
Zebrafish thrombosis models according to the location of thrombus formation
Highlight box
Key findings
• We developed a photochemical thrombosis zebrafish model according to the thrombus location and validated the model using real-time imaging.
What is known and what is new?
• Intravascular thrombosis models using zebrafish were introduced in many studies.
• We presented the site specific (intravascular and intracardiac) thrombosis models using photosensitizer in transgenic zebrafish, and validated the model using real-time imaging and thrombolytic agent.
What is the implication, and what should change now?
• These models are feasible, cost-effective, and intuitive for assessing the thrombolytic agent and can be used for a broad spectrum of future studies.
Introduction
Stroke is associated with high morbidity and mortality, and the global incidence of stroke is increasing. Although the quantity and quality of prevention and treatment strategies for stroke have improved, the need to develop new drugs remains critical. Stroke includes ischemic and hemorrhagic stroke. In ischemic stroke, the diverse mechanisms are attributed to the various causes of the disease. Ischemic stroke develops mainly due to thrombosis from atherosclerosis or arteriosclerosis and thromboembolism from cardiac disease.
The development of animal models for stroke assessment is an indispensable component of research and development for new drugs. Suitable animal models for stroke research can provide insights into stroke mechanisms and allow researchers to designate and localize the ischemic lesion, predict the final ischemic lesion, and potentially perform in vivo functional studies. Moreover, animal models should be easily and economically reproduced. Until now, many animal models have been developed to investigate stroke pathogenesis in which focal or global ischemia was induced by a mechanical or chemical stimulus, with little consideration of stroke mechanisms (1-6). Mainly higher vertebrates have been used for vascular thrombosis animal models and used to discover screen and preclinical testing of new thrombolytic and antithrombotic agents (3,7).
The zebrafish (Danio rerio) has emerged as a popular and cost-effective model because its larvae have translucent body and relatively high genetic and functional similarity with humans (5,6,8). In particular, intravascular thrombosis animal models using larvae and adult zebrafish were introduced in many studies (5,6,8,9). Chemical or irradiation damage introduced intravascular or extravascular manners to make a thrombosis (8-11). Photochemically induced thrombosis using photosensitizer and focal irradiation are more feasible to make site specific intravascular thrombosis than other chemical or irradiation damage induced thrombosis (8). Specially zebrafish larvae present a more translucent body than adult zebrafish (12). Also, genetic modifications of zebrafish maximize in vivo angiographic features. Compared with the AB strain wild-type zebrafish, Tg (flk:gfp) transgenic zebrafish are genetically transformed to express a green fluorescent protein (GFP) within the endothelial cells (12). This transgenic fish allows continuous in vivo observation of the vertebrate embryonic vasculature throughout embryogenesis.
This study developed intracerebral and intracardiac thrombosis models using photochemically induced thrombosis in transgenic zebrafish. The intracranial thrombosis model was validated with a real-time imaging study. To demonstrate the prospective application of our intracardiac photochemical thrombosis model, we evaluated the thrombolytic capability of tissue plasminogen activator (tPA) in zebrafish. We present this article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1265/rc).
Methods
Reagents
We used the following drugs and chemicals in this study: Rose Bengal, rhodamine B-isothiocyanate (RITC)-dextran (MW =70,000 Da),1-phenyl 2-thiourea (PTU), tricaine, O-dianisidine, and human tPA from Sigma-Aldrich (St. Louis, MO, USA). The singlet oxygen from Rose Bengal in the vessels injure the endothelium of blood under light exposure. It triggers the coagulation pathway to make thrombosis in the irradiated area (13).
Zebrafish husbandry
We used transgenic zebrafish larvae Tg (flk:gfp) and Tg (mpeg I: GAL4, UAS:eGFP) in this study. We housed adult zebrafish in an aquaculture facility controlled with standard 14-h-light-10-h-dark photoperiod at 28.5 ℃ and mated to obtain zebrafish larvae. We equipped the container with a multistage filter, fluorescent UV ling, and sterilizing filter (Zebrafish AutoSystem, Genomic Design, Daejeon, Korea). Zebrafish embryos were collected seven days post-fertilization (dpf).
