CBLB502 administration protects gut mucosal tissue in ulcerative colitis by inhibiting inflammation
Introduction
Ulcerative colitis (UC) is a chronic nonspecific intestinal inflammatory disease, with an incidence of 1.2 to 20.3 cases per 100,000 individuals per year. The highest estimates are observed in people aged 20–35 years (1), and the etiology and pathogenesis of the disorder are still unclear (2). The incidence of UC has risen in recent years, and UC influences the overall health of human beings (3). Due to a lack of effective clinical treatments and the unclear pathogenesis and etiology of the disease, UC has been recognized as a refractory disease by the WHO (2). Further characterization of the pathogenesis and the development of new medical treatments for UC have been a challenge (4). It has been reported that tauroursodeoxycholate (5), lactobacillus plantarum 21 (LAB 21) (6), and amentoflavone (7) have protection effect in UC. However, past and ongoing therapeutic strategies for UC have only been moderately successful. UC is still considered a more ‘benign’ disease than Crohn’s disease.
CBLB502 contains the complete N- and C-terminal domains of Salmonella flagellin separated by a flexible linker (8), and is an agonist of toll-like receptor 5 (TLR5). CBLB502 exerts radioprotective activity and is characterized as safe and highly effective in reducing the risk of death by activating nuclear factor kappa B (NF-κB) pathway (8). CBLB502 inhibits acute renal ischemic failure and is protective when given 30 min before and after ischemic kidney reperfusion (9). CBLB502 can significantly reduce the severity of dermatitis and oral mucositis caused by local radiation, and these properties are mediated via the activation of TLR5 signaling (10). TLR5 binding to bacterial flagella activates signaling through the transcription factor NF-κB pathway and triggers an innate immune response to the invading pathogen. This proposed signaling mechanism is supported by structure-guided mutagenesis and the deletion analysis of CBLB502 (11). Granulocyte colony-stimulating factor (G-CSF) and interleukin-6 (IL-6) are candidate biomarkers of the radioprotective efficacy of CBLB502, and both G-CSF and IL-6 activity is TLR5-dependent and CBLB502 dose-dependent (12). The no-observed adverse effect level (NOAEL) for the developmental toxicity of CBLB502 in Wistar rats was estimated to be ≥300 μg/kg/day (13). CBLB502 stimulates a robust antitumor response by directly activating NF-κB nuclear translocation and TLR5-expressing accessory CD11b(+) and CD11c(+) cells (14). STAT3 and activator protein 1 (AP-1)-driven pathways were strongly activated by CBLB502 along with NF-κB. CBLB502 exerts significant hepatoprotective activities and exhibits anticancer effects (15).
We have previously demonstrated that CBLB502 possesses antioxidant and free radical scavenging activities in vitro (16). Now that CBLB502 has been shown to protect the gastrointestinal tract through TLR, IL and NF-κB signaling, can it treat UC, which is related to TLR and IL? We examined the protective effect of CBLB502 on 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced UC in this study. By constructing a UC model and treatment with CBLB502, our results suggest that CBLB502 can treat UC via the TLR and NF-κB pathways.
Methods
TNBS-induced colitis
Male BALB/c mice (6–8 weeks old) were obtained from Experimental Animal Center of Academy of Military Medical Sciences (Beijing, China). Mice were provided sterile food and water, and were maintained in a 12 h light/dark cycle. All experiments were conducted with the approval of the Institutional Animal Care and Use Committee of Beijing Institute of Radiation Medicine, and the experiments adhered strictly to the NIH Guide for the Care and Use of Laboratory Animals. All experiments were designed to minimize suffering and the number of animals used.
The UC model was induced according to a previously published method (17) with minor modifications. Briefly, mice (food-deprived for 36 h) were anesthetized with isoflurane using an anesthesia machine; the oxygen flow rate of the anesthesia machine was adjusted to 0.5–0.7 nL/min. To induce colitis, 37.5, 75, 150, or 200 mg/kg of TNBS (Sigma-Aldrich) in 38% ethanol (to break the intestinal epithelial barrier) was administered by the mouse gavage needle equipped with a 1 mL syringe. The gavage needle was advanced into the rectum until the tip was 4 cm proximal to the anal verge; mice were held in a vertical position for 60 s after rectal administration. Control mice received 38% ethanol via the same method described above. The total injection volume was 100 μL.
