areas (Figure 2J-2L). Immunohistochemistry showed positivity for both thyroid transcription factor-1 (TTF-1) and epithelial membrane antigen (EMA), with cytokeratin 7 (CK7) and napsin-A positivity on the surface cuboidal cells, and an approximately 15% positivity in the Ki-67 hot spot region (Figure 3). Furthermore, fibroplasia and chronic inflammatory cell infiltration, accumulation of foamy histiocytes, and multifocal multinucleated giant cell reactions were observed in the extracapsular lung tissue. No tumor cells were found in the extracapsular lung or lymph nodes. The patient’s cough and chest tightness were alleviated, 6 months postoperatively, but with occasional right chest pain. Otherwise, the patient’s work and life were as usual. The possible cause of the pain was given to the patient and relevant medical advice was given to her. At the recent follow-up, we were pleased to learn that she was already free from chest pain. A follow-up of chest CT will be scheduled.

Discussion
PSP is most common among Asian middle-aged women with no smoking history. The male to female ratio of PSP is 1:5 (3-5), which may be associated with the positive expression of the estrogen and progesterone receptors (6). The clinical manifestations of PSP lack specificity. Some patients present with nonspecific respiratory symptoms, such as cough, expectoration, shortness of breath, chest tightness, hemoptysis, and chest pain, while PSP is only occasionally found in imaging examinations (7). In rare cases, fever is the first symptom of PSP (8). Some patients with PSP have concurrent neuroendocrine disorder and autoimmune disease, such as scleroderma and rheumatoid arthritis (9,10). In our case, the respiratory symptoms were obvious and manifest as dry cough and dyspnea, which was considered to be related to the compression of the surrounding tissues.
Imaging examination is a significant auxiliary method for detecting PSP. Typical cases usually show isolated round or quasi round nodules or masses, often have a clear boundary and uniform density, and are accompanied by calcification and cystic degeneration, while few are lobulated (11). Moreover, 73.7% of isolated PSPs are smaller than 3 cm in average diameter (4), and rare cases of giant PSP have also been reported, including cases with tumors measuring 14 and 19 cm in size (12,13). PSP is more commonly found in a peripheral pattern and mainly distributed in the RML or lower left lobe (LLL) or right lobe (RLL) of the lungs (14). Multiple or diffuse PSP is rare, even in the mediastinum and bronchial lumen (11,15). The boundary in more than one-third of cases is not clear, and the pathological findings show invasive growth to the surrounding tissues (16). In some cases, invasive growth is distributed near the pleura or hilum, or there are malignant signs such as burrs and pleural depression, which are easily misdiagnosed as lung cancer and require careful clinical decision-making. In one report, enhanced CT combined with texture analysis was used to locate calcification, and arterial phase CT values could assist in differentiating PSP from peripheral lung cancer (17). Depending on the different tissue structures of the lesion, different degrees of enhancement may occur in the early stage after enhanced scanning (14). Other less common CT signs of PSP, such as the halo sign, air crescent sign, caudal sign, and hemagglutination sign (11,18), are required to differentiate PSP from pulmonary aspergillosis, tuberculosis (TB), hamartoma, and other diseases. Shin et al. (19) reported a 19-year-old male patient who simulated active pulmonary TB. His CT showed aground-glass shadow in the right upper lobe, but no significant improvement was observed after anti-TB treatment, and the final pathology confirmed PSP. In patients suspected of TB but with a poor response to anti-TB treatment, PSP should be additionally considered, especially in younger patients. Our case had a large isolated mass in the right lung with calcification, a clear boundary, and delayed enhancement, which required a definitive differential diagnosis from sarcoma, teratoma, and inflammatory pseudotumor using further histopathology. 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET)-CT may have certain utility for diagnosis but is somewhat limited in this regard. PSP mostly shows mild to moderate uptake of FDG, and the maximum standardized uptake value (SUVmax) may inform speculation upon the specific pathological type and is positively correlated with the tumor size of typical PSP (20,21). However, it should be noted that false-positive high uptake results mimic the presentation of malignancy or lung metastases and may affect clinical decision-making, especially in patients with a primary tumor (22,23).
