
Twenty-five years with HER2 targeted therapy
Introduction
On September 25, 1998, the Food and Drug Administration (FDA) approved trastuzumab (Herceptin, Roche/Genentech) for the treatment of patients with metastatic breast cancer whose tumors overexpress the human epidermal growth receptor 2 (HER2). The approval of trastuzumab was granted along with the companion diagnostic assay (CDx) HercepTest (Dako/Agilent) to determine HER2 protein expression in breast cancer tissues (1,2). Trastuzumab became a “game changer” for treatment of women with HER2 positive breast cancer resulting in a reduction in cancer recurrence and improvement of survival (1,3). Not only was it a major scientific and medical achievement, but it also paved the way for the drug-diagnostic co-development model in which a predictive biomarker assay is developed in parallel to the drug (4). The clinical enrichment trial design developed in relation to trastuzumab has had a significant impact on cancer drug development in the 21st century. In 2019, the importance of this achievement was further emphasized when Dennis Slamon, Axel Ullrich, and Michael Shepard received the Lasker-DeBakey Clinical Research Award for their work on HER2 in breast cancer and for the development of trastuzumab (1).
The potential of HER2 as a target for cancer therapy was initially explored in relation to breast cancer, and later for gastric cancer. Furthermore, HER2 targeted therapy has recently been approved for the treatment of non-small cell lung cancer (NSCLC). Trastuzumab is not the only drug developed to target HER2; over the years, it has been complemented by a number of other compounds, including monoclonal antibodies, small molecule tyrosine kinase inhibitors (TKI), and antibody-drug conjugates (ADC). At the beginning of 2023, the FDA have so far approved eight different HER2 targeted drugs and in addition to these compounds, several biosimilar versions of trastuzumab and reformulations of existing drugs can be added (5). With the development of trastuzumab as a reference point, this article will provide a brief summary of the efficacy of HER2 targeted therapy, including testing for HER2 positivity, as it has evolved over the past 25 years.
Trastuzumab
In 1987, Dennis Slamon et al. published an article in Science, where they described a link between HER2 amplification and a poor prognosis in women with breast cancer (6,7). Based on this observation, they concluded that the HER2 gene product might function as a growth factor receptor that plays a role in the pathogenesis of breast cancer, and the identification of its ligand and development of specific antagonists could have important therapeutic implications. This conclusion became the basis of one of the most significant cancer drug development projects in the 20th century. The antagonist mentioned in their Science publication years later became the monoclonal antibody trastuzumab (2).
In the laboratories of Genentech a mouse monoclonal antibody (4D5) was developed that showed selective growth inhibitory effects on human breast cancer cell lines with HER2 amplification (8). Furthermore, in vivo studies in tumor xenograft models using human breast cancer cells overexpressing HER2 showed that the 4D5 antibody exhibited a significant growth inhibitory effect (9). However, if a mouse monoclonal antibody is used in humans, it can result in an immunogenetic reaction, which could affect both efficacy and safety as it will be recognized by the patient as being of foreign origin (10,11). Based on a newly developed technique using molecular grafting, the researchers at Genentech made it possible to alter the mouse antibody to a humanized version. This humanized version of the 4D5 antibody showed potency similar to that of the murine version, with respect to blocking the proliferation of HER2 expressing SK-BR-3 human breast cancer cells (11). In a xenograft model, the humanized antibody demonstrated a pronounced growth inhibitory effect on human breast tumors overexpressing HER2 (12). Furthermore, it was shown to be much more efficient in supporting immunological antibody-dependent cellular cytotoxicity (ADCC) against SK-BR-3 cells, which has subsequently been shown to be an important part of the mechanism of action of monoclonal antibody therapy (11,13,14). ADCC mediated by natural killer (NK) cells is likely to be the main mechanism of action of trastuzumab in relation to its antitumor effect (15). Another important discovery from the preclinical studies was the additive effect of combining a cytotoxic agent with trastuzumab, which later had a significant impact on the design of clinical trials (16,17). The humanized version of the 4D5 antibody targeting the extracellular domain of the HER2 protein was now ready for clinical development.
Metastatic breast cancer
Preclinical studies had demonstrated a link between the level of HER2 overexpression and trastuzumab inhibition of tumor cell growth (9,11,12,18). This information was essential when trastuzumab entered clinical development, and in order to select the right patient population for the clinical trials, Genentech developed an assay. This clinical trial assay (CTA) was a semiquantitative immunohistochemistry (IHC) assay that could detect the HER2 expression levels in tumor tissue. This CTA assay was used throughout the clinical development of trastuzumab for metastatic breast cancer to select the most likely responding patients, thereby introducing what is known today as the clinical enrichment trial design in cancer drug development (2). In 1992, phase I clinical trials were initiated, which enrolled approximately 50 HER2-postive metastatic breast cancer patients in three individual trials (19). The aim of these trials was to investigate the safety, maximum tolerated dose and pharmacokinetics of trastuzumab. In the subsequent year, trastuzumab entered phase II clinical development. This program consisted of three trials, and here, trastuzumab was administered either as monotherapy or in combination with cisplatin (20-22). During the phase II program, standardized scoring criteria for HER2 overexpression, as we know from today’s IHC assays, were developed. Table 1 shows the FDA-approved cell membrane staining intensity scoring criteria for the HerceptTest assay in breast cancer (23). However, it is important to note that, during the development of trastuzumab for metastatic breast cancer, the criteria for HER2 positivity were either IHC2+ or IHC3+. In one of phase II trials, trastuzumab was administered as monotherapy to 222 patients with HER2-postive metastatic breast cancer (22). The objective response rate (ORR) for all patients in the trial was 15%; however, subgroup analysis showed that patients with an IHC3+ score had a higher ORR than those with an IHC2+ score (18% vs. 6%).
Table 1
| Staining pattern | HER2 protein overexpression assessment | Score |
|---|---|---|
| No staining is observed or membrane staining is observed in less than 10% of the tumor cells | Negative | 0 |
| A faint/barely perceptible membrane staining is detected in more than 10% of the tumor cells. The cells are only stained in part of their membrane | Negative | 1+ |
| A weak to moderate complete membrane staining is observed in more than 10% of the tumor cells | Weakly positive | 2+ |
| A strong complete membrane staining is observed in more than 10% of the tumor cells | Strongly positive | 3+ |
FDA, Food and Drug Administration; HER2, human epidermal growth receptor 2.