Treatment of TNBS-induced colitis
BALB/c mice were treated with CBLB502 (0.2–6.4 mg/kg) from 0.5 to 8 h after TNBS administration. Mice were monitored for the appearance of diarrhea, changes in body weight, and overall mortality. At the end of the experiment, surviving mice were killed, blood samples were collected by cardiac puncture, and a 7-cm segment of the colon was excised for macroscopic and microscopic damage evaluation.
ELISA assay
The IL and tumor necrosis factor-α (TNF-α) protein levels in plasma were measured via ELISA; kits were provided by the Beijing gersion Bio-Technology Co., Ltd. A 0.5–1.0 mL blood sample was collected, placed in a tube containing EDTA and aprotinin, incubated at 4–8 °C for 15 min, and centrifuged at 5,000 rpm for 2 min at 4 °C to separate the plasma. Plasma samples were stored at −20 °C until analysis. All samples were added in duplicate to the Microelisa strip plate, and set standards and testing samples were added to the wells. Standard solution (50 μL) was added to the standard well, 10 μL of each test sample was added to the well along with 40 μL of sample diluent, and the blank well was empty. Then, 100 μL of HRP-conjugate reagent was added to each well, covered with an adhesive strip and incubated for 60 minutes at 37 °C. Each well was then aspirated and washed, and the process was repeated four times for a total of five washes. Washing consisted of filling each well with wash solution (400 μL) using a squirt bottle, and complete removal of liquid was ensured at each step. After the last wash, any remaining wash solution was removed by aspirating or decanting, inverting the plate and blotting it against clean paper towels. Chromogen solution A (50 µL) and chromogen solution B (50 µL) were added to each well, before gently mixing and incubating for 15 minutes at 37 °C, protected from light. After 50 μL of Stop Solution was added to each well, the color in the wells was expected to change from blue to yellow; if the color in the well was green or the color change did not appear uniform, then the plate was gently tapped to ensure thorough mixing. The optical density was measured at a wavelength of 450 nm using a microtiter plate reader within 15 minutes. The final results from plasma were expressed as pg/mL for IL and TNF-α.
RT-PCR
The colon samples were removed at the indicated times, washed with phosphate-buffered saline (pH 7.2), and cut into small pieces. Total RNA of colon samples was extracted by Total RNA kits II (Omega, Japan) according to the manufacturers’ instructions, and then reverse-transcribed by the Reverse Transcription System (Sigma, USA). Subsequently, TLR and β-actin were amplified by PCR using the following primers (Table 1). PCR products were identified with 2% agarose-gel electrophoresis followed by image analysis using Image Pro Plus (version 5.1; Media Cybernetics, Bethesda, MD, USA) and then plotted using the Origin 8.0 software. All experiments were repeated at least 3 times.

Full table
Western blotting
The colon samples were removed at the indicated times, washed with phosphate-buffered saline (pH 7.2), and cut into small pieces. The protein concentration was determined in the supernatants using a BCA protein assay kit with bovine serum albumin as a standard. The samples were boiled with equal amounts (vol/vol) of 2× sample buffer at 95 °C for 6 min. Samples containing equal amounts of protein (50 µg per lane) were loaded onto a 12.5% SDS-polyacrylamide gel, electrophoresed and subsequently transferred onto a nitrocellulose membrane (Millipore Corporation, USA). The membrane was blocked in 5% non-fat milk and probed with primary antibodies (NF-κB p65: 1:3,000, Abcam; β-actin: 1:1,000, Sigma), and developed with the HRP-conjugated goat anti-mouse secondary antibody (1:5,000 in TBST, Beijing Zhong-Shan Biotechnology, China) by incubation for 1 h at room temperature. The specific reaction was visualized with the chemiluminescence substrate luminol reagent (GE, USA) and autoradiography. Protein bands were visualized using Image Pro Plus (version 5.1; Media Cybernetics, Bethesda, MD, USA) and then plotted using the Origin 8.0 software. All experiments were repeated at least 3 times.