The histological morphology of PSP is complex and consists of 4 histomorphologies (hemangioma-like areas, adenoid papillary areas, solid cellular areas, fibrosclerotic areas) and 2 types of tumor cells (cuboidal cells and stromal cells) (2). FNA is valuable for the diagnosis and differential diagnosis of PSP but is associated with the risk of misdiagnosis or missed diagnosis. Interestingly, PSP may be misdiagnosed as a papillary or solid subtype of lung adenocarcinoma or neuroendocrine tumor in patients with atypical histopathology or clinical outcomes of recurrence or metastasis (24). The cytomorphologic manifestations of typical PSP may partially overlap with those of patients with well-differentiated lung adenocarcinoma. Nonetheless, the results of immunohistochemistry, specifically positivity for TTF-1 and EMA in surface cuboidal-like cells and round interstitial cells, may facilitate differential diagnosis, as was found in our case (25). In addition, the challenges posed by intraoperative frozen diagnosis of PSP are substantial. Yang et al. (24) retrospectively analyzed 59 patients with PSP who underwent intraoperative frozen section examination and identified the diagnostic accuracy to be only 44.1%, with a misdiagnosis rate of 16.9%. Shang et al. (26) found that patients with PSP of a size of smaller than 1 cm were misdiagnosed at an 11.1% rate, and they suggested that failing to identify a double cell population and cellular atypia caused misdiagnosis. Given the limited tissue representation, we recommend further definitive pathohistological diagnosis after complete resection of the mass.
We completed a literature review to identify the borderline features of PSP. Relevant case reports were retrieved from PubMed and Web of Science using the search terms “Pulmonary sclerosing pneumocytoma”, “metastasis”, and “progression”. All case reports of PSP with malignant biological behavior were included in the analysis. Instead of focusing on histologic atypia, we defined malignant biological behavior as rapid tumor proliferation, metastasis, or invasion. All data collected (such as patient age, gender, symptom, tumor size, tumor location, metastatic site, Ki-67 proliferation index, treatment, and diagnosis) were entered into SPSS Statistics 25.0 (IBM Corp.) for statistical analysis. In Table 1 and Table 2, we provide an inductive summary of the clinical features of 38 patients with PSP with malignant biological behavior. Asymptomatic middle-aged females were predominant. The median mass size was 35 [interquartile range (IQR), 24.00–80.00] mm, and most masses were located in the LLL. For metastatic sites, 86.49% showed lymphatic metastasis, few showed endobronchial invasion or pleural metastasis, and extrapulmonary metastases such as liver and bone were also reported.
Table 1
| Patient no. | Source, year | Age (years)/sex | Symptoms | Tumor location | Primary tumor size (mm) | Metastatic site | Ki-67 (%) | Treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | Spencer et al., 1986 (27) | NA/NA | NA | NA | NA | Lymph node | NA | NA |
| 2 | Tanaka et al., 1986 (28) | 22/M | None | RLL | 50 | Hilar lymph node | NA | NA |
| 3 | Devouassoux-Shisheboran et al., 2000 (4) | 18/F | None | LLL | 35 | Hilar lymph node | NA | NA |
| 4 | Nicholson et al., 2002 (29) | 53/M | Chest pain | LLL | 35 | Peribronchial lymph node | NA | NA |
