The emerging role of immunotherapy in colorectal cancer
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in the United States with a 5-year survival rate for those with metastatic disease of 12% (1,2). Treatment modalities currently being used for metastatic CRC have been shown to have only modest efficacy and are also associated with significant toxicities (3). This unmet need for effective treatment of metastatic CRC has driven the search for novel strategies to improve survival while minimizing toxicities experienced by patients.
Immunotherapy has had promising results in solid tumors originally thought to be non-immunogenic, for example, lung cancer and melanoma (4). The immune system plays an intricate and complex role in all aspects of cancer from carcinogenesis to treatment (5). Over the past two decades in particular, great advances have been made in our understanding of the interplay between the immune system and cancer (Figure 1). This has led to the development of therapies such as cancer vaccines and adoptive cell therapy (ACT). These therapies have been shown to have an alternative side-effect profile in comparison to traditional chemotherapy yet, until recently, they had not been shown to be particularly effective (6). The FDA approval of immunotherapies such as the cancer vaccine sipuleucel-T and the immunomodulatory monoclonal antibodies (Abs) ipilimumab, nivolumab and pembrolizumab, heralds an exciting change in the direction of cancer therapeutics. This review will focus on the use of immunotherapy in CRC.
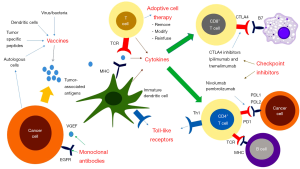
Vaccines
Cancer vaccines have been used to facilitate the immune destruction of cancer cells in many different tumor types. They work on the premise that the natural immune response of recognizing and destroying altered self-antigens is deficient in cancer. Some factors which contribute to this failure are the tumor’s ability to prevent immune activation by hiding tumor-associated antigens and also its ability to suppress the immune system here instead once it has been activated. Cancer vaccines overcome this failure by helping to activate and maintain an anti-tumor immune response. The following categories of vaccines autologous, peptide, dendritic cell and viral, are discussed.
Autologous vaccines
Autologous vaccines use cells which have been removed directly from a patient’s own tumor. This approach guarantees that the vaccines will contain all tumor-associated antigens specific to each individual patient (7). However, whole cell vaccines contain antigens in isolated cells, the majority of which are also present on normal tissue. Therefore, the response generated by the vaccine is generally not specific enough to have a substantial impact on the tumor burden. For this reason autologous vaccines to date have demonstrated limited efficacy (8).
In an effort to improve immunogenicity, whole cell vaccines have been modified in various ways. One phase III trial combined an autologous whole cell vaccine and the BCG vaccine to determine whether surgical resection plus the vaccine was more beneficial than resection alone in 412 stage II and III colon cancer patients (9). Patients were randomized to observation or to receive three weekly intradermal vaccines consisting of 1×107 irradiated autologous tumor cells which had been digested from their primary tumor and stored as a cell suspension. Vaccines were administered with BCG organisms for 2/3 administrations of which the first two injections contained BCG organisms and overall these produced local reactions in 79% of patients. After a median of 7.6 years of follow-up, there were no statistically significant differences between the groups in terms of disease-free and overall survival. However, the 5-year survival rate was 84.6% for those with indurations greater than 10 mm, compared with 45% for those with indurations less than 5 mm suggesting that those who developed a greater local reaction may have benefited from the vaccine-induced immune response. No specific antibody responses were measured in this study so it is unclear whether a cutaneous reaction can serve as a surrogate marker of response.
Autologous cell vaccines have also been modified to secrete granulocyte macrophage colony-stimulating factor (GM-CSF) which can induce anti-tumor immunity. While the trials have not been shown to have any significant impact on overall survival, the vaccine has been shown to augment antitumor immunity in melanoma (10) and lung cancer (11). In a phase I study, nine patients with metastatic CRC were treated with a CRC vaccine composed of irradiated, allogeneic human colon cancer cells and GM-CSF-producing bystander cells in combination with a single dose of cyclophosphamide in order to deplete regulatory T (Treg) cells (12). GM-CSF acts as an important differentiation factor for dendritic cells thereby improving their capacity to present tumor antigens. The vaccine was found to be well tolerated and immunological correlates showed that this vaccine enhanced the production of anti-MUC1 Abs which suggests T cell targeting. At last follow-up, six patients had survived longer than 36 months and four of these patients were without disease recurrence.
Another way of modifying the immune response which has been explored in more detail in CRC uses Newcastle disease virus-infected (NDV), irradiated whole cell tumor vaccines. This method was adopted following the results of previous trials which demonstrated that infecting autologous cells with NDV results in potent up-regulation of both the innate and adaptive immune system (13). A phase II study administered this vaccine to 23 patients after R0 resection of CRC liver metastases (14). Patients received autologous, irradiated metastasis-derived tumor cells incubated with 32 hemagglutinin units of NDV 2 weeks post-operatively and the vaccine was administered at 14-day intervals followed by a single boost 3 months later. After a follow-up of 18 months, 61% of patients had developed tumor recurrence whereas 87% of matched control patients (had similar surgery but were not vaccinated) developed recurrences (P=0.05). Around 40% of patients experienced delayed-type hypersensitivity reactivity when challenged with autologous tumor cells but correlations between reactivity and survival were not performed.