In 1995, a phase III trial was initiated to evaluate the efficacy of trastuzumab combined with chemotherapy. In this trial, 469 patients with metastatic HER2 positive breast cancer were randomized to receive trastuzumab plus anthracycline and cyclophosphamide or paclitaxel depending on previous treatment or chemotherapy alone (24). The trial showed that the addition of trastuzumab to chemotherapy resulted in an increased ORR (50% vs. 32%; P<0.001), longer duration of response (median 9.1 vs. 6.1 months; P<0.001), and longer median survival (25.1 vs. 20.3 months; P=0.01). Similar to the phase II trials, patients with an IHC3+ score benefited most from treatment with trastuzumab compared to IHC2+ patients.
During the conduct of the phase III trial, it was realized that the CTA was too complex and likely not robust enough to be developed for broader use in general pathology laboratories. To simplify the assay and make it more reliable and robust, Genentech began to cooperate with the diagnostic company Dako, resulting in the development of the HercepTest assay (2,25). When Genentech submitted the documentation on trastuzumab to the FDA, Dako simultaneously submitted their documentation on the HercepTest, and on September 25, 1998, both the drug and diagnostic assay obtained regulatory approval. This is a procedure that the FDA has followed numerous times subsequently, which makes a lot of sense, as the CDx assay needs to be available at the same time as the drug in order to guide the patient selection process.
Adjuvant breast cancer
A few years after the approval of trastuzumab for the treatment of metastatic HER2-positive breast cancer, two adjuvant clinical trials sponsored by the National Cancer Institute were initiated (26). These two trials were led by the National Surgical Adjuvant Breast and Bowel Project (NSABP) and North Central Cancer Treatment Group (NCCTG). In the NSABP B-31 trial, HER2 positive early-stage breast cancer patients were randomized to receive doxorubicin and cyclophosphamide followed by paclitaxel or the same chemotherapy regimen plus trastuzumab for one year. The NCCTG N9831 trial had a slightly different design; here, the group of patients who received trastuzumab was divided in an arm B and an arm C. In arm B, trastuzumab was given for one year following 12 weeks of paclitaxel treatment where’s in arm C trastuzumab was administered concomitantly with trastuzumab for 12 weeks followed by 40 weeks of monotherapy with trastuzumab. When the adjuvant trials were set up, the criteria for HER2 positivity were changed from IHC2+ or IHC3+ to either IHC3+ or HER2 amplification using fluorescence in situ hybridization (FISH) (HER2/CEN17 ≥2.0) (26).
In the analyses presented to the FDA for the adjuvant breast cancer indication, the data from the two trials were pooled and comprised 3,752 patients (27). The primary efficacy endpoint in these trials was disease-free survival (DSF), and the results showed a significant difference in favor of the trastuzumab group [hazard ratio (HR), 0.48; P<0.0001]. Furthermore, the studies demonstrated that trastuzumab therapy was associated with a 33 percent relative reduction in the risk of death (P=0.015) (26,27). In November 2006, based on positive results from the NSABP B-31 and NCCTG N9831 trials, trastuzumab was approved by the FDA for adjuvant treatment of patients with early stage HER2-overexpressing, node-positive breast cancer as part of a regimen containing doxorubicin, cyclophosphamide, and paclitaxel. Other large adjuvant trials with trastuzumab, such as the HERA trial and the trial by the Breast Cancer International Research Group, have shown similar positive results with respect to the primary endpoint of DSF (28,29). In 2014, the final survival analysis was conducted for the NSABP B-31 and NCCTG N9831 trials, with a median follow-up time of 8.4 years (3). The results of this analysis showed that the combination of trastuzumab and chemotherapy led to a 37 percent relative improvement in overall survival (OS) (HR 0.63; P<0.001) and an increase in the 10-year survival rate from 75.2 to 84.0 percent.
Gastric cancer
HER2 amplification and/or HER2 over-expression has been reported in malignancies other than breast cancer, such as ovarian, prostate, lung, and gastric cancers (30). As early as 1986, HER2 amplification and overexpression of HER2 were reported in gastric cancer (31,32). Subsequently, several studies have confirmed these findings, and several have shown that HER2 positivity is a negative prognostic marker in patients with gastric cancer (33). Positive results from different preclinical in vitro and in vivo studies with trastuzumab in different gastric cancer models, as well as a few positive clinical case studies, led to the initiation of a clinical phase III trial. However, due to marked differences in tumor biology between breast and gastric cancer, especially with respect to tumor heterogeneity, changes to the scoring criteria for the HercepTest were made before the start of the trial in order to select the right patient population (2,34). Table 2 shows the FDA-approved cell membrane staining intensity scoring criteria for the HerceptTest assays for gastric and gastroesophageal junction cancer (35).
Table 2
| Staining pattern surgical specimen | Staining pattern biopsy specimen | HER2 protein overexpression assessment | Score |
|---|---|---|---|
| No reactivity or membranous reactivity in <10% of tumor cells | No reactivity or no membranous reactivity in any (or <5 clustered) tumor cell | Negative | 0 |
| Faint/barely perceptible membranous reactivity in ≥10% of tumor cells; cells are reactive only in part of their membrane | Tumor cell cluster (≥5 cells) with a faint/barely perceptible reactivity irrespective of percentage of tumor cells stained | Negative | 1+ |
| Weak to moderate complete, basolateral or lateral membranous reactivity in ≥10% of tumor cells | Tumor cell cluster (≥5 cells) with a weak to moderate complete, basolateral or lateral membranous reactivity irrespective of percentage of tumor cells stained | Equivocal | 2+ |
| Strong complete, basolateral or lateral membranous reactivity in ≥10% of tumor cells | Tumor cell cluster (≥5 cells) with a strong complete, basolateral or lateral membranous reactivity irrespective of percentage of tumor cells stained | Positive | 3+ |
FDA, Food and Drug Administration; HER2, human epidermal growth receptor 2.