| 5 | Miyagawa-Hayashino et al., 2003 (30) | 10/F | Flu-like | RML | 47 | Regional lymph node | NA | Lobectomy and lymph node dissection |
| 6 | Miyagawa-Hayashino et al., 2003 (30) | 45/F | Flu-like | RUL | 25 | Hilar lymph node | NA | Lobectomy and lymph node dissection |
| 7 | Miyagawa-Hayashino et al., 2003 (30) | 45/M | None | LLL | 37 | Mediastinal lymph node | NA | Lobectomy and lymph node dissection |
| 8 | Miyagawa-Hayashino et al., 2003 (30) | 56/F | None | LLL | 15 | Peribronchial lymph node | NA | Lobectomy and lymph node dissection |
| 9 | Kim et al., 2003 (31) | 19/F | Cough | LLL | 100 | Interlobular and hilar lymph nodes | NA | Lobectomy, lymph node dissection, chemotherapy |
| 10 | Chan et al., 2003 (32) | 19/M | Chest pain, cough | LUL | 30 | Interlobular lymph node | NA | Lobectomy |
| 11 | Kim et al., 2004 (6) | 37/F | NA | LLL | 20 | Peribronchial lymph node | NA | Lobectomy |
| 12 | Katakura et al., 2005 (33) | 35/M | None | LLL | 30 | Hilar lymph node | NA | Lobectomy |
| 13 | Wani et al., 2007 (34) | 47/F | Cough | RUL | 48 | Right bronchus, segmental bronchus, and peripheral bronchioles | NA | Lobectomy, bronchoplasty, and lymph node dissection |
| 14 | Chien et al., 2009 (35) | 18/M | None | LUL | 80 | Lymph node | NA | Lobectomy and lymph node dissection |
| 15 | Vaideeswar, 2009 (36) | 23/M | Cough, hemoptysis | RUL | 90 | Regional lymph node | NA | Lobectomy |
| 16 | Anan et al., 2010 (37) | 38/M | None | RML | 20 | lymph node | NA | Lobectomy |
| 17 | Park et al., 2011 (38) | NA/NA | NA | LUL | NA | Left upper pulmonary vein lymph node | NA | Lobectomy and lymph node dissection |
| 18 | Suzuki et al., 2011 (39) | 57/F | None | RLL | 25 | Pleura | NA | Lobectomy |
| 19 | Kita et al., 2013 (40) | 38/F | None | LLL | 39 | Interlobular lymph node | NA | Segmentectomy and lymph node sampling |
| 20 | Adachi et al., 2014 (41) | 40/F | None | LLL | 10 | Hilar lymph node | NA | Lobectomy and lymph node dissection |
| 21 | Kim et al., 2015 (42) | 73/F | None | RLL | NA | Bone (L3), peribronchial, and mediastinal lymph nodes | <5 | Lobectomy and lymph node dissection |
| 22 | Xu et al., 2015 (43) | 26/F | None | RUL | 97 | Peribronchial and hilar lymph nodes | <1, partly 10 | Lobectomy and lymph node dissection |
| 23 | Pokharel et al., 2016 (44) | 33/F | None | LLL | 18 | Peribronchial lymph node | NA | Lobectomy |
| 24 | Jiang et al., 2017 (16) | 66/F | Cough, abnormal sputum | RML | 21 | Multiple intrapulmonary metastases | 2 | No surgery |
| 25 | Soo et al., 2017 (45) | 40/F | NA | RLL | 25 | Interlobular, hilar, and right subcarinal lymph nodes | NA | Lobectomy |
| 26 | Wang et al., 2018 (46) | 26/F | None | LLL | 40 | Mediastinal and regional lymph nodes | 3 | Lobectomy and lymph node dissection |
| 27 | Teng et al., 2019 (47) | 64/M | Hemoptysis | RLL | 30 | Hilar lymph node | 70, 55 | Lobectomy and lymph node dissection |
| 28 | Sakai et al., 2019 (13) | 64/F | Dyspnea, cough | LL | 150 | Endobronchial and vascular invasion | 5, partly 30 | Lobectomy and lymph node dissection |
| 29 | Gao et al., 2020 (48) | 65/F | Chest tightness, dyspnea | LUL | 20 | Hilar lymph node | NA | Lobectomy and lymph node dissection |
| 30 | Gao et al., 2020 (48) | 24/F | None | RUL | 100 | Hilar lymph node | NA | Lobectomy and lymph node dissection |
| 31 | Gao et al., 2020 (48) | 42/M | Chest tightness, abnormal sputum | LUL | 24 | Hilar lymph node | NA | Lobectomy and lymph node dissection |