The resultant phase III study randomized 51 patients with histologically confirmed liver metastases from CRC who underwent metastectomy to receive six doses of NDV-infected autologous tumor cell vaccine or a control group (15). There was no significant difference in terms of overall survival where 48% of patients who received vaccines died during the observation period compared to 64% of patients in the control group. A subgroup analysis showed that patients with colon primary tumors had improved survival rates in terms of overall survival (HR: 3.3; 95% CI, 1–10.4; P=0.042) and metastasis-free survival (HR: 2.7; 95% CI, 1–7.4; P=0.047).
The data from autologous cell vaccines in CRC to date have not altered clinical practice and further efforts to improve the immunogenicity of these vaccines are needed and to determine subgroups of patients most likely to respond to autologous cell vaccines.
Peptide-based vaccines
Peptide vaccines attempt to target more specific components of tumor cells by identifying peptides which are 8–11 amino acids long and are unique to cancer cells. Advantages of peptide vaccines include their low cost, ease of production and storage, their unlimited potential for modifications and their ability to target tumor-specific antigens (16). Trials to date have encountered problems which have limited the effectiveness of peptide vaccines such as: antigenic escape resulting in re-occurrence and HLA-restriction limiting the peptide vaccines to specific HLA haplotypes (16).
Some tumor-associated antigens have been targeted by peptide vaccines in CRC including: EpHA2 (17), β-hCG (18), carcinoembryonic antigen (CEA) (19), SART3 (20), p53 (21), mucin 1 (22), survivin 2B (23) and RNF43/TOMM34 (24). Many of these trials have succeeded in generating an antigen-specific immune response, however, few trials have shown a resultant increase in survival. In a phase II trial, 77 patients were treated with a β-hCG vaccine composed of the COOH terminal peptide of β-hCG conjugated to diphtheria toxoid, of which 56 patients (73%) developed anti-hCG Abs (18). Patients who were found to have β-hCG antibody levels higher than the median had a median survival of 45 weeks in comparison to 24 weeks in patients found to have antibody levels lower than the median (P=0.0002). However, survival did not correlate with the level of antibody mounted to diphtheria toxoid.
There has been an attempt to overcome the limitations of peptide vaccines by targeting multiple epitopes with longer amino acid sequences. A phase II trial was conducted in 96 chemotherapy-naive patients with metastatic CRC using five HLA*2402-restricted peptides (two from VEGF derived receptors and three from oncoantigens) (25). They were administered concurrently with oxaliplatin-based chemotherapy (FOLFOX or XELOX) and the primary objective was to compare the chemotherapy/peptide vaccine in patients with and without the HLA-A*2402 haplotype. However, there were no significant differences in terms of response rate, progression-free survival and overall survival. Given that Treg depletion was not a feature in this trial, it is possible that responses to this combination of peptide vaccines may be enhanced by combining it with checkpoint inhibitor therapies/cyclophosphamide. A similar phase II trial was conducted using IMA910, a vaccine consisting of 13 tumor-associated peptides (TUMAPs), which are naturally presented on major histocompatibility complexes (MHC) molecules of colorectal tumors (26). Patients with HLA-A*02 CRC (n=82) who were clinically stable after 12 weeks of oxaliplatin-based therapy underwent immunomodulation with a single low dose of cyclophosphamide (300 mg/m2) and were immunized with IMA910 in combination with GM-CSF or a with IMA910, GM-CSF and topically applied imiquimod [toll-like receptor (TLR) 7 agonist] . Immune responses were noted in 71% and 72% of patients in both groups respectively. Patients who developed CD8 T-cell responses against numerous TUMAPs had an improved disease control rates compared to other in terms of disease control rate (18% vs. 2% at 6 months; P=0.012) and PFS (HR: 0.652; P=0.039). Further research efforts should be focused on identifying antigens specific to CRC in an attempt to improve clinical outcomes.
Dendritic cell vaccines
Dendritic cells play a key role in the key components of the immune system activation cascade and thus represent an opportunity for targeted immunotherapy (27). Antigens are presented by dendritic cells to T cells on MHC and co-stimulation is provided by a receptor-ligand pair. This combination then triggers the release of cytokines which mediate the immune response.
The original approach to dendritic cell vaccines involved infusing immature dendritic cells which would then pick up antigens in vivo. More novel vaccines have involved harvesting DCs from the patients, pulsing them ex vivo with tumor associated antigens (TAAs) (28), tumor cell lysates (29), apoptotic tumor cells (30), tumor RNA (31) or whole tumor cells (32), and allowing them to mature before re-infusing them with the aim of inducing a tumor-specific immune response. DC vaccines can also be modified to express co-stimulators such as CD40L (33).
