PillCam colon capsule endoscopy (PCCE) in colonic diseases
Introduction
Diseases affecting the colon are common worldwide and can cause a major health problem. Colorectal cancer (CRC) represents a major cause of morbidity and mortality in Western countries (1).
Although colonoscopic screening for colorectal precancerous lesions and removal of polyps has been shown to reduce the risk of CRC (2), the rate of CRC screening acceptance is still low in most western countries (3). This may be explained by the invasive nature of the procedure, which is not free of complications, and the perception of the exam as unpleasant and sometimes painful (4). Moreover, limited resources may further hamper the possibility of colonoscopic screening of the general population. Technical failure in completing colonoscopy may further limit the usefulness of colonoscopy as a screening tool (5), as proximal CRC incidence was found to be increased by up to two folds in these cases (6). Undoubtedly, Increase of the use and enhancement of the quality of CRC screening programs is still needed.
Similarly, colonoscopy is still the gold standard for the diagnosis and surveillance of inflammatory bowel disease (IBD) (7). Monitoring for mucosal inflammation, response to treatment, and suspected complications may require repeated colonoscopic exams at different disease stages, adding inconvenience, discomfort, and risk to the patients (8).
PillCam colon capsule endoscopy (PCCE) is a novel and promising technology that can be useful for the screening and monitoring of colonic diseases. Table 1 summarizes the possible indications for the use of PCCE.

Full table
The aim of this review is to provide up to date information regarding the use and usefulness of this method.
The PCCE system
PCCE is a noninvasive imaging technique that enables the demonstration of the colonic lumen and mucosa without requiring sedation or air insufflation. The first generation PillCam Colon capsule (Given Imaging Ltd., Yoqneam, Israel) was introduced is 2006 and included a 31 mm × 11 mm ingestible capsule, equipped with 2 cameras, enabling it to acquire images from both ends and angle of view of 156°. The capsule captured 4 pictures per second and had a battery life of 10–11 hours. The second generation PCCE (PCCE-2; Covidien/Medtronic, Dublin, Ireland) was introduced later on. It is a wireless capsule (11.6 mm × 31.5 mm) comprised of a light source, 2 cameras (one at each side), battery and a wireless transmitter. A slippery coating allows easy ingestion and prevents adhesion of bowel contents, as it moves via peristalsis from the Mouth to the Anus. The two main modifications from PCCE-1 were that each of the two cameras has and angle of view of 172°, therefore achieving coverage of nearly 360°. The second major modification was that PCCE-2 has an adaptive frame rate-PCCE-2 captures four images per second in an immobile state and 35 images per second when in motion (Figure 1). The new DR3 data recorder records the images and analyzes the pictures in real time and controls the capsule capture rate of images requirement at an adaptive frame rate. When the DR3 recognizes that the capsule is immobile, it reduces the image capture rate to four frames per second. When motion is sensed, the DR3 sets the image capture rate to 35 frames per second.
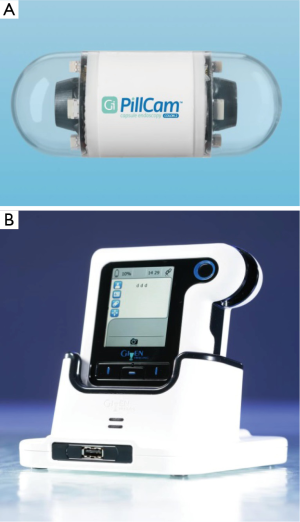
The DR3 has also an important role in guiding the physician and the patient through the procedure. When the video capsule detects intestinal villi, the DR3 buzzes, vibrates and displays instructions to alert the patient to continue the preparation protocol. A recent study that examined the reliability of automatic detection of small bowel mucosa and the subsequent alert for booster ingestion by the DR3 reported a sensitivity of 98.3% (10). After the conclusion of the examination, the recorder is downloaded into a reporting and processing of images and data computer workstation (RAPID 8) and seen as a continuous video film. The new RAPID software includes a graphic interface tool for polyp size estimation. As reading of the video film of the examination is time consuming, a “quick view” (QV) system which reduces the number of the analyzed frames was recently examined. Depending on its setting, the reliability of QV in presenting was notable. At the 30% QV setting, 89% of the significant polyps and 86% of any polyps were reported, and at a 10% QV setting, 98% the polyps could be identified (11). Further research focuses on automatic polyp detection analysis of the examination. At this respect, an algorithm that labels the frame as either containing polyps or not based on geometrical analysis and the texture content of the frame was developed and preliminary results suggested that the proposed geometry-based polyp detection scheme actually works well (12).