| 32 | Wang et al., 2021 (49) | 50/F | None | RUL | 32 | Peribronchial lymph node | <1 | Lobectomy |
| 33 | Mayer et al., 2021 (50) | 57/F | None | LLL | 21 | Lymph node and pleura | <2 | Lobectomy with radical lymphadenectomy, subtotal parietal pleurectomy, partial pericardiectomy, and subtotal diaphragmatic resection |
| 34 | Kocaman et al., 2021 (51) | 25/F | Back pain | LUL | 35 | Lymph node | Very low | Lobectomy and lymph node dissection |
| 35 | Wang et al., 2021 (52) | 23/M | Cough, fever, and chest tightness | RML | 65 | Liver, hilar and cervical lymph nodes | 5 (metastases: 25) | Lobectomy and lymph node dissection |
| 36 | Lee et al., 2021 (12) | 56/F | Dyspnea and cough | LL | 190 | Endobronchial invasion | 1–2 | Lobectomy and partial bronchotomy |
| 37 | Ganga et al., 2022 (53) | 22/F | Chest pain and cough | RML/RLL | 140 | Contralateral lung metastasis | NA | Surgery |
| 38 | Current series | 46/F | Cough and dyspnea | RUL/RML | 100 | NA | 15 | Lung mass resection and lymph node dissection |
PSP, pulmonary sclerosing pneumocytoma; NA, not available/not described; M, male; RLL, right lower lobe; F, female; LLL, left lower lobe; RML, right middle lobe; RUL, right upper lobe; LUL, left upper lobe; LL, left lung.
Table 2
| Variables | Statistical description |
|---|---|
| Sex, n (%) | |
| Male | 11 (28.95) |
| Female | 25 (65.79) |
| NA | 2 (5.26) |
| Age (years), mean (SD) | 39.50 (2.81) |
| Symptoms, n (%) | |
| None | 17 (50.00) |
| Cough | 10 (29.41) |
| Dyspnea | 4 (11.76) |
| Chest tightness | 3 (8.82) |
| Chest pain | 3 (8.82) |
| Flu-like | 2 (5.88) |
| Hemoptysis | 2 (5.88) |
| Abnormal sputum | 2 (5.88) |
| Back pain | 1 (2.94) |
| Fever | 1 (2.94) |
| Primary tumor size (mm), median (IQR) | 35 (24.00, 80.00) |
| Tumor location, n (%) | |
| LLL | 12 (32.43) |
| LUL | 6 (16.22) |
| RUL | 6 (16.22) |
| RLL | 5 (13.51) |
| RML | 4 (10.81) |
| Multiple lobes | 4 (10.81) |
| Metastatic site, n (%) | |
| Lymph node | 32 (86.49) |
| Bronchus | 3 (8.11) |
| Pleural | 2 (5.41) |
| Extrapulmonary organ | 2 (5.41) |
| Contralateral lung | 1 (2.70) |
| Ki-67 (%), n (%) | |
| <5 | 6 (54.55) |
| 5–10 | 2 (18.18) |
| >10 | 3 (27.27) |
PSP, pulmonary sclerosing pneumocytoma; NA, not available; SD, standard deviation; IQR, interquartile range; LLL, left lower lobe; LUL, left upper lobe; RUL, right upper lobe; RLL, right lower lobe; RML, right middle lobe.
The mechanisms of the malignant biological behavior of PSP are not completely understood. The stromal spindle cells may be involved in tumor metastasis. In a middle-aged female patient with peribronchial lymph node metastasis, spindle cells were found in her lymph node metastasis, and immunohistochemistry confirmed interstitial spindle cells to be derived from the tumor (49). A retrospective review of 239 patients with PSP by Gao et al. (48) yielded a lymph node metastasis rate in those with dense spindle stromal cells of 20%. Matrix metalloproteinase-9 (MMP-9) overexpression and epithelial-mesenchymal transition (EMT) of surface cells may also participate in the occurrence and metastasis of PSP (39,47). A few authors also reported AKT1 E17K somatic mutation and TP53 C176Y germline mutation in the whole-exome sequencing of patients with PSP, with the activation of AKT1 and E17K pathways also being related to the underlying malignant PSP phenotype (52,54).