In the Trastuzumab for Gastric Cancer (ToGA) trial, 594 HER2-postive patients with gastric or gastroesophageal junction cancer were randomized to receive either capecitabine plus cisplatin or fluorouracil plus cisplatin and trastuzumab (36). HER2 positivity was defined as either IHC3+, according to the revised scoring criteria for the HercepTest, or HER2 amplification by FISH (HER2/CEN17 ≥2). However, both IHC and FISH testing were performed on nearly all the included patients. In the ToGA trial, the primary efficacy endpoint was OS, and the combination of chemotherapy and trastuzumab was superior to that of chemotherapy alone. The median OS increased from 11.1 to 13.8 months (HR, 0.74; P=0.0046). However, a pre-planned exploratory analysis that examined the efficacy of the different HER2 IHC scoring categories showed that the survival benefit of trastuzumab was dependent on the level of HER2 overexpression. The single subgroup of patients with the greatest survival benefit was the IHC3+ subgroup. Here, the median OS increased to 17.9 months when trastuzumab was added to chemotherapy compared to chemotherapy alone, with an OS of 12.3 months (2,36). In November 2010, based on the results of the ToGA trial, the FDA approved trastuzumab for the treatment of HER2-overexpressing metastatic gastric or gastroesophageal junction adenocarcinomas. Simultaneously with the approval of trastuzumab, the HercepTest and HER2 FISH pharmDx (Dako) assays were approved, both of which were used in the ToGA trial for patient selection (2).
Other HER2 targeted therapies
Since the approval of trastuzumab, several other HER2 targeted therapies have been approved by the FDA, EMA, and other regulatory bodies, as shown in Figure 1. These drugs have been approved for several indications, as shown in Table 3. Grouped according to their primary mechanisms of action, the individual drugs are briefly discussed in the following section.
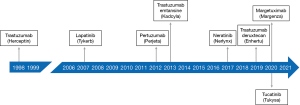
Table 3
| Drugs | Indications |
|---|---|
| Monoclonal antibodies | |
| Trastuzumab (Herceptin) | (I) The treatment of HER2-overexpressing breast cancer |
| (II) The treatment of HER2-overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma | |
| Pertuzumab (Perjeta) | (I) Use in combination with trastuzumab and docetaxel for treatment of patients with HER2-positive metastatic breast cancer who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease |
| (II) Use in combination with trastuzumab and chemotherapy as: | |
| • Neoadjuvant treatment of patients with HER2-positive, locally advanced, inflammatory, or early-stage breast cancer (either greater than 2 cm in diameter or node positive) as part of a complete treatment regimen for early breast cancer | |
| • Adjuvant treatment of patients with HER2-positive early breast cancer at high risk of recurrence | |
| Margetuximab (Margenza) | (I) In combination with chemotherapy, for the treatment of adult patients with metastatic HER2 positive breast cancer who have received two or more prior anti-HER2 regimens, at least one of which was for metastatic disease |
| Tyrosine kinase inhibitors | |
| Lapatinib (Tykerb) | (I) In combination with capecitabine for the treatment of patients with advanced or metastatic breast cancer whose tumors overexpress (HER2) and who have received prior therapy, including an anthracycline, a taxane, and trastuzumab |
| (II) In combination with letrozole for the treatment of postmenopausal women with hormone receptor-positive metastatic breast cancer that overexpresses the HER2 receptor for whom hormonal therapy is indicated | |
| Neratinib (Nerlynx) | (I) As a single agent, for the extended adjuvant treatment of adult patients with early-stage HER2-positive breast cancer, to follow adjuvant trastuzumab based therapy |
| (II) In combination with capecitabine, for the treatment of adult patients with advanced or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 based regimens in the metastatic setting | |
| Tucatinib (Tukysa) | (I) In combination with trastuzumab and capecitabine for treatment of adult patients with advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who have received one or more prior anti-HER2-based regimens in the metastatic setting |
| Antibody drug conjugates | |
| Trastuzumab emtansine (Kadcyla) | (I) As a single agent, for the treatment of patients with HER2-positive, metastatic breast cancer who previously received trastuzumab and a taxane, separately or in combination. Patients should have either: |
| • Received prior therapy for metastatic disease, or | |
| • Developed disease recurrence during or within six months of completing adjuvant therapy | |
| (II) As a single agent for the adjuvant treatment of patients with HER2-positive early breast cancer who have residual invasive disease after neoadjuvant taxane and trastuzumab-based treatment | |
| Trastuzumab deruxtecan (Enhertu) | (I) Adult patients with unresectable or metastatic HER2-positive breast cancer who have received a prior anti-HER2-based regimen either in the metastatic setting, or in the neoadjuvant or adjuvant setting and have developed disease recurrence during or within six months of completing therapy |
| (II) Adult patients with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer who have received a prior chemotherapy in the metastatic setting or developed disease recurrence during or within 6 months of completing adjuvant chemotherapy | |
| (III) Adult patients with unresectable or metastatic NSCLC whose tumors have activating HER2 (ERBB2) mutations and who have received a prior systemic therapy | |
| (IV) Adult patients with locally advanced or metastatic HER2-positive gastric or gastroesophageal junction adenocarcinoma who have received a prior trastuzumab-based regimen |
FDA, Food and Drug Administration; HER2, human epidermal growth receptor 2; IHC, immunohistochemical; ISH, in situ hybridization; NSCLC, non-small cell lung cancer.
Monoclonal antibodies
As described earlier, the mechanism of action of monoclonal antibodies is through an alteration of tyrosine kinase (TK) signaling by binding to the extracellular domain of HER2, and likely more importantly, through an ADCC mediated by NK cells (13-15). Figure 2 shows their binding to HER2 receptor. In addition to trastuzumab two other HER2 targeting monoclonal antibodies have received regulatory approval. These drugs are pertuzumab (Perjeta, Roche/Genentech) and margetuximab (Margenza, MacroGenics). For trastuzumab and pertuzumab, recently two new formulations containing hyaluronidase have been developed for subcutaneous administration (37,38). Hyaluronidase is an endoglycosidase used to increase the dispersion and absorption of the active drug substances from the subcutaneous tissue. The first formulation developed with hyaluronidase was for trastuzumab (Herceptin Hylecta, Roche/Genentech), followed by a combination product of trastuzumab and pertuzumab (Phesgo, Roche/Genentech). These two formulations are not discussed further in this article.