CEA has commonly been the focus of clinical trials involving DC vaccines in CRC due to its known association with CRC. Four early phase trials showed that CEA vaccines were safe and effective in generating a CEA specific immune response (34-37). Following these positive results, there have not been any published results of any phase III trial looking at the efficacy of these vaccines in the CRC population. A phase II trial randomized patients pre-treated for metastatic CRC to receive an autologous tumor lysate dendritic cell vaccine plus best supportive care (BSC) or BSC (38). Although the vaccine was found to generate a tumor specific immune response, there were no benefits seen in terms of PFS (2.7 vs. 2.3 months, P=0.628) and OS (6.2 vs. 4.7 months, P=0.41) compared to BSC, and the study was terminated early due to futility.
Viral/bacterial antigen vaccines
The common theme which is evident in the ultimate failure to date of peptide, autologous and dendritic cell vaccines is the inability to generate an immune response that is specific yet substantial enough to positively affect clinical outcomes. The primary function of the immune system is to protect against foreign pathogens and the human immune system has evolved over time in response to exposure to such antigens. Therefore, it is possible that a mechanism exists which can overcome the vaccine immunogenicity quandary by incorporating the use of pathogenic vectors. However, some disadvantages of vector vaccines are their limited immune responses against the vector, cost, potential for pathogenesis, and potential for insertional mutagenesis (6).
A phase I trial involving vaccinating 58 patients with advanced CEA-expressing cancers with fowlpox-expressing CEA and TRICOM (three costimulatory molecules B7-1, ICAM-1 and LFA-3) resulted in CEA-specific T-cell responses and stabilization of disease in 40% patients for at least 4 months (39). A similar phase II trial randomized 118 patients with metastatic CRC to receive ALVAC virus expressing CEA and costimulatory B7-1 vaccine either: prior to chemotherapy (IFL/FOLFIRI), to receive tetanus toxoid in addition to the viral vaccine or to receive the vaccine if clinically responding after four cycles of chemotherapy (40). All patients developed antibody responses to ALVAC but only three patients developed anti-CEA Abs.
Anti-CEA specific T cell responses were seen in 50% of those who received the vaccine and 40.4% of patients were found to have a clinical response. There were no differences in clinical outcomes between the groups with the majority of clinical responses reported as disease stability (37.5%).
Two other potential pathogen vector vaccine targets which have been targeted in preclinical trials are MUC1 (41,42) and GUCY2C (guanylyl cyclase C) (43). MUC1 is a glycoprotein expressed on the surface of normal glandular epithelia, it has been found to be overexpressed in >70% of human CRC. MUC1 as a target for cytotoxic T-cells has also been used in vaccination of breast, ovarian, and metastatic renal cancer patients (41). GUCY2C (guanylyl cyclase C) is a transmembrane receptor with canonical functions in maintaining intestinal homeostasis that is usually only found on intestinal mucosa but is universally located on metastatic CRC (43). These studies provide good preclinical evidence that these targets merit further study in CRC and more research is needed to determine whether these epitopes can be successfully incorporated into vaccination strategies.
Cytokine therapy
Cytokines play a key role in all aspects of the innate and specific immune response. They function through extracellular signaling and operate in an autocrine and paracrine manner (44). Cytokine therapies which are currently being used in cancer treatment include IL-2 which has FDA approval for use in melanoma and renal cell carcinoma (RCC) and also IFN-α which is being studied in the treatment of multiple hematological malignancies as well as cervical cancer, carcinoid syndrome, medullary thyroid cancer, basal cell and squamous cell carcinoma. There have been limited studies involving cytokine therapies in CRC. A phase I study of PEGylated recombinant IL-10 (AM0010) administered daily subcutaneously for 4 months to 33 patients with advanced solid tumors (including four CRC patients) showed that this had a manageable safety profile and resulted in a sustained systemic Th1 immune stimulation (45). One patient with CRC had sustained disease stability greater than 40 weeks. Cytokine therapy represents an avenue that warrants further investigation in CRC and may be effective in combination with other immunotherapies although their toxicity profiles may likely limit their use in standard clinical practice.