Intracerebral thrombosis and quantitative analysis of thrombus (each n=4)
We anesthetized the seven dpf zebrafish larvae (total n=8) using a solution containing a mixture of Tris buffer and tricaine-3-aminobenzoic acid (0.4 g) in 100 mL ethyl ester, adjusted to pH 7. The mixture of Rose Bengal (800 µg/mL), and a fluorescent agent (RITC-dextran; 10% in dH2O, 2 nL) to stain the blood flow were injected three times into the cardinal vein of the larvae with a microinjector (World Precision Instruments, Friedberg, Germany). The larvae were mounted with 1.5% low melting agarose on the glass-bottom chamber, and an area (measured area =3.99585 µm2) in the skull of the larvae was focused and exposed to a 560 nm confocal laser (Nikon Eclipse Ti, Tokyo, Japan) to induce thrombosis in the intracerebral vessels by photochemical reaction for 10 minutes. Real-time imaging of thrombosis and quantitative analysis of the vascular endothelial cell apoptosis was performed. We validated our zebrafish photochemical thrombosis model with testing the thrombolytic activity of tPA. We removed the larva from the microscope and injected tPA. After injection, we put the larva back in the microscope to observe the thrombolysis. tPA was injected into larvae approximately 120 s after photochemical thrombosis. The blood clots began disappeared when the larva was injected with tPA (intravascular concentration 225 µg·mL−1).
Intracardiac thrombosis and quantitative analysis of thrombus
Zebrafish larvae (7dpf) (n=15) were anesthetized and classified into three groups. Rhodamine and dimethyl sulfoxide (DMSO) were injected into the control group and the Rose Bengal group (rhodamine, DMSO and Rose Bengal were injected). The heart of the mounted zebrafish larvae was exposed to a confocal laser for 15 min in the Rose Bengal + tPA group (rhodamine and tPA were injected after photoactivation). For quantitative analysis of thrombosis in the zebrafish heart, laser-exposed zebrafish larvae were stained with O-dianisidine to quantify the heart red blood cells (RBCs). Larvae placed in 0.1% DMSO were the vehicle control. The larvae were observed after incubation at 28 ℃ for 24 hours. Five zebrafish larvae in each group were stained with 1.0 mg/mL O-dianisidine dye solution for 15 min and washed with DMSO three times as described by Chen et al. (14).
After O-dianisidine staining, zebrafish were mounted with 1.5% low melting agarose, and images were acquired in identical conditions with a microscope. To quantitatively evaluate the RBCs in the zebrafish heart, stained RBC intensity was analyzed by Image J 1.52a software (https://imagej.nih.gov/ij/).
Statistical analysis
All statistical analyses were performed using SPSS 20.0 for Windows (IBM Corporation, Armonk, NY, USA). Data are expressed as mean ± standard deviation (SD) or median (interquartile range) as the appropriate and statistical analysis was performed using the χ2 test or Mann-Whitney U test. P<0.05 was considered to indicate statistical significance.
Ethical statement
The experimental animal procedures and the animals’ care were approved by the Institutional Animal Care and Use Committee at the Korea University College of Medicine (IACUC Number: KOREA-2019-0041) in compliance with the Korean guidelines for the standard operations of the IACUC.
Results
Intracerebral thrombosis model
Formation of intracerebral thrombosis
We induced intracerebral thrombosis in transgenic zebrafish larvae [Tg (flk:gfp)]. In the transgenic zebrafish larvae, in vivo angiography visualized the injury and collapse of the vessels compared with the control group (Figure 1). We found that the blood flow in small-sized cerebral vessels disappeared after microinjection of the photosensitizer mixture and exposure to the confocal laser.

Real-time imaging of intravascular thrombosis
The formation of intracerebral thrombosis after rhodamine injection was recorded through in vivo angiography. We quantitatively analyzed the amount of blood flow per cross-sectional area of the total vasculature pre-and post-exposure with the confocal laser. The dye intensity reflecting the blood flow per cross-sectional area in the Rose Bengal group markedly decreased around the thrombi compared with the control group (Figure 2).