Statistical analysis
All data were presented as the mean ± standard deviation (SD). The results were statistically evaluated for significance using Student’s t-test. P<0.05 was considered a statistically significant difference.
Results
TNBS-induced colitis in BALB/c mice
Based on previous studies demonstrating that the colonic administration of TNBS induces colitis in BALB/c mice (17), we explored the possibility that the administration of TNBS could induce chronic inflammation in the murine colon via an improved enema technique using a gavage needle. We found that BALB/c mice subjected to rectal administration of 150 mg/kg TNBS in 38% ethanol reproducibly developed colitis (18). The control group treated with 38% ethanol alone failed to develop wasting colitis. Morphological and histological analysis revealed that the colons of TNBS-treated BALB/c mice removed 4 d after TNBS administration demonstrated striking hyperemia and inflammation, whereas the colons of control mice treated with 38% ethanol alone demonstrated no macroscopic or histological signs of inflammation.
Previous studies demonstrated that CBLB502 could mitigate radiation-induced injury at an effective dose of 0.2 mg/kg CBLB502 (8). The final dose of CBLB502 in these experiments was set to 3.2 mg/kg after 2 h. CBLB502 exhibited a significant therapeutic effect on TNBS-induced colitis.
CBLB502 changes the concentrations of IL and TNF-α
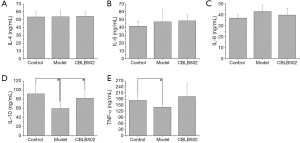
Inflammatory cytokines have increasingly been identified as playing critical roles in the pathogenesis of UC. Among these, TNF-α appears to play an important role (19). IL, which is an immunomodulatory cytokine produced by macrophages and other tissue cells, also plays a major role in inflammatory and immunological responses (20). In the present study, we examined the effects of CBLB502 on the serological levels of IL-4, 6, 8, 10 and TNF-α. IL-4 expression in the serum from animals in the in treatment group, model group, and control group animals was 54.29±5.83, 53.71±7.72, and 53.41±7.28 ng/mL, respectively (Figure 1A). There were no significant differences among these values (P>0.05). No significant differences were detected (P>0.05) for IL-6 and IL-8 protein expression (Figure 1B,C) . In control group animals, IL-10 expression in serum was 91.48±24.38 ng/mL; this was higher than in the model group (59.36±14.46 ng/mL, P<0.05) or the treatment group (54.29±5.83 ng/mL, P<0.05) (Figure 1D). In model group animals, the concentration of TNF-α in serum was 140.11±12.70 ng/mL, which was lower than protein levels in the control group (173.86±29.26 ng/mL, P<0.05) (Figure 1E). There was no effect of CBLB502 on expression of the anti-inflammatory cytokines IL-4, IL-6 and IL-8, which can promote the expression of IL-10. Therefore, CBLB502 can promote the expression of inflammatory cytokines to achieve a protective effect.
CBLB502 reduces the TLRs expression in mice
We analyzed the expression of TLR mRNA in the colon of TNBS treated mice by RT-PCR. As shown in Figure 2, the mRNA levels of TLR1, 2, 3, 4, 6, 7, 8, and 9 in the CBLB502 treatment group were significantly lower than in the model group (P<0.05). The results of RT-PCR demonstrated that CBLB502 can reduce the TLR expression in the mouse colon.

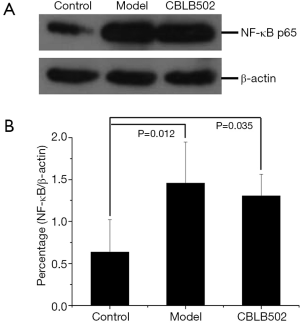
CBLB502 reduces the expression of NF-κB in mice
Protein samples from mouse colons were separated by SDS-PAGE, transferred to a PVDF membrane, and labeled with primary antibodies. Western blot revealed that CBLB502 also reduced NF-κB expression in the mouse colon but that NF-κB expression was not significantly lower than the model group (P>0.05), as shown in Figure 3.