A higher Ki-67 proliferation index is associated with worse tumor grade and prognosis. Unfortunately, we could not draw a clear cutoff point for determining the presence of metastasis or informing the prognosis of PSP. By analyzing the proliferation index of Ki-67 in 12 patients with PSP with malignant biological behavior, we found that the Ki-67 proliferation index was higher in hot spot areas in 27.27% patients, but most (54.55%) patients were below 5%. Although no scholars have summarized differences in Ki-67 proliferation index between metastatic and non-metastatic PSP, we still recommend aggressive surgical intervention for patients with PSP and a high Ki-67 proliferation index, even if no obvious signs of metastasis have been found after a comprehensive examination. In our patient, a Ki-67 proliferation index of 15% indicated an active cell proliferation or underlying malignant biological behavior. It was prudent to choose aggressive surgical treatment and close postoperative follow-up.
Surgical treatment is the preferred treatment for PSP. We evaluated the treatment and prognosis of PSP patients with malignant biological behavior and found that even patients with lymph node or distant metastases can achieve recurrence-free survival with surgery, with only 1 patient being reported as having experienced short-term recurrence (52). In terms of surgical methods, most patients underwent lobectomy with or without lymph node dissection, while a few underwent segmentectomy. Park et al. (38) performed a retrospective analysis of 32 patients with PSP who underwent surgical resection and found limited resection was comparable to lobectomy but could further reduce hospitalization. Zheng et al. (55) confirmed the superior efficacy of sublobectomy compared with lobectomy. If sufficient resection margins are available, limited resection might be superior to lobectomy. Rare patients with bronchial invasion can receive additional partial bronchotomy or bronchoplasty; however, Wani et al. (34) suggested that bronchoplasty might be overtreatment. Others support the rationale of follow-up observation. He et al. (56) reported a 54-year-old female patient who did not receive surgical treatment but showed no signs of progression or metastasis during the 2-year follow-up. Determining whether to proceed with aggressive surgical management requires a comprehensive assessment of the patient’s situation, including economic conditions, willingness, etc. Physicians and pathologists need to make careful decisions concerning nonsurgical treatment owing to the complex histopathological and clinical manifestations of PSP. It is important to note that given the malignant potential of PSP, all patients still need to be closely followed up. Our patient’s tumor was very large and located at the junction of the RUL and RML, and the right whole-lung resection might have seriously affected her quality of life. After comprehensive evaluations, she underwent thoracoscopic right lung resection with mediastinal hilar lymph node dissection. Postoperative pathological examination confirmed PSP with a Ki-67-positive rate of 15%. The patient’s macroscopic findings were discouraging. Although the mass was visibly encapsulated, it also appeared to be infiltrative. Fortunately, careful microscopic examination revealed that this was a chronic inflammation caused by the tumor, the margins were adequate, and the lymph nodes showed no metastasis. As of this writing, the patient is still in postoperative follow-up.
Conclusions
PSP lacks specificity in its clinical and radiological manifestations, and its pathological manifestations are complex. Therefore, preoperative pathological examination may have limited value, and careful differentiation of PSP from other diseases is necessary. Although PSP is considered to be a benign tumor, its potential malignant features still require vigilance. Surgical resection is curative and does not require additional treatment. However, both surgical and nonsurgical patients should be closely followed up.
