
Management of complications following implant-based breast reconstruction: a narrative review
Introduction
Implant-based reconstruction is currently the most common form of breast reconstruction following mastectomy, capturing 75% of all breast reconstruction performed in 2020 (1). Immediate breast reconstruction after mastectomy utilizing either tissue expanders or implants is a well-tolerated operation in the appropriately indicated patients. A variety of techniques exist that include different planes of prosthesis placement, mastectomy patterns, prosthesis types and use of support materials which have all demonstrated appropriate safety data (2-9). Complications after implant-based breast reconstruction, however, are not uncommon, and can be a significant burden to both the patient and provider without appropriate preparation to understand, prevent and treat these issues.
Complications of implant-based breast reconstruction include those inherent to surgery as well as device-related complications. These complications have a wide spectrum of sequelae that include conservative management, medical therapy, reoperation, reconstructive failure and systemic illness. Per the Mastectomy Reconstruction Outcomes Consortium study, overall complication rates for implant-based breast reconstruction range between 26.6–31.3%, while reoperation rates are 15.5–18.8% (10). While severe complications tend to be less frequent, plastic surgeons should be comfortable understanding risk factors that guide decision-making to avoid complications as well as recognize problems early and treat them. Moreover, a thorough comprehension of risk factors for complications, their incidence and the treatment/sequelae is critical for preoperative patient counseling. The purpose of this narrative review is to summarize the current evidence on common complications after implant-based reconstruction, the associated risk factors and guidance on management. We present this article in accordance with the Narrative Review reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1384/rc).
Methods
A review of the literature was performed utilizing the PubMed, OVID MEDLINE and Cochrane Library databases from 2000 to 2023, with MeSH terms and keywords “implant based reconstruction”, “breast reconstruction”, “breast reconstruction complication”, “skin flap necrosis”, “breast implant seroma”, “breast implant hematoma”, “breast implant infection”, “capsular contracture”, “implant malposition”, “breast implant rupture”, “breast implant lymphoma”, and “breast implant illness” as applicable. Inclusion criteria included observational or experimental original articles or systematic reviews of these studies published in peer-reviewed journals indexed to the aforementioned databases. Non-English manuscripts and abstracts were excluded from the review (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | February 1st, 2023 |
| Databases and other sources searched | PubMed, OVID MEDLINE, Cochrane Library |
| Search terms used | “implant based reconstruction”, “breast reconstruction”, “breast reconstruction complication”, “skin flap necrosis”, “breast implant seroma”, “breast implant hematoma”, “breast implant infection”, “capsular contracture”, “implant malposition”, “breast implant rupture”, “breast implant lymphoma”, and “breast implant illness” |
| Timeframe | 01/2000–01/2023 |
| Inclusion and exclusion criteria | Inclusion criteria included observational or experimental original articles or systematic reviews of these studies published in peer-reviewed journals indexed to the aforementioned databases. Non-English manuscripts and abstracts were excluded from review |
| Selection process | Selection process was conducted by authors: Dean H. Meshkin, MD, MS; Joseph M. Firriolo, MD; Ara A. Salibian, MD |
Discussion
Complications after implant-based breast reconstruction can be divided into short and long-term complications (Table 2). Short-term complications can be conceptualized as perioperative complications that have the potential to threaten a reconstruction.
Table 2
| Short-term | Long-term |
|---|---|
| Seroma | Capsular contracture |
| Hematoma | Malposition |
| Infection | Contour deformities & rippling |
| Skin envelope necrosis | Animation deformity |
| Implant rupture | |
| BIA-ALCL | |
| BII |
BIA-ALCL, breast implant associated anaplastic large cell lymphoma; BII, breast implant illness.
Long-term complications may not pose an acute threat of reconstructive failure, but include undesirable aesthetic results (malposition, rippling), more significant reconstructive concerns (severe capsular contracture or animation deformity) and rare systemic illnesses [breast implant-associated anaplastic large cell lymphoma (BIA-ALCL)]. While the chronological acuity of these different complications may have some overlap between the “short” and “long” term categories, we have found that structurally organizing complications in this manner provides easier conceptualization as well as explanation to patients.