Pertuzumab
Pertuzumab is a humanized monoclonal antibody that targets subdomain II of the extracellular domain of the HER2 protein, in contrast to trastuzumab, which binds to subdomain IV (39). Pertuzumab specifically prevents HER2 heterodimerization with other members of the HER family, including epidermal growth factor receptor [EGFR (HER1)], HER3, and HER4, thereby blocking downstream signaling. Similar to trastuzumab, pertuzumab stimulates NK-cell-mediated ADCC. As pertuzumab and trastuzumab target different HER2 subdomains, they possess complementary mechanisms of action, which in HER2-overexpressing xenograft models have been shown to augment antitumor activity. Clinically, a combination of these two drugs has been used to provide a more comprehensive blockade of the HER2 signaling. In several randomized clinical trials, the combination of pertuzumab and trastuzumab with different chemotherapy regimens has been investigated in HER2 positive breast cancer patients in the metastatic and adjuvant settings as well as for neoadjuvant treatment (40-42). In 2012, based on the results of the CLEOPATRA trial, pertuzumab in combination with trastuzumab and docetaxel was approved for the treatment of patients with HER2-positive metastatic breast cancer (39). In the CLEOPATRA trial, 808 HER2-postive breast cancer patients were randomized to receive placebo, trastuzumab, and docetaxel or pertuzumab, trastuzumab, and docetaxel as first-line treatment for metastatic disease (40). The primary endpoint of the trial was PFS, and the pertuzumab group showed to be superior to the placebo group. The median PFS was 12.4 months in the placebo group, as compared with 18.5 months in the pertuzumab group (HR, 0.62; P<0.001). In subsequent years, pertuzumab was approved for both neoadjuvant and adjuvant treatment (41,42). Table 3 shows the approved indications for pertuzumab.
Margetuximab
Margetuximab is a monoclonal antibody that binds to the extracellular domain of HER2. Compared to trastuzumab, margetuximab has an altered Fc receptor affinity, which increases antibody-dependent cellular cytotoxicity through NK-cell activation (43,44).
In the randomized SOPHIA trial, margetuximab plus chemotherapy was compared with trastuzumab plus chemotherapy in 536 metastatic HER2-positive breast cancer patients who had progressed on two or more previous anti-HER2 therapies (44). The primary trial endpoint of PFS showed a small improvement with margetuximab compared with trastuzumab (HR, 0.76; P=0.03). The median PFS for margetuximab was 5.8 versus 4.9 months for trastuzumab. Based on interim data from the SOPHIA trial, margetuximab obtained FDA approval for the treatment of metastatic breast cancer in December 2020. Table 3 shows the approved indications for margetuximab. A recent final analysis of the other primary efficacy endpoint of OS showed no statistically significant difference between the two treatment groups, with 21.6 months in the margetuximab group and 21.9 months in the trastuzumab group (45).
TKI
TKIs are small molecules that cross the cell membrane and inhibit intracellular TK activity of the HER2 receptor, as shown in Figure 2. The first TKI to obtain FDA approval was lapatinib (Tykerb, Novartis), followed by neratinib (Nerlynx, Puma Biotechnology) and tucatinib (Tukysa, Seattle Genetics), as shown in Figure 1 (46-48).
Lapatinib
Lapatinib exhibit a reversible dual inhibition of the TK domains of both EGFR and HER2 (46). In vitro and in vivo studies have demonstrated tumor growth-inhibitory effects of EGFR and HER2 expressing cells. Furthermore, in vitro studies have shown an additive effect of lapatinib and 5-FU, the active metabolite of capecitabine. Furthermore, other in vitro studies demonstrated no cross-resistance between lapatinib and trastuzumab. Based on the results of the preclinical studies, a clinical development program was initiated and here, lapatinib was combined with capecitabine in HER2 positive metastatic breast cancer patients.
In a Phase III trial, 399 women with HER2-positive metastatic breast cancer, who had progressed after prior treatment with regimens containing anthracyclines, taxanes, and trastuzumab, were randomized to receive either lapatinib plus capecitabine or capecitabine alone. The primary efficacy endpoint in the trial was time to progression (TTP) and here, the addition of lapatinib was superior to capecitabine alone (46,49,50). The TTP in the lapatinib plus capecitabine group was 27.1 months against 18.6 months in the capecitabine group (HR, 0.57; P<0.001). In 2007, based on a phase III trial, lapatinib in combination with capecitabine was approved by the FDA for metastatic breast cancer as shown in Figure 1. A few years later, the indication was expanded when the combination of lapatinib with the aromatase inhibitor letrozole was approved by the FDA for the treatment of HER2-positive postmenopausal women with hormone receptor-positive metastatic breast cancer (46,51). The approved indications for lapatinib are listed in Table 3.
Neratinib
Neratinib is a multikinase inhibitor that irreversibly binds to the TK domains of EGFR, HER2, and HER4 (47). In vitro studies with neratinib have demonstrated antitumor activity in EGFR and HER2 expressing tumor cell lines, as well as inhibition of the TK domains of EGFR, HER2, and HER4. In a mouse xenograft model with tumor cell lines expressing EGFR and HER2, the oral administration of neratinib inhibited tumor growth.
The first clinical indication for neratinib to be developed was as an extended adjuvant treatment after conventional adjuvant trastuzumab-based therapy (47,52). In the ExteNET trial, 2,840 women with early-stage HER2-positive breast cancer were randomized to receive either neratinib or placebo administered once daily for one year. The primary efficacy endpoint in the trial was invasive disease-free survival (iDFS). At two years follow-up a small but significant difference in iDFS was detected between the two treatment arms of the trial. In the neratinib group, iDFS was 93.9% and 91.6% in the placebo group (HR, 0.67; P=0.009). In 2017, based on the results of the ExteNET trial, neratinib obtained FDA approval as monotherapy for the extended adjuvant treatment of patients with early-stage HER2-positive breast cancer. Subsequently, neratinib in combination with capecitabine was approved for the treatment of patients with advanced or metastatic HER2-positive breast cancer based on the NALA phase III trial (47,53). The approved indications for neratinib are shown in Table 3.
Tucatinib
Tucatinib exhibit a dual inhibition of the TK domains of both HER2 and HER3 (48). In vitro studies in HER2 expressing tumor cells have demonstrated suppression of downstream signaling and inhibition of cell proliferation. In vivo studies with tucatinib showed growth inhibition in HER2 expressing tumors. Furthermore, in vitro and in vivo studies have demonstrated increased antitumor activity when tucatinib is combined with trastuzumab compared to the administration of either drug alone.