Acknowledgments
We are deeply grateful to the physicians, nurses, and other staff at the Shandong Key Laboratory of Infectious Respiratory Diseases and especially to the patient and her family.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4049/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4049/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liebow AA, Hubbell DS. Sclerosing hemangioma (histiocytoma, xanthoma) of the lung. Cancer 1956;9:53-75. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Yalcin B, Bekci TT, Kozacioglu S, et al. Pulmonary sclerosing pneumocytoma, a rare tumor of the lung. Respir Med Case Rep 2019;26:285-7. [Crossref] [PubMed]
- Devouassoux-Shisheboran M, Hayashi T, Linnoila RI, et al. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF-1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am J Surg Pathol 2000;24:906-16. [Crossref] [PubMed]
- Nasr Y, Bettoli M, El Demellawy D, et al. Sclerosing Pneumocytoma of the Lungs Arising in a Child With PTEN Mutation. Pediatr Dev Pathol 2019;22:579-83. [Crossref] [PubMed]
- Kim GY, Kim J, Choi YS, et al. Sixteen cases of sclerosing hemangioma of the lung including unusual presentations. J Korean Med Sci 2004;19:352-8. [Crossref] [PubMed]
- Liu X, Huang Z, Zhang J. Analysis of Clinical Characteristics of 35 Cases of Pulmonary Sclerosing Pneumocytoma. Zhongguo Fei Ai Za Zhi 2020;23:1049-58. [PubMed]
- Zhou L, Sun C, Huang Y, et al. Pulmonary sclerosing hemangioma with a rare symptom: A case report and review of the literature. Mol Clin Oncol 2017;6:221-4. [Crossref] [PubMed]
- Abalı H, Tokgöz Akyıl F, Tural Önür S, et al. Coexistence of Multiple Pulmonary Sclerosing Pneumocytoma and Scleroderma-Rheumatoid Arthritis Overlap Syndrome: A Case Report. Turk Thorac J 2022;23:302-5. [Crossref] [PubMed]
- Mlika M, Rais A, Abdelkbir A, et al. Collision lung tumor associating typical carcinoid tumor to sclerosing hemangioma. Clin Case Rep 2022;10:e6237. [Crossref] [PubMed]
- Shin SY, Kim MY, Oh SY, et al. Pulmonary sclerosing pneumocytoma of the lung: CT characteristics in a large series of a tertiary referral center. Medicine (Baltimore) 2015;94:e498. [Crossref] [PubMed]
- Lee HS, Kim J, Moon DH, et al. Huge Pulmonary Sclerosing Pneumocytoma with Endobronchial Invasion: A Case Report with a Literature Review. J Chest Surg 2021;54:528-31. [Crossref] [PubMed]
- Sakai T, Miyoshi T, Umemura S, et al. Large pulmonary sclerosing pneumocytoma with massive necrosis and vascular invasion: a case report. Oxf Med Case Reports 2019;2019:omz066. [Crossref] [PubMed]
- Xu G, Wang Z, Xiong Z, et al. Correlation Between Pulmonary Sclerosing Pneumocytoma Features and MSCT Imaging Manifestations in 34 Patients: Implications for Precision Medicine. Front Med (Lausanne) 2021;8:650996. [Crossref] [PubMed]
- Fan X, Lin L, Wang J, et al. Genome profile in a extremely rare case of pulmonary sclerosing pneumocytoma presenting with diffusely-scattered nodules in the right lung. Cancer Biol Ther 2018;19:13-9. [Crossref] [PubMed]
- Jiang L, Sun L, Liu L. Benign tumor behaves malignantly: a case report of bilateral multiple pulmonary sclerosing pneumocytoma. Int J Clin Exp Pathol 2017;10:8735-40. [PubMed]
- Luo C, Song Y, Liu Y, et al. Analysis of the value of enhanced CT combined with texture analysis in the differential diagnosis of pulmonary sclerosing pneumocytoma and atypical peripheral lung cancer: a feasibility study. BMC Med Imaging 2022;22:16. [Crossref] [PubMed]