In this regard, preoperative patient counseling and discussion is a central tenet to the management of complications in implant-based reconstruction. Studies have demonstrated the lack of informed decision-making in breast reconstruction which requires a thorough discussion and moreover understanding of the issues at hand by patients (11). Critical topics of discussion include incidence of specific complications, implications for the reconstruction and the patient, short and long-term sequalae, risk factors and potential necessary treatments if complications do occur. Several risk calculators are available to help guide both patients and providers in understanding the relative risks of these procedures in an individualized manner (12,13).
Short-term complications
Hematoma
Hematoma is estimated to occur in approximately 1–3% of cases of implant-based breast reconstruction (7,14-19). Most hematomas occur within the first postoperative week and often present with breast swelling, ecchymosis, tenderness, and high drain outputs with frank blood. The majority of hematomas have been found to originate from chest wall muscle (principally pectoralis major) or within the axillary region (18). While traditionally considered a short-term complication, late hematomas do occur and should be considered by plastic surgeons when evaluating a swollen breast in the setting of alloplastic reconstruction (15,20).
Careful pocket dissection and meticulous hemostasis are key to hematoma prevention. Preoperatively, drugs with antiplatelet and/or anticoagulant properties should be discontinued as appropriate. Bleeding diatheses and coagulopathies must be addressed. Drain placement does not prevent hematoma, but may lead to earlier detection of hematoma and relieve mastectomy flaps of pressure secondary to hematoma formation (21).
Few specific patient characteristics, operative techniques, or oncologic factors have proven to be risk factors for hematoma formation (15,18). Hematoma rates appear similar between those with prepectoral and subpectoral breast reconstruction (22,23). Additionally, intraoperative use of ketorolac is not associated with hematoma formation (24). Administration of intravenous tranexamic acid (TXA), an anti-fibrinolytic medication, has demonstrated success in significantly decreasing hematoma rates (25). Other studies have confirmed comparable or superior efficacy of topical tranexamic acid, while having significantly lower blood concentrations than intravenous administration, thereby decreasing the risk of thromboembolic phenomena (26).
Hematoma increases the risk of mastectomy skin flap necrosis, capsular contracture, and poor aesthetic outcome. In severe cases, hematoma can lead to hemodynamic compromise and necessitate the transfusion of blood products. Most hematomas require return to the operating room for breast exploration, hematoma evacuation, and restoration of hemostasis (18,25). Infrequently, small hematomas may be aspirated, and less frequently, they are treated with compression (although this intervention lacks evidence).
Infection
Infection is a dreaded complication of implant-based breast reconstruction that can result in implant loss, delay of adjuvant oncological treatment, and compromise of the final aesthetic outcome. Infection in implant-based reconstruction has been observed at greater rates than in cosmetic breast augmentation (27). Infection occurs in an estimated 7–14% of cases of prosthetic reconstruction; however, rates have been recorded as high as 30% (28-30). The clinical presentation of infection is highly variable and includes mild peri-incisional cellulitis, whole breast erythema, localized abscess in the breast pocket, and turbid or purulent drainage.
Several risk factors have been identified for the development of infection following implant-based breast reconstruction (29,31). These include high body mass index (BMI), a history of preoperative radiation, a history of chemotherapy, smoking, and the development of noninfectious complications (skin necrosis, hematoma, and seroma). Acellular dermal matrix (ADM) has also been implicated in infection, with some investigators citing a three- to five-fold risk associated with ADM use (14,32). While the underlying mechanisms are not completely understood, it is likely that this infection risk is in part associated with increased seroma formation with ADM.