In the clinical HER2CLIMB phase III trial, 612 HER2-postive metastatic breast cancer patients were randomized (2:1) to receive either tucatinib, trastuzumab and capecitabine or placebo, trastuzumab and capecitabine. The enrolled patients had been previously treated with trastuzumab, pertuzumab, or trastuzumab deruxtecan in any setting (54). The primary efficacy endpoints were PFS and OS. The trial results showed that the median PFS was 7.8 months in the tucatinib group compared to 4.9 months in the placebo group (HR, 0.57; P<0.00001). Similar superiority of tucatinib was observed with respect to OS. Here the median OS for the tucatinib group was 24.7 versus 19.2 months for the placebo group (HR, 0.73; P=0.004). In 2020, based on the results of the HER2CLIMB trial, tucatinib in combination with trastuzumab and capecitabine, was approved for metastatic HER2-positive breast cancer (48). Table 3 shows the approved indication for tucatinib.
Antibody drug conjugates
ADCs are designed to deliver cytotoxic compounds directly to the cancer cells (55). An ADC molecule consists of three main components: carrier antibody, linker, and cytotoxic payload. After administration, the ADC will home to the tumor and binds to the specific extracellular antigen receptor of the cancer cells, as shown in Figure 2. After binding to the receptor, the ADC undergoes receptor-mediated internalization and subsequent lysosomal degradation, resulting in intracellular release of its cytotoxic payload and tumor cell death. The use of the ADC technology should also be seen as an effective way to improve the therapeutic index of cytotoxic agents (56). So far, two ADCs targeting HER2 have been regulatory approved by the FDA both with trastuzumab as carrier antibody, which are trastuzumab emtansine (Kadcyla, Roche/Genentech) and trastuzumab deruxtecan (Enhertu, Daiichi Sankyo/AstraZeneca) (57,58).
Trastuzumab emtansine
Trastuzumab emtansine (T-DM1) was the first HER2 targeted ADC to be regulatory approved by the FDA. The cytotoxic agent DM1, a maytansine derivative, is linked to trastuzumab via a stable linker (57). DM1 is a microtubule inhibitor that binds to tubulin and disrupts the microtubule network in the cells, leading to cell cycle arrest and apoptosis. After the linker is cleaved, part of the released DM1 diffuses into the extracellular space, leading to local tumor cell destruction.
In the EMILIA trial, T-DM1 was evaluated for the treatment of HER2-postive metastatic breast cancer and here, 991 patients were randomized to receive either lapatinib plus capecitabine or T-DM1 monotherapy. Before enrolment in the trial, the patients had received treatment with taxane and trastuzumab (59). The primary efficacy endpoints were PFS and OS. The median PFS was 9.6 months in the T-DM1 group compared to 6.4 months in the lapatinib plus capecitabine group (HR, 0.65; P<0.001). For the other primary endpoint of median OS, T-DM1 was superior to lapatinib plus capecitabine with 30.9 versus 25.1 months (HR, 0.68; P<0.001). The results of the EMILIA trial led to FDA approval for the treatment of HER2-postive metastatic breast cancer in 2013. In the subsequent KATHERINE trial, T-DM1 was tested in patients with HER2-positive early-stage breast cancer and was found to be superior to trastuzumab (60). This trial paved the way for the FDA approval of T-DM1 for adjuvant treatment. The indications for T-DM1 are listed in Table 3.
Trastuzumab deruxtecan
Trastuzumab deruxtecan (T-DXd) is another HER2 targeted ADC in which the cytotoxic payload is deruxtecan (DXd), a topoisomerase I inhibitor linked to trastuzumab via a specific linker (58). Following receptor internalization, the linker is cleaved intracellularly by lysosomal enzymes, and the DXd molecule is released, resulting in DNA damage and apoptotic cell death. Owing to the high membrane permeability of DXd, it diffuses into the extracellular environment and causes local tumor cell death.
The first indication developed for T-DXd was HER2-postive metastatic breast cancer. In a single-arm phase II trial (DESTINY-Breast01), 184 patients with HER2-positive metastatic breast cancer were treated with T-DXd (61). Patients enrolled in the trial had previously received two or more prior anti-HER2 therapies. The primary endpoint of the trial was the ORR, with PFS as one of the secondary endpoints. Following T-DXd treatment, an ORR of 60.9% was observed, with a median PFS of 16.4 months. By the end of 2019, T-DXd had obtained FDA approval for the treatment of HER2-postive metastatic breast cancer through accelerated approval (58). The efficacy of T-DXd in metastatic breast cancer was further confirmed in the DESTINY-Breast03 trial. In this trial, 524 patients with HER2-positive metastatic breast cancer were randomized to receive either T-DXd or T-DM1 (62). The primary end point was PFS, and at 12 months 75.8% of those treated with T-DXd were alive compared to 34.1% in the T-DM1 group (HR, 0.28; P<0.001).
Until recently, the criterion for receiving HER2 targeted therapy was HER2-positivity, defined as IHC3+ or gene amplification by in situ hybridization (ISH); however, with the results of the DESTINY-Breast04 trial, this criterion will need to be changed, at least for T-DXd (63). In the DESTINY-Breast04 trial, HER2-low patients, defined as IHC1+ or IHC2+ and negative for gene amplification by ISH, were randomized (2:1) to receive T-DXd or chemotherapy (physician’s choice). Both hormone receptor-positive (HR+) and hormone receptor-negative (HR−) patients were enrolled. The primary end point was PFS and in the HR+ cohort, which consisted of 494 of the 557 enrolled patients, the median PFS was 10.1 months in the T-DXd group and 5.4 months in the chemotherapy group (HR, 0.51; P<0.001). OS was a secondary endpoint with 23.9 months in the T-DXd group and 17.5 months for chemotherapy (HR, 0.64; P=0.003). In 2022, based on the DESTINY-Breast04, the FDA approved T-DXd for the treatment of HER2-low metastatic breast cancer patients together with the Pathway anti-Her2/neu assay (Ventana Medical Systems) as CDx (57,64).