TLR agonists
The vast majority of immunotherapeutic interventions in cancer have focused on adaptive immunity yet some recent approaches, including TLR agonists, target the innate immune system (46). Following tumor cell death, damage-associated molecular pattern molecules (DAMPs) are present on TLRs, which are found on innate immune cells (47). Currently ten human TLRs (TLR-1 to TLR-10) have been identified (48) and investigations in CRC have focused on TLR-9. TLR-9 has been shown to have a potential protective role against malignant transformation in colorectal mucosa (49). Early clinical trials implementing the use of CpG-oligodeoxynucleotides (CpG-ODN) as TLR agonists in various cancers were associated with both narrow clinical efficacy and significant toxicity (50,51). Next generation TLR-9 agonists (such as MGN1703 and double stem loop immunomodulators, dSLIM) have shown more promise than their predecessors (52). A phase II trial evaluated the use of MGN1703 as maintenance therapy in patients with metastatic CRC. Fifty-nine patients with mCRC who had stable disease after standard first-line chemotherapy were randomized to MGN1703 60 mg (n=43) or placebo (n=16). MGN1703 was found to not only be well-tolerated but also to induce durable and prolonged PFS (HR: 0.50; 95% CI, 0.31–1.02; P=0.02) (53). Retrospectively, certain pretreatment characteristics were found to be predictive of improved PFS including normalized CEA, objective response, and the presence of activated NK T cells at the end of induction chemotherapy, were also found to be predictive of OS (HR: 0.63, median 24.5 vs. 15.1 months, not statistically significant) however these data were premature as only 35% patients who received MGN1703 had a survival event (compared to 50% of control patients) (54). A phase III trial, IMPALA trial of MGN1703 is being designed as maintenance therapy in patients with metastatic CRC after standard first-line therapy (ClinicalTrials.gov: NCT02077868).
ACT
Adoptive cell transfer is a form of passive immunotherapy that involves transferring immune cells or molecules to the host. The most successful approaches to ACT have involved harvesting autologous T cells from a tumor (TILs), then activating and expanding them to larger numbers ex vivo prior to reinfusing them back to the patient. Modifying T cells in this way allows T cells to overcome immune inhibition and tolerance which may have occurred in vivo (55). This method has had success in certain tumor types classically associated to be highly immunogenic, particularly melanoma (56). However, some of the difficulties encountered by ACT are the lack of immune memory, the time and cost associated with producing T cells and poor persistence in vivo (6).
Attempts to improve the efficacy of ACT include the genetic modification of T cells to express high avidity for T cell receptors (TCRs) (57). Antibody-based chimeric antigen receptors (CARs) combine intracellular signaling domains with the expression of a single chain variable fragment derived from a tumor-associated antigen capable of recognizing monoclonal antibody (58). There has been considerable interest in CAR T cells due to success with CD19-targeting T cells in hematologic malignancies (58).
Three patients with mCRC were treated with T cells which had been modified to express high avidity for CEA specific murine TCR in a phase I study (59). The murine TCR specifically mediates the recognition of peptide-loaded HLA-A*201+ T2 cells and CEA+ human CRC cell lines in vitro and genetically modified autologous T lymphocytes were adoptively transferred into patients with IL-2 after receiving lymphodepleting chemotherapy comprising cyclophosphamide and fludarabine. All three patients experienced reductions in CEA levels (74–99%) and one patient had an objective clinical response (partial) although they all developed severe transient inflammatory colitis constituting grade 3 diarrhea with subsequent halting of study accrual. It is plausible that colonic flora contribute to the development of colitis in the presence of lymphopenic conditions due to TLR stimulation. The potential toxicities of inadvertently targeting expression of antigens in normal tissues were also seen in a case report of a patient with mCRC who was treated with Her2-specific CAR T cells (60). In this case, a patient developed severe respiratory distress shortly after the infusion of 1×1010 autologous CAR T cells over-expressing ERBB2 resulting in the death of this patient possibly due to a cytokine storm triggered by the recognition of native ERBB2 expression in normal lung tissue. A phase I/II study of adjuvant immunotherapy involving sentinel lymph node (SLN) T lymphocytes in 55 patients with mCRC did not observe any treatment-related toxicity (61). At 24 months, 55.6% of the SLN T lymphocyte group were alive vs. 17.5% of the control group (P=0.02). Overall survival was significantly improved in those who received SLN-T lymphocyte compared to control (28 vs. 1 month, respectively). This study represents a significant breakthrough in CRC ACT however, further studies and a phase III study is needed to further assess efficacy.
Monoclonal antibody-based therapy
Monoclonal Abs have been shown to have clinical utility in the treatment of metastatic CRC (62). These Abs incur significant production costs and can cause hypersensitivity reactions in patients due to foreign (murine) protein recognition. Abs which have been approved include: cetuximab (63) or panitumumab (64) which targets EGFR and anti-VEGF Abs bevacizumab (65) and aflibercept (66). Regorafenib, a tyrosine kinase inhibitor, has also been approved for use in refractory metastatic CRC (67). There are several ongoing clinical trials involving these targeted therapies in CRC (Table 1). An in-depth discussion of the use of monoclonal Abs is beyond the scope of this review.
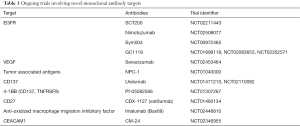
Full table
Checkpoint inhibition
The most notable development in immunotherapy in the last decade has been the emergence of checkpoint inhibitors. Checkpoint inhibitors are monoclonal Abs which modify MHC-TCR signaling pathways by targeting co-inhibitory molecules such as PD-1, PD-L1/2, CTLA-4, lymphocyte-activation gene 3 (LAG-3), T-cell immunoglobulin mucin-3 (TIM-3), and B and T lymphocyte attenuator (BTLA). Co-inhibitory molecules suppress the immune system by inducing T-cell apoptosis or dysfunction. Tumors harness these pathways in order to evade the human immune system (68).