Meticulous sterile technique and intraoperative antibiotic prophylaxis are the current standard of care for the prevention of surgical site infection. It is not uncommon for extended antibiotic prophylaxis (>24 hours postoperatively) to be prescribed in the setting of implant-based breast reconstruction; however, this practice does not appear to reduce infection nor implant loss (33,34). A recent prospective, multicenter, randomized control trial recommended against multiple-dose intravenous antibiotic prophylaxis due to both its lack of efficacy and its increased antibiotic-related adverse events (33).
Broadly, the management of infections involves antimicrobial therapy (oral or intravenous) and management of concurrent complications (including necrosis, seroma, and/or hematoma). When indicated, escalation may occur to interventional drainage and operative washout with implant removal. Upon diagnosis of infection, prompt initiation of empiric antibiotics is paramount, followed by close clinical observation to determine the appropriateness of implant salvage. Mild infections may be treated with oral antibiotics (10- to 14-day course of typically clindamycin, trimethoprim-sulfamethoxazole, linezolid, or ciprofloxacin) (27,29,35). However, moderate to severe cases of infection necessitate intravenous antibiotics, with coverage of methicillin-resistant Staphylococcus aureus (MRSA), coagulase-negative staphylococci, and gram-negative bacteria (e.g., vancomycin and piperacillin-tazobactam) (27,29,35,36). If rapid improvement is observed while on intravenous antibiotics, patients may be transitioned to an oral regimen. If no improvement is noted within with intravenous antibiotics, operative washout with implant removal is indicated (37).
Patients who undergo debridement and implant removal are treated with 10–14 days of antibiotics, guided by intraoperative cultures. Reimplantation should be delayed until 4–6 months following the completion of antibiotic therapy (37). Implant salvage rates are reported in the range of 37–64% based on retrospective cohort studies, with approximately 25% salvage rate by means of antibiotic therapy alone and 12% following implant exchange, although this stratification is scarce in the literature (31,38,39). Higher white blood cell counts and certain causative organisms (most notably MRSA) have been identified as risk factors for failed implant salvage (31,38,39).
Seroma
The incidence of seroma following implant-based breast reconstruction has been reported to be as high as 20% (40) in prior literature reviews. In addition to causing patient distress and discomfort, seromas may delay progression to adjuvant therapy, and potentially escalate to infection and prosthesis loss. The etiology of seroma formation is multifactorial; however, post-mastectomy inflammation, dead space formation, lymphatic disruption, and electrocautery damage all likely play a role (41-43). Furthermore, foreign body response may also contribute to the development of seroma which may be further potentiated by contamination and the presence of biofilm (18,44,45).
Elevated BMI is a major risk factor for seroma formation. Among obese patients (i.e., BMI >30 kg/m2), every unit increase in BMI has been demonstrated to incur a 7–14% increased risk of seroma (14,40). ADM use is also associated with seroma formation. When compared with submuscular breast reconstruction, various meta-analyses have determined a two- to four-fold increase in seroma rate associated with ADM use (7,16,17,19,40). Preoperative radiation has also been associated with a 3.2-fold increase in likelihood of seroma formation (40) in a recent meta-analysis.
Several strategies are available to prevent seroma formation. Closed-suction drains are considered a standard part of implant-based reconstruction and are most commonly removed once output is decreased below a certain low threshold. A recent randomized controlled study, however, demonstrated low seroma rates and improved quality of life with early drain removal protocols (46). Intraoperative elimination of dead space can also aid in seroma prevention and may be achieved in various ways, including excision/tailoring of skin flaps, judicious intraoperative tissue expansion fills, and quilting sutures. The topical application of TXA has also been demonstrated to reduce drain outputs and decrease complications in a recent double-blind, randomized controlled trial (47).
Intraoperative sterile technique is paramount to decrease bacterial load and avoid the development of biofilm, which may serve to heighten the postoperative inflammatory response. Fenestration or meshing of the ADM can be employed to promote fluid egress and most newer iterations of ADM include fenestrated designs. Activity restrictions, particularly as they pertain to limitations in shoulder and arm movement, may also decrease seroma formation (43).