- Han J, Xiang H, Ridley WE, et al. Tail sign: Pulmonary sclerosing pneumocytoma. J Med Imaging Radiat Oncol 2018;62:49. [Crossref] [PubMed]
- Shin SY, Kim MY, Lee HJ, et al. Clustered pulmonary sclerosing pneumocytoma in a young man: a case report. Clin Imaging 2014;38:532-5. [Crossref] [PubMed]
- Xu J, Dong Y, Yin G, et al. (18) F-FDG PET/CT imaging: A supplementary understanding of pulmonary sclerosing pneumocytoma. Thorac Cancer 2019;10:1552-60. [Crossref] [PubMed]
- Liu H, Dang H, Wang R, et al. Analysis of the F-18 FDG PET/CT features of pulmonary sclerosing pneumocytoma. Nucl Med Commun 2021;42:665-71. [Crossref] [PubMed]
- De Luca G, Martucci N, Setola S, et al. Sclerosing hemangioma of the lung mimicking pulmonary metastasis. Lung 2015;193:447-8. [Crossref] [PubMed]
- Kamaleshwaran KK, Rajan F, Mehta S, et al. Multiple pulmonary sclerosing hemangiomas (pneumocytoma) mimicking lung metastasis detected in fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography. Indian J Nucl Med 2014;29:168-70. [Crossref] [PubMed]
- Yang CH, Lee LY. Pulmonary sclerosing pneumocytoma remains a diagnostic challenge using frozen sections: a clinicopathological analysis of 59 cases. Histopathology 2018;72:500-8. [Crossref] [PubMed]
- Maleki Z, Muller S, Layfield L, et al. Pulmonary sclerosing pneumocytoma: Cytomorphology and immunoprofile. Cancer Cytopathol 2020;128:414-23. [Crossref] [PubMed]
- Shang Z, Han Y, Shao J, et al. Challenging of frozen diagnoses of small sclerosing pneumocytoma. J Clin Pathol 2021;74:730-4. [Crossref] [PubMed]
- Spencer H, Nambu S. Sclerosing haemangiomas of the lung. Histopathology 1986;10:477-87. [Crossref] [PubMed]
- Tanaka I, Inoue M, Matsui Y, et al. A case of pneumocytoma (so-called sclerosing hemangioma) with lymph node metastasis. Jpn J Clin Oncol 1986;16:77-86. [PubMed]
- Nicholson AG, Magkou C, Snead D, et al. Unusual sclerosing haemangiomas and sclerosing haemangioma-like lesions, and the value of TTF-1 in making the diagnosis. Histopathology 2002;41:404-13. [Crossref] [PubMed]
- Miyagawa-Hayashino A, Tazelaar HD, Langel DJ, et al. Pulmonary sclerosing hemangioma with lymph node metastases: report of 4 cases. Arch Pathol Lab Med 2003;127:321-5. [Crossref] [PubMed]
- Kim KH, Sul HJ, Kang DY. Sclerosing hemangioma with lymph node metastasis. Yonsei Med J 2003;44:150-4. [Crossref] [PubMed]
- Chan NG, Melega DE, Inculet RI, et al. Pulmonary sclerosing hemangioma with lymph node metastases. Can Respir J 2003;10:391-2. [Crossref] [PubMed]
- Katakura H, Sato M, Tanaka F, et al. Pulmonary sclerosing hemangioma with metastasis to the mediastinal lymph node. Ann Thorac Surg 2005;80:2351-3. [Crossref] [PubMed]
- Wani Y, Notohara K, Tsukayama C, et al. Sclerosing hemangioma with florid endobronchial and endobronchiolar growth. Virchows Arch 2007;450:221-3. [Crossref] [PubMed]
- Chien NC, Lin CW, Tzeng JE. Sclerosing haemangioma with lymph node metastasis. Respirology 2009;14:614-6. [Crossref] [PubMed]
- Vaideeswar P. Sclerosing hemangioma with lymph nodal metastases. Indian J Pathol Microbiol 2009;52:392-4. [Crossref] [PubMed]
- Anan E, Shirai R, Hirat N, et al. Two cases, of pulmonary sclerosing hemangioma, and peripheral lung carcinoid, in which the diagnoses were difficult by intraoperative frozen section examinations. Nihon Kokyuki Gakkai Zasshi 2010;48:253-9. [PubMed]