Preparation for PCCE
The preparation of the colon before and during the procedure serves for four proposals: cleansing the colon in order to achieve the best possible view, facilitating the capsule movement during the procedure and improving the vision by inducing a submarine view of the submerged capsule, and allowing its excretion while still photographing.
The patients are on a clear liquid diet the day before the procedure. On the evening before the examination, the patients ingest 2 L of polyethylene glycol (PEG) solution, followed by an additional 2 L in the morning of the procedure. Afterwards the patient swallows the capsule. When the capsule is detected in the small bowel, the patients ingest 40 mL of a NaP solution (Fleet Phosphosoda) with 1 L of water in which 50 mL of gastrografin are put in order to facilitate the progression of the capsule. The progression of the capsule is assessed using the rapid access real-time viewing system, and the patient is released only after confirming that the capsule passed into the colon. If the capsule is not excreted, 3 hours after the first boost a second boost consisting of 25 mL of NaP solution +0.5 L of water +25 mL gastrografin is given, followed a 10 mg bisacodyl suppository 2 h after the second boost if the capsule was not expelled (13). With this regimen PCCE completion rate reaches 98%. Other possible preparations that were compared to the conventional preparation method include a 1-day preparation that includes a fiber-free diet and 3 L of PEG on day 0, with no PEG on day 2 (14), and a reduced volume method, in which the patients ingest 2 L of PEG only in the morning before the procedure, followed by boosters of 100 g magnesium citrate mixed with 900 mL water 2 and 6 hours after ingestion (15). There were no significant differences in terms of colon cleanliness between the conventional and both described.
Polyp detection rate using the PCCE system
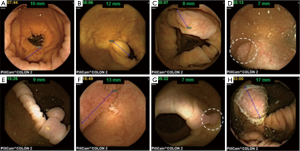
After the initial positive signal from a multicenter Israeli study (16), the 1st generation colon capsule was evaluated in a multicenter European study including 328 patients with known or suspected colonic disease (17). Capsule exertion while photographing was 92.8%, and acceptable colonic visualization in 72%. The sensitivity and specificity of PCCE-1 for detecting polyps >6 mm in size were 64% and 84%, respectively, and for detecting advanced adenoma, 73% and 79%, respectively. The sensitivity for detection of CRC was 74% (14/19 cases). These rather disappointing results led to the development of the 2nd generation colon capsule. The accuracy of PCCE-2 for detection of colonic polyps and cancer was examined both on average risk population and as well as on patients with suspected or known colonic disease Figure 2 demonstrates typical endoscopic findings using PCCE-2 in this study. In a cohort of 98 patients (32% screening, 68% suspected or known colonic disease), the colonic cleanliness was adequate in 78%. The sensitivity and specificity for the detection of patients with polyps ≥6 mm were 89% and 76%, respectively, and for the detection of polyps ≥10 mm 88% and 89%, respectively (9). In another prospective, multicenter trial, including 117 patients (21% of which were for CRC screening), the overall cleansing level was adequate in 81%, and 88% of the capsules were naturally egested within 10 hours. Per patient PCCE-2 sensitivity and specificity for the detection of polyps ≥6 mm was 84% and 64%, and for polyps ≥10 mm it was 88% and 95%, respectively. All three invasive carcinomas were detected by the capsule (18). Recently, the results of a large prospective multicenter study comparing PCCE-2 to colonoscopy in average risk population screened for CRC were published (19). Of the 695 patients included in the study, 77 were excluded because the capsule did not reach the colon by 12 hours after ingestion, or did not leave the cecum. Bowel preparation was adequate for the entire colon in 80% of patients. The capsule was excreted within 12 hours in 92%. The capsule sensitivity and specificity for detecting subjects with any polyp ≥6 mm was 81% and 93%, respectively, and for the detection of polyps ≥10 mm was 80% and 97%, respectively. PCCE-2 sensitivity and specificity for the detection of subjects with conventional adenomas ≥6 mm was 88% and 82%, respectively, and for the detection of conventional adenoma ≥10 mm were 92% and 95%, respectively. Conventional colonoscopy detected 4 cancers in 4 patients. The capsule identified three cancers for a per-lesion sensitivity of 75%. The cancer that was missed was a 10 mm sessile lesion in the sigmoid colon that was seen retrospectively by another reader (Table 2).