Discussion
UC has been suggested to be caused by infection and circumstantial evidence links Escherichia coli (E. coli) with the condition. Clinical results suggest that treatment with a non-pathogenic E. coli (Nissle 1917) was as effective as mesalazine in maintaining UC remission. The beneficial effect of live E. coli may provide clues to the cause of UC (21). The use of anti-TNF antibodies in UC is less effective than in Crohn’s disease (22). Immunosuppressive therapy is an alternative choice for patients with UC. The dose-dependent use of oral tacrolimus is efficacious and safe for UC. The optimal dose range appears to be 10–15 ng/mL with 2-week therapy duration (23). Heparin has been reported exert a therapeutic role in inflammatory bowel disease (IBD), particularly in UC. Some studies suggest that heparin can reduce the severity of symptoms and improve healing in patients with UC (24,25). Infliximab 4–5 mg/kg is an effective and safe rescue therapy in patients experiencing severe UC (26). The use of the helminth Trichuris suis in the treatment of UC appears to be safe and effective in patients; however, additional larger clinical trials are required to refine the therapeutic role of intestinal helminthes in treating UC (27). Oral aminosalicylates are well established in the treatment of active mild/moderate UC. However, the combined oral and enema treatment with Pentasa (mesalazine) is superior to oral therapy alone in patients with extensive mild/moderate active UC (28).
UC is characterized by a Th2 immune response with inflammation and epithelial barrier dysfunction. IL-13 is a crucial effector Th2 cytokine in UC that impacts epithelial barrier function by affecting epithelial apoptosis, tight junctions, and restitution velocity (29). IL-13 is associated with an IL-1 receptor antagonist gene polymorphism and UC (30). IL2 and IFN-gamma could exacerbate colonic epithelial cell injury in UC (31). Increased concentrations of TNF-α were also found during intestinal perfusion of patients, and the TNF-α concentration correlated with IL-8 expression. The local mucosal recruitment/activation of neutrophils in UC is mediated by enhanced IL-8 synthesis, and TNF-α may be a stimulus for IL-8 synthesis (32). IL-33 expression was significantly elevated in active lesions from patients with UC, but was not detected in inactive lesions in UC patients or in lesions from patients with either active or inactive Crohn’s disease. IL-33 is specifically enhanced in inflamed mucosa of UC, and may play an important role in the pathophysiology of UC (33).
TLRs are membrane-bound receptors that affect both the innate and adaptive immune systems, particularly in inflammatory responses against pathogen infection (34). TLR5 ligation in epithelial cells can activate a number of genes with anti-apoptotic functions (35). Therefore, flagellin is a key target of mucosal innate immunity (36). Bacterial flagellin can activate basolaterally expressed TLR5 to induce epithelial-driven inflammatory responses and proinflammatory gene expression. TLR5 is expressed exclusively on the basolateral surface of intestinal epithelia, and provides a molecular basis for the innate immune response (37). Flagellins from most species activate TLR5 and are natural ligands of the innate immune sensor TLR5. Activation of TLR5 by flagellin initiates a powerful host response, indicating that the flagellin-TLR5 interaction is critical in regulating both the innate and adaptive immune responses in maintaining intestinal immune homeostasis (38). Flagellin is a principal component of bacterial flagella. Bacterial flagellin can activate basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. This response depends on both extracellular leucine-rich repeats and the intracellular Toll/IL-1R homology region of TLR5 (37). The innate immune response to bacterial flagellin is mediated by TLR5, and TLR5 has evolved to permit mammals to specifically detect flagellated bacterial pathogens (39).
CBLB502 exerts radioprotective activities and inhibits acute renal ischemic failure (8-10,12). In this study, we examined the treatment of UC using CBLB502 via the effects of CBLB502 on TLR responses and IL and NF-κB signaling pathways. CBLB502 could be used as a potential drug for the treatment of UC.