HER2-postive gastric or gastroesophageal junction adenocarcinoma is another indication that has been evaluated for T-DXd treatment (65). In the DESTINY-Gastric01 trial, 187 HER2-positive patients were randomized (2:1) to receive T-DXd or chemotherapy (physician’s choice). HER2 positivity was defined as IHC3+ or IHC2+ and gene amplification by ISH. The primary endpoint of the trial was ORR, with OS as a secondary endpoint. The results showed an ORR of 51% for patients who had received T-DXd compared to 14% in the chemotherapy group (P<0.001). Subgroup analysis showed that the IHC3+ group had a somewhat higher ORR than that of the IHC2+/ISH group (58.2% vs. 28.6%). These results are interesting because they are in agreement with those observed in the ToGA trial following trastuzumab treatment (36). In terms of median OS, T-DXd demonstrated superiority over chemotherapy, with 12.5 versus 8.4 months (HR, 0.59; P=0.01). Based on positive results from the DESTINY-Gastric01 trial, T-DXd obtained FDA approval for metastatic HER2-positive gastric or gastroesophageal junction adenocarcinoma in 2020 (58). T-DXd has also shown to be effective in the treatment of patients with HER2-mutated metastatic NSCLC, which resulted in FDA approval in 2022, together with the Guardant360 CDx (Guardant Health) and Oncomine Dx Target Test (Life Technologies Corporation) as CDx assays (57,64,66). The approved indications for T-DXd are presented in Table 3.
Companion diagnostics for HER2 targeted therapies
When trastuzumab was developed, one of the challenges was to select the right patient population. For this purpose, Genentech developed a CTA, which was later replaced by the HercepTest (2). The purpose of this assay was to select the right patient population for trastuzumab treatment by determining the level of HER2 expression in the tumor tissue. To standardize and improve the accuracy of HER2 testing in breast cancer, the American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAP) issued a clinical practice guideline in 2007 (67). One of the problems with this first version of the guideline was that the scoring criteria deviated from the FDA-approved scoring criteria, which until then had been used in all pivotal clinical trials with trastuzumab (68,69). However, with the revision of the ASCO/CAP guidelines in 2013 and 2018, the scoring criteria became similar to the original FDA-approved criteria (70,71). In 2017, the ASCO/CAP issued a similar HER2 guideline for gastroesophageal adenocarcinoma (72). Since 1998, when the first CDx assay for HER2 targeted therapy was approved, and until 2023, the number of HER2 assays that have obtained FDA approval is 14 as shown in Table 4. In addition to IHC, both ISH and next-generation sequencing (NGS) have been used to detect HER2 positivity.
Table 4
| Assay | Breast cancer | Gastric cancer | Lung cancer |
|---|---|---|---|
| IHC assays | |||
| Bond Oracle HER2 IHC System (Leica Biosystems) | Trastuzumab | ||
| HercepTest (Agilent Technologies/Dako Denmark) | Trastuzumab | Trastuzumab | |
| Pertuzumab | |||
| Trastuzumab emtansine | |||
| InSite Her-2/neu KIT (Biogenex Laboratories) | Trastuzumab | ||
| PATHWAY anti-Her2/neu (Ventana Medical Systems) | Trastuzumab | ||
| Trastuzumab emtansine | |||
| Trastuzumab deruxtecan | |||
| ISH assays | |||
| HER2 FISH pharmDx Kit (Agilent Technologies/Dako Denmark) | Trastuzumab | Trastuzumab | |
| Pertuzumab | |||
| Trastuzumab emtansine | |||
| HER2 CISH pharmDx Kit (Agilent Technologies/Dako Denmark) | Trastuzumab | ||
| INFORM HER-2/neu (Ventana Medical Systems) | Trastuzumab | ||
| INFORM HER2 Dual ISH DNA Probe Cocktail (Ventana Medical Systems) | Trastuzumab | ||
| Trastuzumab emtansine | |||
| PathVysion HER-2 DNA Probe Kit (Abbott Molecular) | Trastuzumab | ||
| SPOT-LIGHT HER2 CISH Kit (Life Technologies Corporation) | Trastuzumab | ||
| VENTANA HER2 Dual ISH DNA Probe Cocktail (Ventana Medical Systems) | Trastuzumab | ||
| NGS assays | |||
| FoundationOne CDx (Foundation Medicine) | Trastuzumab | ||
| Pertuzumab | |||
| Trastuzumab emtansine | |||
| Guardant 360 CDX (Guardant Health) | Trastuzumab deruxtecan | ||
| Oncomine Dx Target Test (Life Technologies Cooperation) | Trastuzumab deruxtecan | ||
FDA, Food and Drug Administration; HER2, human epidermal growth receptor 2; IHC, immunohistochemical; ISH, in situ hybridization; NGS, next-generation sequencing.
With regard to the development of trastuzumab, it has been said that if an assay did not exist to enrich the trial population with HER2-postive patients, the phase III trial in metastatic breast cancer would almost certainly have failed (10,73). This statement was supported by alternative sample size calculations performed after the conclusion of the trial (74,75). The calculations showed that had an all-comers trial design been used, without testing for HER2, it would have required more than 8,000 patients to demonstrate a statistically significant difference between the trastuzumab and control arms, instead of the 469 patients enrolled in the actual phase III trial (24).
In 2005, the FDA issued a draft concept paper on drug-diagnostic co-development, which was inspired by the way that trastuzumab had been developed in parallel with a predictive biomarker assay. Years later, this concept paper was followed by different guidelines discussing the co-development of a therapeutic product with a CDx assay (76,77). In 2014, the FDA issued a guidance document in which they defined a CDx assay for the first time. According to this guideline, a CDx is an in vitro diagnostic device that provides information that is essential for the safe and effective use of a corresponding therapeutic product (76).
Conclusions
A tumor is a dynamic entity, and resistance develops when patients are treated with anti-cancer drugs including HER2 targeted therapy. This underlines the need to develop new treatment modalities based on a better understanding of the mechanisms of resistance and tumor vulnerability. As described recently in a comprehensive review in Nature Review Drug Discovery, several new approaches for the treatment of HER2-positive tumors are under development (78). These approaches include combination therapy with immune checkpoint inhibitors, new mono- and bispecific antibodies, new ADCs, TKIs, and vaccines targeting HER2.