Full table
Another interesting study examined the uptake of PCCE-2 versus conventional colonoscopy for the screening of asymptomatic first degree relatives (FDRs) of CRC patients (20). Among the 120 eligible FDRs who were assigned to undergo colon capsule endoscopy (CCE), only 68 (56.6%) agreed to participate in the study. Overall, 29 (24.2%) accepted the assigned strategy and 28 (23.3%) finally underwent CCE, whereas 39 (32.5%) who declined CCE preferred colonoscopy. Among the 113 eligible FDRs who were invited to undergo colonoscopy, 63 (55.8%) agreed to participate. Of these 42 (37.1%) underwent colonoscopy, whereas 19 (16.8%) preferred CCE, and 17 (15.0%) performed the procedure. Therefore, Contrary to expectations, screening uptake was similar between CCE and colonoscopy in the study population.
PCCE in patients with incomplete colonoscopy
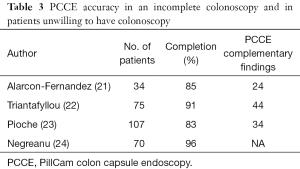
Failure to reach the cecum during a “difficult colonoscopy” is estimated to occur in 1–43% of colonoscopies (5). The place of PCCE in case of incomplete colonoscopy was examined in few studies (Table 3). In a prospective study including 34 patients with incomplete colonoscopy, PCCE exceeded the most proximal point reached by conventional colonoscopy in 85.3% of patients and allowed formulation of a specific management plan in 58.8% (21). In another prospective study, PCCE was compared to computed tomographic colonography (CTC) in 100 patients with previous incomplete colonoscopy (12). PCCE detected more polyps ≥6 mm than the CTC in the area that was not examined by standard colonoscopy, although this was not statistically significant. In a third prospective study that included 75 patients with incomplete colonoscopy, PCCE reached or went beyond the colon segment at which colonoscopy stopped in 68 patients (91%), and additional significant findings were diagnosed in 36% of the same-day cases and in 48% of the rescheduled ones (22).
Full table
Early in 2014, The FDA approved the use of CCE-2 in patients following incomplete colonoscopy.
PCCE in patients with high risk for colonoscopy
The yield of PCCE-1 for the detection of colonic polyps in a combined population of high risk patients unable to go through anesthesia and technical failure was examined in a French multicenter study in 107 patients (28% high risk) (23). Complete colorectal examination by capsule was achieved in 83.2 % of cases. A significant diagnosis was made in 33.6 % of patients, and a medical or surgical treatment scheduled in 21 % of them. In another study that included 70 high-risk patients who were unable or unwilling to undergo, PCCE-2 demonstrated significant findings in 34% of the patients, including four with colon cancers, one gastric cancer, and one with small bowel cancer (24). Table 3 summarizes the significant studies in this population.
PCCE versus CTC
Two prospective studies compared PCCE-2 to CTC. The larger mention above by Spada et al. compared both modalities in 100 patients with incomplete colonoscopy (13). The relative sensitivity of PCCE for detection of polyps ≥6 mm compared to CTC was 2, and for polyps ≥10 mm was 1.67. Another study by Rondonotti et al. compared 50 such patients with similar sensitivities and specificities between the two procedures (25).
PCCE as screener after a positive fecal immunochemical test (FIT)
The role of PCCE as a screening procedure after a positive FIT examination has been evaluated in two rather small studies. The authors of both studies found PCCE’s sensitivity and specificity for detection of polyps equal or larger than 6 or 10 mm was rather good and ranged between 90–95% (25,26) (Table 4).