Analysis of vascular endothelial cell damage
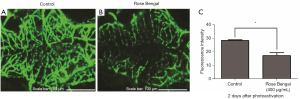
We found that the number of vascular endothelial cells decreased in the intracerebral vessel of transgenic zebrafish larvae [Tg (flk:gfp)] 2 days after photothrombosis. The control group (n=2) showed intact fluorescence of the vascular endothelial cells (Figure 3A). Photochemical dye-injected larvae showed decreased fluorescence intensity of damaged vascular endothelial cell layers (Figure 3B). The intensity of fluorescence of endothelial cells in the Rose Bengal group was significantly lower than that in the control group (P<0.01) ((Figure 3C).
Validation of intracerebral photothrombosis model through tPA
The thrombolytic activity of tPA was visually confirmed by in vivo angiography in zebrafish larvae and measured by a confocal microscope. Figure 4A shows the dorsal longitudinal vein of zebrafish larvae taken by a confocal laser microscope before and after exposure to the confocal laser. The tPA group showed a reduction of the thrombus (Figure 4B). The area of the thrombus in the control group (n=4) was 568.5 (100%), and that in the tPA group was 283 (49.78%±7.090%, mean ± standard deviation) (Figure 4C).

Intracardiac thrombosis model
We induced intracardiac thrombus formation by the same photothrombosis method and simultaneously validated the model. We established three groups: control group (n=5), Rose Bengal group (n=5), and Rose Bengal + tPA group (n=5). After exposure to a confocal laser for 15 min, comparing with the control (Figure 5A), the RBC intensity in the heart markedly decreased in the Rose Bengal group (24.33%±2.899%) (Figure 5B). The RBC intensity in the heart in Rose Bengal + tPA groups (63.53%±14.07%) did not decreased (Figure 5C). The quantitative image analysis of the heart RBC intensity validated the intracardiac thrombosis formation and thrombolytic activity of tPA (Figure 5D).

Discussion
This study developed vascular thrombosis models according to the location of the thrombosis and validated the models as an assessment tool for thrombosis. We achieved intracerebral and intracardiac thrombosis formation on 7dpf transgenic zebrafish larvae by injecting photosensitizing agents and exposing the larvae to a confocal laser (560 nm). We validated thrombosis formation using real-time imaging software and quantitatively assessed the thrombolysis.
Zebrafish demonstrate advantages as a model system for assessing drug efficacy, toxicity, and safety (11,15). Zebrafish larvae allow for both ease of in vitro fertilization as well as transparency (10). Additionally, they are similar to humans in many aspects (16). In particular, the hematological system in zebrafish, including the platelets and coagulation factors, show similarity with their counterparts in humans (17). Therefore, zebrafish is a useful model for research on thrombus formation and exploring the antithrombotic effect of various drugs. In this study, we used transgenic zebrafish expressing fluorescent vascular endothelial cells. Transgenic zebrafish Tg (flk:gfp) are used for in vivo angiography in the animal cerebrovascular model (18). We confirmed vascular endothelial cell damage as the main mechanism in photochemically induced thrombosis through in vivo angiography in the larvae. A weaker intensity of fluorescence indicates a smaller number of vascular endothelial cells. The number of cells can decrease due to apoptosis or necrosis. Other research studies using rodent models showed that apoptosis and necrosis might cause cell damage from ROS produced by Rose Bengal and photostimulation (19,20). As zebrafish in larval stage have transparent body, this feature enables the unique possibility of inducing thrombogenesis in specific cerebral vessels and provoking ischemic stroke.
The laser-induced vascular injury model enables intravascular thrombosis in animal cardiovascular disease models. However, in other animal models such as rats and rabbits, establishing an intravascular thrombosis model requires the sacrifice of many mammals. Conventional rat models of vascular thrombosis generally involve complicated procedures that may show irreducibility or risk of inducing extensive thrombosis and inflammation (2). Models of intracardiac thrombus are challenging to establish and have rarely been reported (21). Compared with these models, our transgenic zebrafish models using photochemical thrombosis are highly reproducible and easy to establish. These features may provide real-time visualization of the formation of intracerebral thrombosis and intracardiac RBC aggregation according to the location of the thrombosis. In contrast, higher degree primate models generally require complicated surgery and a substantial investment of resources to assess the effect of new drugs and hence, might suffer from unsatisfactory reproducibility. The photochemical thrombosis were employed in rodents by Nishimura and coworkers (22). However, skull surgery was required to access the cerebral vessels of these animals and confirm the induction of the thrombus.