Acknowledgements
Funding: This work was supported by the National Basic Research Project (973 program) (2012CB518200), the General Program (81371232, 81100979) of the Natural Science Foundation of China, Beijing Natural Science Foundation (7132171), Special Key Programs for Science and Technology of China (2012ZX09102301-016), the General Program of the State Key Laboratory of Proteomics (SKLP-Y201105), and Science and Technology Program of Suzhou, Jiangsu Province (ZXY2012017).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional ethic review board of Beijing Institute of Radiation Medicine.
References
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004;126:1504-17. [Crossref] [PubMed]
- Campieri M, Gionchetti P. Bacteria as the cause of ulcerative colitis. Gut 2001;48:132-5. [Crossref] [PubMed]
- Yamamoto-Furusho JK, Uscanga LF, Vargas-Alarcón G, et al. Clinical and genetic heterogeneity in Mexican patients with ulcerative colitis. Hum Immunol 2003;64:119-23. [Crossref] [PubMed]
- Meier J, Sturm A. Current treatment of ulcerative colitis. World J Gastroenterol 2011;17:3204-12. [PubMed]
- Yang Y, He J, Suo Y, et al. Tauroursodeoxycholate improves 2,4,6-trinitrobenzenesulfonic acid-induced experimental acute ulcerative colitis in mice. Int Immunopharmacol 2016;36:271-6. [Crossref] [PubMed]
- Satish Kumar CS, Kondal Reddy K, Reddy AG, et al. Protective effect of Lactobacillus plantarum 21, a probiotic on trinitrobenzenesulfonic acid-induced ulcerative colitis in rats. Int Immunopharmacol 2015;25:504-10. [Crossref] [PubMed]
- Sakthivel KM, Guruvayoorappan C. Amentoflavone inhibits iNOS, COX-2 expression and modulates cytokine profile, NF-κB signal transduction pathways in rats with ulcerative colitis. Int Immunopharmacol 2013;17:907-16. [Crossref] [PubMed]
- Burdelya LG, Krivokrysenko VI, Tallant TC, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 2008;320:226-30. [Crossref] [PubMed]
- Fukuzawa N, Petro M, Baldwin WM 3rd, et al. A TLR5 agonist inhibits acute renal ischemic failure. J Immunol 2011;187:3831-9. [Crossref] [PubMed]
- Burdelya LG, Gleiberman AS, Toshkov I, et al. Toll-like receptor 5 agonist protects mice from dermatitis and oral mucositis caused by local radiation: implications for head-and-neck cancer radiotherapy. Int J Radiat Oncol Biol Phys 2012;83:228-34. [Crossref] [PubMed]
- Yoon SI, Kurnasov O, Natarajan V, et al. Structural basis of TLR5-flagellin recognition and signaling. Science 2012;335:859-64. [Crossref] [PubMed]
- Krivokrysenko VI, Shakhov AN, Singh VK, et al. Identification of granulocyte colony-stimulating factor and interleukin-6 as candidate biomarkers of CBLB502 efficacy as a medical radiation countermeasure. J Pharmacol Exp Ther 2012;343:497-508. [Crossref] [PubMed]
- Chow CP, Faqi AS. Developmental toxicity study of CBLB502 in Wistar rats. Reprod Toxicol 2014;46:12-9. [Crossref] [PubMed]
- Leigh ND, Bian G, Ding X, et al. A flagellin-derived toll-like receptor 5 agonist stimulates cytotoxic lymphocyte-mediated tumor immunity. PLoS One 2014;9:e85587. [Crossref] [PubMed]
- Burdelya LG, Brackett CM, Kojouharov B, et al. Central role of liver in anticancer and radioprotective activities of Toll-like receptor 5 agonist. Proc Natl Acad Sci U S A 2013;110:E1857-66. [Crossref] [PubMed]
- Li W, Ge C, Yang L, et al. CBLB502, an agonist of Toll-like receptor 5, has antioxidant and scavenging free radicals activities in vitro. Int J Biol Macromol 2016;82:97-103. [Crossref] [PubMed]
- Neurath MF, Fuss I, Kelsall BL, et al. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med 1995;182:1281-90. [Crossref] [PubMed]
- Xu Yang, Liu Haifeng. Department of Gastroenterology, et al. Preparation of a mouse model of TNBS-induced ulcerative colitis: technology improvement and optimal dose exploration. World Chinese Journal of Digestology 2012;20:106-12.