CTLA-4
CTLA-4 is a receptor found on the surface of CD4 and CD8 T cells. It prevents immune stimulation by binding to B7 ligands on antigen presenting cells (APCs) and preventing immune stimulation which is normally provided by B7 binding to a CD28 receptor on T cells (69). Ipilimumab and tremelimumab are CTLA-4 inhibitors which have been developed for use in humans. Ipilimumab was approved by the FDA in 2011 for use in metastatic melanoma when two landmark phase III clinical trials showed that it was associated with improved survival (4). However these checkpoint inhibitors have failed to demonstrate similar success in CRC. A phase II trial administered with tremelimumab IV every 90 days until progression to 47 patients who had failed standard therapies (70). This treatment was relatively well tolerated in comparison to checkpoint inhibitor studies with 63.8% experiencing treated-related adverse events and 19.1% of patients had ≥ grade 3 toxicities. The vast majority of patients (n=44) did not receive a second dose due to progressive disease with only a single partial response recorded. Although this study included heavily pre-treated patients, it does not suggest further study of CTLA4 blockade as monotherapy in metastatic CRC.
PD-1
PD-1 belongs to the same CD28 receptor family as CTLA-4, while CTLA-4 prevents naïve T cell activation, PD-1 mainly induces exhaustion or anergy in effector T cells. PD-1 is particularly over-expressed in chronic inflammatory states and malignancy (71) and PD-1 inhibitors have been associated with impressive durable clinical responses in non-small cell lung cancer, RCC and bladder cancer (72-77).
Until recently however only a minority of patients with CRC demonstrated significant responses to PD-1 blockade. A phase I study looked at 39 patients with multiple tumor types including metastatic melanoma, CRC, castrate-resistant prostate cancer, NSCLC, or RCC. One durable complete response (CRC) and two partial responses (melanoma, RCC) were observed in this study. A phase II study looked at 296 patients with similar tumor types and response rates for NSCLC, melanoma and RCC were 18%, 28% and 27% respectively while no objective response was seen in patients with CRC or prostate cancer (76). The results of these trials raised important questions regarding the low response rates in patients with CRC and what factors distinguished the single patient who did respond from other patients with CRC (78).
It was hypothesized that the response rates were directly related to the mutational burden of the tumor (78). CRC is usually associated with a low mutational burden unless the tumor is deficient in mismatch repair (dMMR) (also known as microsatellite instability). The solitary patient with CRC who had responded to the PD-1 inhibitor in the trial was subsequently found to have dMMR. A phase II trial was conducted in order to test this hypothesis which aimed to determine the clinical activity of pembrolizumab (PD-1 inhibitor) in 41 patients with mCRC with or without dMMR. Patients with dMMR had improved response rates compared to those without dMMR (78% vs. 40%) and immune-related progression free survival rates were also improved in those with dMMR CRC (11% vs. 0%). A significant difference in somatic mutations per tumor was noted following whole-exome sequencing with a mean of 1,782 somatic mutations in the dMMR cohort vs. 73 somatic mutations proficient mismatch repair (pMMR) tumors (P=0.007). This increase in mutational burden was in turn found to be associated with prolonged overall survival (P=0.02). More research is needed to determine the role of checkpoint inhibitors in those with proficient mismatch repair CRC, particularly finding strategies to overcome resistance to immune checkpoint blockade CRC.
Combination therapy
Due to the complex nature of tumorigenesis and modest levels of success of immunotherapies as monotherapy, recent trials have investigated whether clinical benefits can be improved using combinatorial therapies. Preclinical trials have had some success with various combinations of radiofrequency ablation, vaccines and checkpoint inhibitors. In patients with metastatic melanoma, a phase I study combined nivolumab and ipilimumab in 53 patients and found that the maximum tolerated dose induced an objective response in 53% of patients (80% or more reduction in tumor growth) (79). There are numerous ongoing trials examining the utility of combination immunotherapy for patients with advanced CRC (Table 2). The major challenge with these trials is to improve clinical benefit while developing regimens with acceptable toxicity profiles. As oncologists increasingly prescribe immunotherapeutic agents, it is imperative that they optimize patient safety by recognizing and promptly managing the autoimmune complications that can occur with these agents (80). In addition, the future landscape of immunotherapy in CRC will likely incorporate combinations of immunotherapeutic drugs with non-immunotherapeutic agents e.g., anti-angiogenic drugs (81).