In 2001, when Slamon et al. published the results of the phase III trial of trastuzumab in metastatic breast cancer in New England Journal of Medicine, it was accompanied by an editorial written by Elisabeth A. Eisenhauer (79). Here, she praised the study as a landmark trial and continued: “On a broader level, this trial demonstrates that a rationally selected therapy directed against a molecular determinant of malignant transformation can improve outcome. This is the beginning of an important new era in cancer treatment, since many more such targeted therapies are now undergoing clinical evaluation.” Looking back over the past decades, one must agree with Elisabeth Eisenhauer, this was definitely the beginning of an important new era in cancer therapy. Since trastuzumab was approved in 1998, a significant number of targeted cancer therapies guided by CDxs have been developed and approved by the FDA (80). The development of these therapies is indebted to trastuzumab, which demonstrated how critical it is to understand the molecular pathophysiology and drug mechanism of action. In relation to both research and routine clinical practice, we have learned the importance of selecting patients for treatment based on objective molecular criteria. The enrichment trial design, in which a predictive biomarker assay is used for patient selection, has made the clinical development of cancer therapies much more rational and the subsequent use of these therapies in the clinic more predictable to the benefit of the individual cancer patients.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-153/coif). The author serves as an unpaid editorial board member of Annals of Translational Medicine from November 2022 to October 2024. The author has also worked as a consultant and advisor for Agilent Technologies, Alligator Biosciences, Argenx, AstraZeneca, Biovica, Visiopharm, Leo Pharma, and received royalties from Elsevier, and served as a Board member of Danish Society of Cyto- and Histochemistry. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hayes DF. HER2 and Breast Cancer - A Phenomenal Success Story. N Engl J Med 2019;381:1284-6. [Crossref] [PubMed]
- Jørgensen JT, Winther H, Askaa J, et al. A Companion Diagnostic With Significant Clinical Impact in Treatment of Breast and Gastric Cancer. Front Oncol 2021;11:676939. [Crossref] [PubMed]
- Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 2014;32:3744-52. [Crossref] [PubMed]
- Jørgensen JT, Hersom M. Clinical and Regulatory Aspects of Companion Diagnostic Development in Oncology. Clin Pharmacol Ther 2018;103:999-1008. [Crossref] [PubMed]
- FDA. Drugs@FDA: FDA-Appproved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. [Accessed Januray 2, 2023].
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82. [Crossref] [PubMed]
- Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989;244:707-12. [Crossref] [PubMed]
- Hudziak RM, Lewis GD, Winget M, et al. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol 1989;9:1165-72. [PubMed]
- Shepard HM, Lewis GD, Sarup JC, et al. Monoclonal antibody therapy of human cancer: taking the HER2 protooncogene to the clinic. J Clin Immunol 1991;11:117-27. [Crossref] [PubMed]
- Sawyers CL. Herceptin: A First Assault on Oncogenes that Launched a Revolution. Cell 2019;179:8-12. [Crossref] [PubMed]
- Carter P, Presta L, Gorman CM, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A 1992;89:4285-9. [Crossref] [PubMed]
- Pietras RJ, Pegram MD, Finn RS, et al. Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene 1998;17:2235-49. [Crossref] [PubMed]
- Wong JKM, Dolcetti R, Rhee H, et al. Weaponizing natural killer cells for solid cancer immunotherapy. Trends Cancer 2023;9:111-21. [Crossref] [PubMed]
- Moasser MM. Inactivating Amplified HER2: Challenges, Dilemmas, and Future Directions. Cancer Res 2022;82:2811-20. [Crossref] [PubMed]
- Mandó P, Rivero SG, Rizzo MM, et al. Targeting ADCC: A different approach to HER2 breast cancer in the immunotherapy era. Breast 2021;60:15-25. [Crossref] [PubMed]
- Pietras RJ, Fendly BM, Chazin VR, et al. Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene 1994;9:1829-38. [PubMed]
- Baselga J, Norton L, Albanell J, et al. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 1998;58:2825-31. [PubMed]
- Sarup JC, Johnson RM, King KL, et al. Characterization of an anti-p185HER2 monoclonal antibody that stimulates receptor function and inhibits tumor cell growth. Growth Regul 1991;1:72-82. [PubMed]
- Baselga J. Phase I and II clinical trials of trastuzumab. Ann Oncol 2001;12:S49-55. [Crossref] [PubMed]
- Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol 1996;14:737-44. [Crossref] [PubMed]
- Pegram MD, Lipton A, Hayes DF, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol 1998;16:2659-71. [Crossref] [PubMed]
- Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999;17:2639-48. [Crossref] [PubMed]
- FDA. Summary of Safety and Effectiveness Data DAKO HercepTest. September 25, 1998. https://www.accessdata.fda.gov/cdrh_docs/pdf/P980018.pdf. [Accessed March 9, 2023]
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [Crossref] [PubMed]
- Jørgensen JT, Winther H. The Development of the HercepTest - From Bench to Bedside. In: Winther H, Jørgensen JT, editors. Molecular Diagnostics – The Key Driver of Personalized Cancer Medicine. Singapore: Pan Stanford Publishing; 2010.
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673-84. [Crossref] [PubMed]
- FDA. Herceptin (trastuzumab) labeling text. November 11, 2006. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/103792s5150lbl.pdf. [Accessed December 4, 2022].
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005;353:1659-72. [Crossref] [PubMed]
- Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273-83. [Crossref] [PubMed]
- Jørgensen JT. Targeted HER2 treatment in advanced gastric cancer. Oncology 2010;78:26-33. [Crossref] [PubMed]
- Yamamoto T, Ikawa S, Akiyama T, et al. Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature 1986;319:230-4. [Crossref] [PubMed]
- Sakai K, Mori S, Kawamoto T, et al. Expression of epidermal growth factor receptors on normal human gastric epithelia and gastric carcinomas. J Natl Cancer Inst 1986;77:1047-52. [PubMed]
- Jørgensen JT, Hersom M. HER2 as a Prognostic MGastric Cancer - A Systematic Analysis of Data from the Literature. J Cancer 2012;3:137-44. [Crossref] [PubMed]
- Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 2008;52:797-805. [Crossref] [PubMed]
- FDA. Summary of Safety and Efffectivness Data. HeceprTest. October 20, 2010. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf/P980018S010b.pdf. [Accessed March 12, 2023].
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-esophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- FDA. Pertuzumab, trastuzumab, and hyaluronidase (Phesgo). Full Prescribing Information. Revised 06/2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761170s000lbl.pdf. [Accessed December 10, 2022].