Full table
PCCE in IBD
Ulcerative colitis (UC)
In the recent years, some data regarding the yield of PCCE in surveillance of IBD has been published mainly on UC. In a study examining the feasibility of PCCE 1 and 2 in 42 patients with UC, bowel preparation was considered adequate in 80% of the patients and no serious adverse events related to the PCCE procedure or bowel preparation were reported. The correlation between optical colonoscopic disease severity and that estimated by the PCCE, as well as estimation of disease extent by the two modalities were good (κ=0.79; 95% confidence interval: 0.62–0.96) and (κ=0.71; 95% confidence interval: 0.52–0.90), respectively. Moreover, the ability of PCCE to assess a broad segment of distal ileum led to a change in the diagnosis from UC to ileocolonic Crohn’s disease in three patients (27). Another study examined disease severity in 25 UC patients (28). Again, adequate colonic cleanliness was achieved in 80% of subjects. The correlation of disease severity and extent were good (κ=0.751, P<0.001 and κ=0.522, P<0.001, respectively). In the largest study performed to date, the sensitivity of CCE to detect active colonic inflammation was 89 % and specificity was 75 %. No serious adverse event related to the CCE procedure or preparation was reported (29). PCCE-2 was also examined in a cohort of 29 pediatric UC patients (8). At an adopted cutoff of the modified Matts score, the sensitivity of PCCE-2 in detecting disease activity was 96% and the specificity was 100%. An additional analysis considering the accuracy of the capsule to discriminate between “mild” and “severe” disease demonstrated a perfect accuracy of CCE-2 in classifying patients with “severe” inflammation, with sensitivity of 100% and specificity of 100%.
Crohn’s disease
Even less data exist regarding the use of PCCE in Crohn’s disease. A recent study by D’Haens et al. (30) compared PCCE-2 to optical colonoscopy in assessing disease extent and severity using the SES-CD grading system. The correlation between the two modalities was relatively good, although PCCE-2 downgraded disease severity. The correlation was much better in the terminal ileum and right colon compared to other regions.
Another small 12 patients study from Portugal demonstrated the ability of PCCE-2 to determine pan enteric mucosal healing post treatment for Crohn’s disease involving both small and large bowel (31).
As to date, the European Crohn’s and Colitis Organization does not support the use of PCCE for the diagnostic work-up or in the surveillance of patients with UC due to lack of enough data (32).
Conclusions
The PCCE-2 system offers a new convenient and accurate method for colon examination and CRC screening both in moderate and high risk patients. It is also very useful in cases of incomplete colonoscopy due to technical difficulties, as well as in patients with a high risk for anesthesia.
Although PCCE-2 seems to be accurate in IBD, its precise place in the framework of UC and Crohn’s disease is yet to be determined.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116:544-73. [Crossref] [PubMed]
- Bujanda L, Sarasqueta C, Zubiaurre L, et al. Low adherence to colonoscopy in the screening of first-degree relatives of patients with colorectal cancer. Gut 2007;56:1714-8. [Crossref] [PubMed]
- Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med 2007:1403-12. [PubMed]
- Villa NA, Pannala R, Pasha SF, et al. Alternatives to Incomplete Colonoscopy. Curr Gastroenterol Rep 2015;17:43. [Crossref] [PubMed]
- Brenner H, Chang-Claude J, Jansen L, et al. Role of colonoscopy and polyp characteristics in colorectal cancer after colonoscopic polyp detection: a population-based case-control study. Ann Intern Med 2012;157:225-32. [Crossref] [PubMed]
- Van Assche G, Dignass A, Reinisch W, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: Special situations. J Crohns Colitis 2010;4:63-101. [Crossref] [PubMed]
- Oliva S, Di Nardo G, Hassan C, et al. Second-generation colon capsule endoscopy vs. colonoscopy in pediatric ulcerative colitis: a pilot study. Endoscopy 2014;46:485-92. [Crossref] [PubMed]
- Eliakim R, Yassin K, Niv Y, et al. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy 2009;41:1026-31. [Crossref] [PubMed]
- Adler S, Hassan C, Metzger Y, et al. Accuracy of automatic detection of small-bowel mucosa by second-generation colon capsule endoscopy. Gastrointest Endosc 2012;76:1170-4. [Crossref] [PubMed]
- Farnbacher MJ, Krause HH, Hagel AF, et al. QuickView video preview software of colon capsule endoscopy: reliability in presenting colorectal polyps as compared to normal mode reading. Scand J Gastroenterol 2014;49:339-46. [Crossref] [PubMed]
- Figueiredo PN, Figueiredo IN, Prasath S, et al. Automatic polyp detection in pillcam colon 2 capsule images and videos: preliminary feasibility report. Diagn Ther Endosc 2011;2011:182435.