Full table
Conclusions
Currently the prognosis for individuals with metastatic CRC remains poor. There has been limited success with traditional immunotherapeutic approaches in the past. However, immunotherapy now represents a possible avenue for improving survival for a subset of patients with CRC. Our understanding of the interaction between the immune system and the tumor microenvironment has progressed substantially in the last decade and this has resulted in the development of novel targeted therapies such as checkpoint inhibitors which have been shown significant promises. The success of these novel therapies has largely been confined to malignancies which are associated with a high mutational burden such as RCC, melanoma and non-small cell lung cancer. However, this success has been replicated in patients with deficient mismatch repair CRC. Ongoing trials in CRC are focused on expanding the use of these new immunotherapies and combining these therapies aiming to improve clinical outcomes. The challenge remains in identifying subsets of patients who are likely to respond to these therapies in addition to producing novel regimens with acceptable toxicity profiles.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104-17. [Crossref] [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- Gallagher DJ, Kemeny N. Metastatic colorectal cancer: from improved survival to potential cure. Oncology 2010;78:237-48. [Crossref] [PubMed]
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Markman JL, Shiao SL. Impact of the immune system and immunotherapy in colorectal cancer. J Gastrointest Oncol 2015;6:208-23. [PubMed]
- Xiang B, Snook AE, Magee MS, et al. Colorectal cancer immunotherapy. Discov Med 2013;15:301-8. [PubMed]
- Koido S, Ohkusa T, Homma S, et al. Immunotherapy for colorectal cancer. World J Gastroenterol 2013;19:8531-42. [Crossref] [PubMed]
- Klebanoff CA, Acquavella N, Yu Z, et al. Therapeutic cancer vaccines: are we there yet? Immunol Rev 2011;239:27-44. [Crossref] [PubMed]
- Harris JE, Ryan L, Hoover HC, et al. Adjuvant active specific immunotherapy for stage II and III colon cancer with an autologous tumor cell vaccine: Eastern Cooperative Oncology Group Study E5283. J Clin Oncol 2000;18:148-57. [PubMed]
- Soiffer R, Hodi FS, Haluska F, et al. Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanoma. J Clin Oncol 2003;21:3343-50. [Crossref] [PubMed]
- Salgia R, Lynch T, Skarin A, et al. Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non-small-cell lung carcinoma. J Clin Oncol 2003;21:624-30. [Crossref] [PubMed]
- Zheng L, Edil BH, Soares KC, et al. A safety and feasibility study of an allogeneic colon cancer cell vaccine administered with a granulocyte-macrophage colony stimulating factor-producing bystander cell line in patients with metastatic colorectal cancer. Ann Surg Oncol 2014;21:3931-7. [Crossref] [PubMed]
- Liebrich W, Schlag P, Manasterski M, et al. In vitro and clinical characterisation of a Newcastle disease virus-modified autologous tumour cell vaccine for treatment of colorectal cancer patients. Eur J Cancer 1991;27:703-10. [Crossref] [PubMed]
- Schlag P, Manasterski M, Gerneth T, et al. Active specific immunotherapy with Newcastle-disease-virus-modified autologous tumor cells following resection of liver metastases in colorectal cancer. First evaluation of clinical response of a phase II-trial. Cancer Immunol Immunother 1992;35:325-30. [Crossref] [PubMed]
- Schulze T, Kemmner W, Weitz J, et al. Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: results of a prospective randomized trial. Cancer Immunol Immunother 2009;58:61-9. [Crossref] [PubMed]
- Bartnik A, Nirmal AJ, Yang SY. Peptide Vaccine Therapy in Colorectal Cancer. Vaccines 2012;1:1-16. [Crossref] [PubMed]
- Yamaguchi S, Tatsumi T, Takehara T, et al. EphA2-derived peptide vaccine with amphiphilic poly(gamma-glutamic acid) nanoparticles elicits an anti-tumor effect against mouse liver tumor. Cancer Immunol Immunother 2010;59:759-67. [Crossref] [PubMed]
- Moulton HM, Yoshihara PH, Mason DH, et al. Active specific immunotherapy with a beta-human chorionic gonadotropin peptide vaccine in patients with metastatic colorectal cancer: antibody response is associated with improved survival. Clin Cancer Res 2002;8:2044-51. [PubMed]
- Bilusic M, Heery CR, Arlen PM, et al. Phase I trial of a recombinant yeast-CEA vaccine (GI-6207) in adults with metastatic CEA-expressing carcinoma. Cancer Immunol Immunother 2014;63:225-34. [Crossref] [PubMed]
- Miyagi Y, Imai N, Sasatomi T, et al. Induction of cellular immune responses to tumor cells and peptides in colorectal cancer patients by vaccination with SART3 peptides. Clin Cancer Res 2001;7:3950-62. [PubMed]
- Speetjens FM, Kuppen PJK, Welters MJP, et al. Induction of p53-specific immunity by a p53 synthetic long peptide vaccine in patients treated for metastatic colorectal cancer. Clin Cancer Res 2009;15:1086-95. [Crossref] [PubMed]
- Kimura T, McKolanis JR, Dzubinski LA, et al. MUC1 vaccine for individuals with advanced adenoma of the colon: a cancer immunoprevention feasibility study. Cancer Prev Res (Phila) 2013;6:18-26. [Crossref] [PubMed]
- Idenoue S, Hirohashi Y, Torigoe T, et al. A potent immunogenic general cancer vaccine that targets survivin, an inhibitor of apoptosis proteins. Clin Cancer Res 2005;11:1474-82. [Crossref] [PubMed]
- Okuno K, Sugiura F, Hida JI, et al. Phase I clinical trial of a novel peptide vaccine in combination with UFT/LV for metastatic colorectal cancer. Exp Ther Med 2011;2:73-9. [PubMed]
- Hazama S, Nakamura Y, Tanaka H, et al. A phase II study of five peptides combination with oxaliplatin-based chemotherapy as a first-line therapy for advanced colorectal cancer (FXV study). J Transl Med 2014;12:108. [Crossref] [PubMed]
- Mayer F, Mayer-Mokler A, Nowara E, et al. A phase I/II trial of the multipeptide cancer vaccine IMA910 in patients with advanced colorectal cancer (CRC). J Clin Oncol 2012;30:abstr 555.
- Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013;39:38-48. [Crossref] [PubMed]
- Celluzzi CM, Mayordomo JI, Storkus WJ, et al. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med 1996;183:283-7. [Crossref] [PubMed]
- Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumorlysate-pulsed dendritic cells. Nat Med 1998;4:328-32. [Crossref] [PubMed]
- Berard F, Blanco P, Davoust J, et al. Cross-priming of naive CD8 T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. J Exp Med 2000;192:1535-44. [Crossref] [PubMed]
- Koido S, Kashiwaba M, Chen D, et al. Induction of antitumor immunity by vaccination of dendritic cells transfected with MUC1 RNA. J Immunol 2000;165:5713-9. [Crossref] [PubMed]
- Gong J, Chen D, Kashiwaba M, et al. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med 1997;3:558-61. [Crossref] [PubMed]
- Liu Y, Zhang X, Zhang W, et al. Adenovirus-mediated CD40 ligand gene-engineered dendritic cells elicit enhanced CD8(+) cytotoxic T-cell activation and antitumor immunity. Cancer Gene Ther 2002;9:202-8. [Crossref] [PubMed]
- Morse MA, Deng Y, Coleman D, et al. A Phase I study of active immunotherapy with carcinoembryonic antigen peptide (CAP-1)-pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembryonic antigen. Clin Cancer Res 1999;5:1331-8. [PubMed]
- Fong L, Hou Y, Rivas A, et al. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci U S A 2001;98:8809-14. [Crossref] [PubMed]
- Itoh T, Ueda Y, Kawashima I, et al. Immunotherapy of solid cancer using dendritic cells pulsed with the HLA-A24-restricted peptide of carcinoembryonic antigen. Cancer Immunol Immunother 2002;51:99-106. [Crossref] [PubMed]
- Lesterhuis WJ, de Vries IJM, Schuurhuis DH, et al. Vaccination of colorectal cancer patients with CEA-loaded dendritic cells: antigen-specific T cell responses in DTH skin tests. Ann Oncol 2006;17:974-80. [Crossref] [PubMed]
- Maurel J, Caballero-Baños M, Mila J, et al. Phase II randomized trial of autologous tumor lysate dendritic cell vaccine (ADC) plus best supportive care (BSC) compared with BSC, in pre-treated advanced colorectal cancer patients. J Clin Oncol 2015;33:abstr 3048.
- Marshall JL, Gulley JL, Arlen PM, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol 2005;23:720-31. [Crossref] [PubMed]
- Kaufman HL, Lenz HJ, Marshall J, et al. Combination chemotherapy and ALVAC-CEA/B7.1 vaccine in patients with metastatic colorectal cancer. Clin Cancer Res 2008;14:4843-9. [Crossref] [PubMed]
- Chen D, Koido S, Li Y, et al. T cell suppression as a mechanism for tolerance to MUC1 antigen in MUC1 transgenic mice. Breast Cancer Res Treat 2000;60:107-15. [Crossref] [PubMed]
- Mukherjee P, Pathangey LB, Bradley JB, et al. MUC1-specific immune therapy generates a strong anti-tumor response in a MUC1-tolerant colon cancer model. Vaccine 2007;25:1607-18. [Crossref] [PubMed]
- Snook AE, Stafford BJ, Li P, et al. Guanylyl cyclase C-induced immunotherapeutic responses opposing tumor metastases without autoimmunity. J Natl Cancer Inst 2008;100:950-61. [Crossref] [PubMed]
- Omrane I, Medimegh I, Baroudi O, et al. Involvement of IL17A, IL17F and IL23R Polymorphisms in Colorectal Cancer Therapy. PLoS One 2015;10:e0128911. [Crossref] [PubMed]
- Infante JR, Naing A, Papadopoulos KP, et al. A first-in-human dose escalation study of PEGylated recombinant human IL-10 (AM0010) in advanced solid tumors. J Clin Oncol 2015;33:abstr 3017.