We present the possibility of establishing the cardiac origin of thromboembolism as the cause of stroke. This method allows selective coagulation. To make intracardiac thromboses, we quantitatively analyzed heart RBCs stained with O-dianisidine. Moreover, tPA thrombolysis was induced. O-dianisidine staining has been used to identify RBCs in the intracardiac thrombosis model (23). We checked the thrombolytic capability of tPA in our model in vivo. These results show that our method can be a possible tool for screening and preclinical testing new thrombolytic and antithrombotic agents.
Despite the attractive features of zebrafish models, our study has three limitations. First, zebrafish are considerably different from humans in physical aspects. The lesser density of thrombocytes (24) and the shorter clotting time of zebrafish plasma than that of humans were the different aspects (25). Second, these thrombosis models do not reflect the diverse and complex aspects of human stroke development that are attributed to risk factors, genetic factors, and environmental factors. However, these are inherent problems that can be encountered in animal models in general. Third, although the small laser power employed in our method does not induce extensive photochemical damage, a local thermal effect associated with prolonged exposure may have existed. Therefore, an endothelial injury might aggravate, and the initiation of the thrombi might be partially affected by the high temperature. However, photo stimulated thrombogenesis in transgenic zebrafish was monitored in real-time and confirmed through in vivo angiography and injury of the endothelial cells. These models could be a reproducible standard for a thrombosis animal model.
Conclusions
In this study, we developed and validated two zebrafish thrombosis models that could be suitable for in vivo screening and efficacy assessment of antithrombotic agents. These zebrafish thrombosis models are precise and readily available with a short testing time and may increase the research and development of antithrombotic drugs.
Acknowledgments
We acknowledge Mi Young Kim, the librarian in Korea university medical library.
Funding: This work was supported by a 2016 Korea University Ansan Hospital R&D Support Project through the support of the Vice President for Medical Affairs of Korea University Special Research Funds (No. K1613821), and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (Information & Communications Technology) (No. NRF-2020R1C1C1009294).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1265/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1265/dss
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1265/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1265/coif). All authors report that this work was supported by a 2016 Korea University Ansan Hospital R&D Support Project through the support of the Vice President for Medical Affairs of Korea University Special Research Funds (No. K1613821), and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (Information & Communications Technology) (No. NRF-2020R1C1C1009294). JMJ has received: lecture honoraria from Pfizer, Sanofi-Aventis, Otsuka, Dong-A, Hanmi Pharmaceutical Co., Ltd., and Boryung Pharmaceutical Co., Ltd.; a study grant from Il-Dong and Cheiljedang Pharmaceutical Co., Ltd.; and consulting fees from Daewoong Pharmaceutical Co., Ltd. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yoshizawa K, Kissling GE, Johnson JA, et al. Chemical-induced atrial thrombosis in NTP rodent studies. Toxicol Pathol 2005;33:517-32. [Crossref] [PubMed]
- Westrick RJ, Winn ME, Eitzman DT. Murine models of vascular thrombosis (Eitzman series). Arterioscler Thromb Vasc Biol 2007;27:2079-93. [Crossref] [PubMed]