- Watkins PE, Warren BF, Stephens S, et al. Treatment of ulcerative colitis in the cottontop tamarin using antibody to tumour necrosis factor alpha. Gut 1997;40:628-33. [Crossref] [PubMed]
- Braat H, Peppelenbosch MP, Hommes DW. Interleukin-10-based therapy for inflammatory bowel disease. Expert Opin Biol Ther 2003;3:725-31. [Crossref] [PubMed]
- Rembacken BJ, Snelling AM, Hawkey PM, et al. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet 1999;354:635-9. [Crossref] [PubMed]
- Ochsenkühn T, D'Haens G. Current misunderstandings in the management of ulcerative colitis. Gut 2011;60:1294-9. [Crossref] [PubMed]
- Ogata H, Matsui T, Nakamura M, et al. A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut 2006;55:1255-62. [Crossref] [PubMed]
- Gaffney PR, Doyle CT, Gaffney A, et al. Paradoxical response to heparin in 10 patients with ulcerative colitis. Am J Gastroenterol 1995;90:220-3. [PubMed]
- Folwaczny C, Wiebecke B, Loeschke K. Unfractioned heparin in the therapy of patients with highly active inflammatory bowel disease. Am J Gastroenterol 1999;94:1551-5. [Crossref] [PubMed]
- Järnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 2005;128:1805-11. [Crossref] [PubMed]
- Summers RW, Elliott DE, Urban JF Jr, et al. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology 2005;128:825-32. [Crossref] [PubMed]
- Marteau P, Probert CS, Lindgren S, et al. Combined oral and enema treatment with Pentasa (mesalazine) is superior to oral therapy alone in patients with extensive mild/moderate active ulcerative colitis: a randomised, double blind, placebo controlled study. Gut 2005;54:960-5. [Crossref] [PubMed]
- Heller F, Florian P, Bojarski C, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005;129:550-64. [Crossref] [PubMed]
- Carter MJ, di Giovine FS, Jones S, et al. Association of the interleukin 1 receptor antagonist gene with ulcerative colitis in Northern European Caucasians. Gut 2001;48:461-7. [Crossref] [PubMed]
- Hibi T, Ohara M, Watanabe M, et al. Interleukin 2 and interferon-gamma augment anticolon antibody dependent cellular cytotoxicity in ulcerative colitis. Gut 1993;34:788-93. [Crossref] [PubMed]
- Raab Y, Gerdin B, Ahlstedt S, et al. Neutrophil mucosal involvement is accompanied by enhanced local production of interleukin-8 in ulcerative colitis. Gut 1993;34:1203-6. [Crossref] [PubMed]
- Kobori A, Yagi Y, Imaeda H, et al. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol 2010;45:999-1007. [Crossref] [PubMed]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010;140:805-20. [Crossref] [PubMed]
- Zeng H, Wu H, Sloane V, et al. Flagellin/TLR5 responses in epithelia reveal intertwined activation of inflammatory and apoptotic pathways. Am J Physiol Gastrointest Liver Physiol 2006;290:G96-G108. [Crossref] [PubMed]
- Vijay-Kumar M, Gewirtz AT. Flagellin: key target of mucosal innate immunity. Mucosal Immunol 2009;2:197-205. [Crossref] [PubMed]
- Gewirtz AT, Navas TA, Lyons S, et al. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol 2001;167:1882-5. [Crossref] [PubMed]
- de Zoete MR, Keestra AM, Wagenaar JA, et al. Reconstitution of a functional Toll-like receptor 5 binding site in Campylobacter jejuni flagellin. J Biol Chem 2010;285:12149-58. [Crossref] [PubMed]
- Hayashi F, Smith KD, Ozinsky A, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001;410:1099-103. [Crossref] [PubMed]