- Wittig B, Schmidt M, Scheithauer W, et al. MGN1703, an immunomodulator and toll-like receptor 9 (TLR-9) agonist: from bench to bedside. Crit Rev Oncol Hematol 2015;94:31-44. [Crossref] [PubMed]
- Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev 2008;18:11-8. [Crossref] [PubMed]
- Thompson MR, Kaminski JJ, Kurt-Jones EA, et al. Pattern recognition receptors and the innate immune response to viral infection. Viruses 2011;3:920-40. [Crossref] [PubMed]
- Eiró N, González L, González LO, et al. Study of the expression of toll-like receptors in different histological types of colorectal polyps and their relationship with colorectal cancer. J Clin Immunol 2012;32:848-54. [Crossref] [PubMed]
- Hirsh V, Paz-Ares L, Boyer M, et al. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2011;29:2667-74. [Crossref] [PubMed]
- Machiels JP, Kaminsky MC, Keller U, et al. Phase Ib trial of the Toll-like receptor 9 agonist IMO-2055 in combination with 5-fluorouracil, cisplatin, and cetuximab as first-line palliative treatment in patients with recurrent/metastatic squamous cell carcinoma of the head and neck. Invest New Drugs 2013;31:1207-16. [Crossref] [PubMed]
- Weihrauch MR, Richly H, von Bergwelt-Baildon MS, et al. Phase I clinical study of the toll-like receptor 9 agonist MGN1703 in patients with metastatic solid tumours. Eur J Cancer 2015;51:146-56. [Crossref] [PubMed]
- Schmoll HJ, Wittig B, Arnold D, et al. Maintenance treatment with the immunomodulator MGN1703, a Toll-like receptor 9 (TLR9) agonist, in patients with metastatic colorectal carcinoma and disease control after chemotherapy: a randomised, double-blind, placebo-controlled trial. J Cancer Res Clin Oncol 2014;140:1615-24. [Crossref] [PubMed]
- Riera-Knorrenschild J, Arnold D, Kopp HG, et al. A subgroup with improved overall survival from the phase 2 IMPACT study: Maintenance therapy of metastatic colorectal cancer patients with the TLR-9 agonist MGN1703. J Clin Oncol 2015;33:abstr 3049.
- Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 2012;12:269-81. [Crossref] [PubMed]
- Rosenberg SA, Yang JC, Sherry RM, et al. Durable Complete Responses in Heavily Pretreated Patients with Metastatic Melanoma Using T-Cell Transfer Immunotherapy. Clin Cancer Res 2011;17:4550-7. [Crossref] [PubMed]
- Robbins PF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011;29:917-24. [Crossref] [PubMed]
- Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509-18. [Crossref] [PubMed]
- Parkhurst MR, Yang JC, Langan RC, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 2011;19:620-6. [Crossref] [PubMed]
- Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010;18:843-51. [Crossref] [PubMed]
- Zhen YH, Liu XH, Yang Y, et al. Phase I/II study of adjuvant immunotherapy with sentinel lymph node T lymphocytes in patients with colorectal cancer. Cancer Immunol Immunother 2015;64:1083-93. [Crossref] [PubMed]
- Kurniali PC, Hrinczenko B, Al-Janadi A. Management of locally advanced and metastatic colon cancer in elderly patients. World J Gastroenterol 2014;20:1910-22. [Crossref] [PubMed]
- Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408-17. [Crossref] [PubMed]
- Giusti RM, Shastri KA, Cohen MH, et al. FDA drug approval summary: panitumumab (Vectibix). Oncologist 2007;12:577-83. [Crossref] [PubMed]
- Cohen MH, Gootenberg J, Keegan P, et al. FDA drug approval summary: bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. Oncologist 2007;12:356-61. [Crossref] [PubMed]
- Wang TF, Lockhart AC. Aflibercept in the treatment of metastatic colorectal cancer. Clin Med Insights Oncol 2012;6:19-30. [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [Crossref] [PubMed]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011;331:1565-70. [Crossref] [PubMed]
- Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res 2013;19:4917-24. [Crossref] [PubMed]
- Chung KY, Gore I, Fong L, et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol 2010;28:3485-90. [Crossref] [PubMed]
- Ito A, Kondo S, Tada K, et al. Clinical Development of Immune Checkpoint Inhibitors. Biomed Res Int 2015;2015:605478.
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [Crossref] [PubMed]
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. [Crossref] [PubMed]
- Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558-62. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Topalian SL, Sznol M, McDermott DF, et al. Survival, Durable Tumor Remission, and Long-Term Safety in Patients With Advanced Melanoma Receiving Nivolumab. J Clin Oncol 2014;32:1020-30. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122-33. [Crossref] [PubMed]
- Teply BA, Lipson EJ. Identification and management of toxicities from immune checkpoint-blocking drugs. Oncology (Williston Park) 2014;28:30-8. [PubMed]
- Hodi FS, Lawrence D, Lezcano C, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res 2014;2:632-42. [Crossref] [PubMed]