- Bodary PF, Eitzman DT. Animal models of thrombosis. Curr Opin Hematol 2009;16:342-6. [Crossref] [PubMed]
- Levi M, Dörffle-Melly J, Johnson GJ, et al. Usefulness and limitations of animal models of venous thrombosis. Thromb Haemost 2001;86:1331-3. [Crossref] [PubMed]
- Jagadeeswaran P, Cooley BC, Gross PL, et al. Animal Models of Thrombosis From Zebrafish to Nonhuman Primates: Use in the Elucidation of New Pathologic Pathways and the Development of Antithrombotic Drugs. Circ Res 2016;118:1363-79. [Crossref] [PubMed]
- Semyachkina-Glushkovskaya OV, Sokolovski SG, Goltsov A, et al. Laser-induced generation of singlet oxygen and its role in the cerebrovascular physiology. Prog Quantum Electron 2017;55:112-28. [Crossref]
- Myers DD Jr. Nonhuman primate models of thrombosis. Thromb Res 2012;129:S65-9. [Crossref] [PubMed]
- Lee IJ, Yang YC, Hsu JW, et al. Zebrafish model of photochemical thrombosis for translational research and thrombolytic screening in vivo. J Biophotonics 2017;10:494-502. [Crossref] [PubMed]
- Yu X, Li YV. Zebrafish (Danio rerio) Developed as an Alternative Animal Model for Focal Ischemic Stroke. Acta Neurochir Suppl 2016;121:115-9. [Crossref] [PubMed]
- Weyand AC, Shavit JA. Zebrafish as a model system for the study of hemostasis and thrombosis. Curr Opin Hematol 2014;21:418-22. [Crossref] [PubMed]
- Zhu JJ, Xu YQ, He JH, et al. Human cardiotoxic drugs delivered by soaking and microinjection induce cardiovascular toxicity in zebrafish. J Appl Toxicol 2014;34:139-48. [Crossref] [PubMed]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol 2002;248:307-18. [Crossref] [PubMed]
- Bacigaluppi M, Comi G, Hermann DM. Animal models of ischemic stroke. Part two: modeling cerebral ischemia. Open Neurol J 2010;4:34-8. [Crossref] [PubMed]
- Chen Y, Chen PD, Bao BH, et al. Anti-thrombotic and pro-angiogenic effects of Rubia cordifolia extract in zebrafish. J Ethnopharmacol 2018;219:152-60. [Crossref] [PubMed]
- Zhou J, Guo SY, Zhang Y, et al. Human prokinetic drugs promote gastrointestinal motility in zebrafish. Neurogastroenterol Motil 2014;26:589-95. [Crossref] [PubMed]
- Williams CH, Hong CC. Multi-step usage of in vivo models during rational drug design and discovery. Int J Mol Sci 2011;12:2262-74. [Crossref] [PubMed]
- Lang MR, Gihr G, Gawaz MP, et al. Hemostasis in Danio rerio: is the zebrafish a useful model for platelet research? J Thromb Haemost 2010;8:1159-69. [Crossref] [PubMed]
- Chico TJ, Ingham PW, Crossman DC. Modeling cardiovascular disease in the zebrafish. Trends Cardiovasc Med 2008;18:150-5. [Crossref] [PubMed]
- Yao H, Sugimori H, Fukuda K, et al. Photothrombotic middle cerebral artery occlusion and reperfusion laser system in spontaneously hypertensive rats. Stroke 2003;34:2716-21. [Crossref] [PubMed]
- Kim GW, Sugawara T, Chan PH. Involvement of oxidative stress and caspase-3 in cortical infarction after photothrombotic ischemia in mice. J Cereb Blood Flow Metab 2000;20:1690-701. [Crossref] [PubMed]
- Wang Y, Hsi DH, Yuan W, et al. New experimental animal model of intracardiac thrombus created with epicardial echocardiographic guidance. Am J Transl Res 2019;11:3092-100. [PubMed]
- Nishimura N, Schaffer CB, Friedman B, et al. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci U S A 2007;104:365-70. [Crossref] [PubMed]
- Sung JJ, Jeon J, Lee JJ, et al. Zebrafish Jak2a plays a crucial role in definitive hematopoiesis and blood vessel formation. Biochem Biophys Res Commun 2009;378:629-33. [Crossref] [PubMed]
- Lin HF, Traver D, Zhu H, et al. Analysis of thrombocyte development in CD41-GFP transgenic zebrafish. Blood 2005;106:3803-10. [Crossref] [PubMed]
- Jagadeeswaran P, Sheehan JP, Craig FE, et al. Identification and characterization of zebrafish thrombocytes. Br J Haematol 1999;107:731-8. [Crossref] [PubMed]