- Spada C, Hassan C, Barbaro B, et al. Colon capsule versus CT colonography in patients with incomplete colonoscopy: a prospective, comparative trial. Gut 2015;64:272-81. [Crossref] [PubMed]
- Ramos L, Alarcón O, Adrian Z, et al. One-day versus two-day cleansing for colon capsule endoscopy: a prospective randomized pilot study. Gastroenterol Hepatol 2014;37:101-6. [Crossref] [PubMed]
- Kakugawa Y, Saito Y, Saito S, et al. New reduced volume preparation regimen in colon capsule endoscopy. World J Gastroenterol 2012;18:2092-8. [Crossref] [PubMed]
- Eliakim R, Fireman Z, Gralnek IM, et al. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy 2006;38:963-70. [Crossref] [PubMed]
- Van Gossum A, Munoz-Navas M, et al. Fernandez-Urien I, Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med 2009;361:264-70. [Crossref] [PubMed]
- Spada C, Hassan C, Munoz-Navas M, et al. Second-generation colon capsule endoscopy compared with colonoscopy. Gastrointest Endosc 2011;74:581-589.e1. [Crossref] [PubMed]
- Rex DK, Adler SN, Aisenberg J, et al. Accuracy of capsule colonoscopy in detecting colorectal polyps in a screening population. Gastroenterology 2015;148:948-957.e2. [Crossref] [PubMed]
- Adrián-de-Ganzo Z, Alarcón-Fernández O, Ramos L, et al. Uptake of Colon Capsule Endoscopy vs Colonoscopy for Screening Relatives of Patients With Colorectal Cancer. Clin Gastroenterol Hepatol 2015;13:2293-301.e1. [Crossref] [PubMed]
- Alarcón-Fernández O, Ramos L, Adrián-de-Ganzo Z, et al. Effects of colon capsule endoscopy on medical decision making in patients with incomplete colonoscopies. Clin Gastroenterol Hepatol 2013;11:534-40.e1. [Crossref] [PubMed]
- Triantafyllou K, Viazis N, Tsibouris P, et al. Colon capsule endoscopy is feasible to perform after incomplete colonoscopy and guides further workup in clinical practice. Gastrointest Endosc 2014;79:307-16. [Crossref] [PubMed]
- Pioche M, de Leusse A, Filoche B, et al. Prospective multicenter evaluation of colon capsule examination indicated by colonoscopy failure or anesthesia contraindication. Endoscopy 2012;44:911-6. [Crossref] [PubMed]
- Negreanu L, Babiuc R, Bengus A, et al. PillCam Colon 2 capsule in patients unable or unwilling to undergo colonoscopy. World J Gastrointest Endosc 2013;5:559-67. [Crossref] [PubMed]
- Rondonotti E, Borghi C, Mandelli G, et al. Accuracy of capsule colonoscopy and computed tomographic colonography in individuals with positive results from the fecal occult blood test. Clin Gastroenterol Hepatol 2014;12:1303-10. [Crossref] [PubMed]
- Holleran G, Leen R, O'Morain C, et al. Colon capsule endoscopy as possible filter test for colonoscopy selection in a screening population with positive fecal immunology. Endoscopy 2014;46:473-8. [Crossref] [PubMed]
- San Juan-Acosta M, Caunedo-Álvarez A, Argüelles-Arias F, et al. Colon capsule endoscopy is a safe and useful tool to assess disease parameters in patients with ulcerative colitis. Eur J Gastroenterol Hepatol 2014;26:894-901. [Crossref] [PubMed]
- Ye CA, Gao YJ, Ge ZZ, et al. PillCam colon capsule endoscopy versus conventional colonoscopy for the detection of severity and extent of ulcerative colitis. J Dig Dis 2013;14:117-24. [Crossref] [PubMed]
- Sung J, Ho KY, Chiu HM, et al. The use of Pillcam Colon in assessing mucosal inflammation in ulcerative colitis: a multicenter study. Endoscopy 2012;44:754-8. [Crossref] [PubMed]
- D'Haens G, Löwenberg M, Samaan MA, et al. Safety and Feasibility of Using the Second-Generation Pillcam Colon Capsule to Assess Active Colonic Crohn's Disease. Clin Gastroenterol Hepatol 2015;13:1480-6.e3. [Crossref] [PubMed]
- Boal Carvalho P, Rosa B, Dias de Castro F, et al. PillCam COLON 2 in Crohn's disease: A new concept of pan-enteric mucosal healing assessment. World J Gastroenterol 2015;21:7233-41. [PubMed]
- Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis 2012;6:965-90. [Crossref] [PubMed]