- FDA. Trastuzumab and hyaluronidase (Herceptin Hylecta). Full Prescribing Information. Revised 02/2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761106Orig1s000lbl.pdf. [Accessed December 10, 2022].
- FDA. Pertuzumab (Perjeta) Full Prescribing Information, Revised 01/2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103792s5345lbl.pdf. [Accessed December 10, 2022].
- Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109-19. [Crossref] [PubMed]
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25-32. [Crossref] [PubMed]
- von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med 2017;377:122-31. [Crossref] [PubMed]
- FDA. Margenza (margetuximab) Full Prescribing Information. Revised 12/2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761150s000lbl.pdf. [Accessed December 11, 2022].
- Rugo HS, Im SA, Cardoso F, et al. Efficacy of Margetuximab vs Trastuzumab in Patients With Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol 2021;7:573-84. [Crossref] [PubMed]
- Rugo HS, Im SA, Cardoso F, et al. Margetuximab Versus Trastuzumab in Patients With Previously Treated HER2-Positive Advanced Breast Cancer (SOPHIA): Final Overall Survival Results From a Randomized Phase 3 Trial. J Clin Oncol 2023;41:198-205. [Crossref] [PubMed]
- FDA. Lapatinib (Tykerb) Full Prescribing Information. Revised 03/2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/022059s031lbl.pdf. [Accessed December 15, 2022].
- FDA. Neratinib (Nerlynx) Full Prescribing Information. Revised 06/2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/208051s009lbl.pdf. [Accessed December 15, 2022].
- FDA. Tucatinib (Tukysa) Full Prescribing Information. Revised 04/2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213411s000lbl.pdf. [Accessed December 16, 2022].
- Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006;355:2733-43. [Crossref] [PubMed]
- Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat 2008;112:533-43. [Crossref] [PubMed]
- Johnston SRD, Hegg R, Im SA, et al. Phase III, Randomized Study of Dual Human Epidermal Growth Factor Receptor 2 (HER2) Blockade with Lapatinib Plus Trastuzumab in Combination with an Aromatase Inhibitor in Postmenopausal Women With HER2-Positive, Hormone Receptor-Positive Metastatic Breast. J Clin Oncol 2021;39:79-89. [Crossref] [PubMed]
- Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2016;17:367-77. [Crossref] [PubMed]
- Saura C, Oliveira M, Feng YH, et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. J Clin Oncol 2020;38:3138-49. [Crossref] [PubMed]
- Curigliano G, Mueller V, Borges V, et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann Oncol 2022;33:321-9. [Crossref] [PubMed]
- Burris HA. Developments in the use of antibody-drug conjugates. Am Soc Clin Oncol Educ Book 2013; [Crossref] [PubMed]
- Nakada T, Sugihara K, Jikoh T, et al. The Latest Research and Development into the Antibody-Drug Conjugate, [fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 Cancer Therapy. Chem Pharm Bull (Tokyo) 2019;67:173-85. [Crossref] [PubMed]
- FDA. Trastuzumab emtansin (Kadcyla). Full Prescribing Information, Revised 02/2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125427s111lbl.pdf. [Accessed December 17, 2022].
- FDA. Trastuzumab deruxtecan (Enhertu). Full Prescribing Information, Revised 11/2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761139s024lbl.pdf. [Accessed December 18].
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783-91. [Crossref] [PubMed]
- von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med 2019;380:617-28. [Crossref] [PubMed]
- Modi S, Saura C, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med 2020;382:610-21. [Crossref] [PubMed]
- Cortés J, Kim SB, Chung WP, et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N Engl J Med 2022;386:1143-54. [Crossref] [PubMed]
- Modi S, Jacot W, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med 2022;387:9-20. [Crossref] [PubMed]
- FDA. List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools). Revised 11/2022. Available online: https://www.fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools. [Accessed January 3, 2023].
- Shitara K, Bang YJ, Iwasa S, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med 2020;382:2419-30. [Crossref] [PubMed]
- Li BT, Smit EF, Goto Y, et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N Engl J Med 2022;386:241-51. [Crossref] [PubMed]
- Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007;25:118-45. [Crossref] [PubMed]
- Vang Nielsen K, Jørgensen JT, Schønau A, et al. Human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007;25:4020-author reply 4021-3. [Crossref] [PubMed]
- Hameed O, Chhieng D, Adams AL. Human epidermal growth factor receptor 2 testing in breast cancer: 30% versus 10% cutoff for immunohistochemistry. J Clin Oncol 2008;26:1571. [Crossref] [PubMed]
- Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997-4013. [Crossref] [PubMed]
- Wolff AC, Hammond MEH, Allison KH, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast CancerAmerican Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 2018;36:2105-22. [Crossref] [PubMed]
- Bartley AN, Washington MK, Colasacco C, et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline from the College of American Pathologists, American Society for Clinical Pathology. J Clin Oncol 2017;35:446-64. [Crossref] [PubMed]
- Hortobagyi GN. Opportunities and challenges in the development of targeted therapies. Semin Oncol 2004;31:21-7. [Crossref] [PubMed]
- Simon R, Maitournam A. Evaluating the efficiency of targeted designs for randomized clinical trials. Clin Cancer Res 2004;10:6759-63. [Crossref] [PubMed]
- Correction. Article on Evaluating the Efficiency of Targeted Designs. Clin Cancer Res 2006;12:3229. [Crossref]
- FDA. In Vitro Companion Diagnostic Devices. Guidance for Industry and Food and Drug Administration Staff. August 2014. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/in-vitro-companion-diagnostic-devices. [Accessed December 26, 2022].
- FDA. Principles for Codevelopment of an In Vitro Companion Diagnostic Device with a Therapeutic Product. Draft guidance. July 2016. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/principles-codevelopment-in-vitro-companion-diagnostic-device-therapeutic-product. [Accessed December 26, 2022].
- Swain SM, Shastry M, Hamilton E. Targeting HER2-positive breast cancer: advances and future directions. Nat Rev Drug Discov 2023;22:101-26. [Crossref] [PubMed]
- Eisenhauer EA. From the molecule to the clinic--inhibiting HER2 to treat breast cancer. N Engl J Med 2001;344:841-2. [Crossref] [PubMed]
- Jørgensen JT. Oncology drug-companion diagnostic combinations. Cancer Treat Res Commun 2021;29:100492. [Crossref] [PubMed]