- Park JS, Kim K, Shin S, et al. Surgery for Pulmonary Sclerosing Hemangioma: Lobectomy versus Limited Resection. Korean J Thorac Cardiovasc Surg 2011;44:39-43. [Crossref] [PubMed]
- Suzuki H, Saitoh Y, Koh E, et al. Pulmonary sclerosing hemangioma with pleural dissemination: report of a case. Surg Today 2011;41:258-61. [Crossref] [PubMed]
- Kita H, Shiraishi Y, Katsuragi N, et al. Pulmonary sclerosing hemangioma with lymph node metastasis. Kyobu Geka 2013;66:1141-4. [PubMed]
- Adachi Y, Tsuta K, Hirano R, et al. Pulmonary sclerosing hemangioma with lymph node metastasis: A case report and literature review. Oncol Lett 2014;7:997-1000. [Crossref] [PubMed]
- Kim MK, Jang SJ, Kim YH, et al. Bone metastasis in pulmonary sclerosing hemangioma. Korean J Intern Med 2015;30:928-30. [Crossref] [PubMed]
- Xu HM, Zhang G. A rare case of pulmonary sclerosing hemagioma with lymph node metastasis and review of the literature. Int J Clin Exp Pathol 2015;8:8619-23. [PubMed]
- Pokharel S, Dhillon SS, Ylagan L, et al. Sclerosing Pneumocytoma with Lymph Node Metastasis. J Thorac Oncol 2016;11:1802-4. [Crossref] [PubMed]
- Soo IX, Sittampalam K, Lim CH. Pulmonary sclerosing pneumocytoma with mediastinal lymph node metastasis. Asian Cardiovasc Thorac Ann 2017;25:547-9. [Crossref] [PubMed]
- Wang X, Zhang L, Wang Y, et al. Sclerosing pneumocytoma with metastasis to the mediastinal and regional lymph nodes. Indian J Pathol Microbiol 2018;61:407-9. [Crossref] [PubMed]
- Teng X, Teng X. First report of pulmonary sclerosing pneomucytoma with malignant transformation in both cuboidal surface cells and stromal round cells: a case report. BMC Cancer 2019;19:1154. [Crossref] [PubMed]
- Gao Q, Zhou J, Zheng Y, et al. Clinical and histopathological features of pulmonary sclerosing pneumocytoma with dense spindle stromal cells and lymph node metastasis. Histopathology 2020;77:718-27. [Crossref] [PubMed]
- Wang RQ, Sun Q, Fan XS. Pulmonary sclerosing pneumocytoma with spindle cell characteristics and lymph node metastasis: report of a case. Zhonghua Bing Li Xue Za Zhi 2021;50:1067-9. [PubMed]
- Mayer N, Carboni GL, Thielken A, et al. Sclerosing Pneumocytoma: A Host for a Typical Carcinoid With Pleural Metastasis-A Wolf in Sheep`s Clothing. Chest 2021;159:e1-5. [Crossref] [PubMed]
- Kocaman G, Yenigün MB, Ersöz CC, et al. Pulmonary sclerosing pneumocytoma with mediastinal lymph node metastasis: a case report. Gen Thorac Cardiovasc Surg 2021;69:142-6. [Crossref] [PubMed]
- Wang Q, Lu C, Jiang M, et al. Case Report and Literature Review: Pulmonary Sclerosing Pneumocytoma With Multiple Metastases Harboring AKT1 E17K Somatic Mutation and TP53 C176Y Germline Mutation. Front Med (Lausanne) 2021;8:655574. [Crossref] [PubMed]
- Ganga RT, Ravina M, Sahu D, et al. (18)F-Labeled Fluoro-2-Deoxyglucose Positron Emission Tomography and Computed Tomography in a Large Pulmonary Sclerosing Pneumocytoma with Contralateral Lung Metastasis. Indian J Nucl Med 2022;37:103-4. [Crossref] [PubMed]
- Zhang W, Liu Y, Chai Y, et al. Case Report: Rare Pulmonary Sclerosing Pneumocytoma: Large, Multiple, Metastatic, and Fatal. Front Med (Lausanne) 2021;8:661032. [Crossref] [PubMed]
- Zheng Q, Zhou J, Li G, et al. Pulmonary sclerosing pneumocytoma: clinical features and prognosis. World J Surg Oncol 2022;20:140. [Crossref] [PubMed]
- He C, Fang H, Liu Y, et al. Pulmonary sclerosing hemangioma: report of two cases. World J Surg Oncol 2012;10:182. [Crossref] [PubMed]