Should seromas occur, vigilant treatment is warranted to prevent seeding and superinfection that may threaten the reconstruction. The initiation of prophylactic antibiotics upon seroma detection has been advocated to reduce this risk; however, this practice lacks evidence (40). Needle aspiration may be an adequate treatment for mild to moderate seromas. Often aspiration is performed by interventional radiology under ultrasound guidance for implant protection. This can be paired with interventional drain placement to prevent seroma recurrence. The development of portable ultrasounds have also led plastic surgeons to more safely perform these procedures in the office (48). In the setting of tissue expanders, the expander can be inflated at the time of aspiration to eliminate dead space. Operative incision and drainage are indicated for large seromas, seromas refractory to repeated aspiration, or seromas complicated by infection.
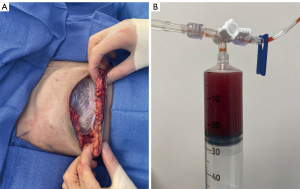
Certain technological advances such as dual-port tissue expanders have allowed for treatment of seromas without the need for ultrasound-guided aspiration or drain placement (Figure 1A,1B). Dual-port tissue expanders have proved beneficial in both the prevention and treatment of seromas. Seromas can be drained through the integrated drain port in the clinic setting, obviating the need for ultrasound-assisted aspiration (49,50). Retrospective reviews by Liu et al. and Momeni et al. report successful treatment of all cases of seroma with in-office aspiration from the integrated drain port (49,51). More recent single center series utilized integrated drain ports for routine aspiration without placing closed-suction drains (52,53). A recent retrospective series by Franck et al. noted a similar complication profile and statistically significant decline in postoperative pain scores with drainless dual-port reconstruction compared to use of standard closed-suction drains (52).
Mastectomy skin envelope necrosis
Mastectomy skin envelope necrosis is characterized by microcirculatory insufficiency at the level of the suprafascial and subdermal plexus responsible for fueling the stages of wound healing (54,55). This compromise in perfusion can lead result in incisional dehiscence as well as partial and full-thickness skin or nipple loss. In the context of implant-based breast reconstruction, the literature’s clinical definition for mastectomy skin envelope necrosis remains inconsistent due to the absence of an accepted, standardized classification system. As such, current diagnostic criteria may refer the area or depth of tissue involvement, type of intervention required, or timing of complication onset, with reported rates of skin envelope necrosis differing significantly based on the preferred criteria (56). For the purposes of this review skin envelope necrosis is considered any degree of necrosis requiring deviation from the normal post-operative care protocol.
Rates of mastectomy flap necrosis in the literature range from 4.5% to 41% based on recent a systematic review (57). Several factors have been identified as independent risk factors for mastectomy flap necrosis in patients undergoing implant-based breast reconstruction (Table 3). Patient comorbidities including tobacco use, BMI of 30 or greater and diagnosis of diabetes mellitus are known to impose deleterious effects on microvascular patency, choke-vessel adaptive responses to metabolic stress and neovascular signaling pathways critical to wound healing following mastectomy and breast reconstruction (58-61). Similar concern has been speculated for nicotine-containing electronic cigarettes and vape pens, although the association has yet to be studied clinically (62). Radiation therapy is also an important factor due its deleterious effects on tissues due to proinflammatory factors and reactive oxygen species that cause hypercoagulability and microvascular thrombosis (63,64). Data comparing outcomes with radiation therapy prior to implant-based reconstruction show higher rates of skin flap necrosis, infection and seroma when the onset of these changes precedes reconstructive surgery, likely due to impaired ability of irradiated tissues to adapt to the stress imposed by permanent implants or expanders (65,66).
Table 3
| Patient characteristics | External factors | Reconstructive factors |
|---|---|---|
| BMI ≥0 | Tobacco use | Poor mastectomy flap quality |
| Diabetes mellitus | Radiation therapy | Peri-areolar incisions† |
| Macromastia† | Chemotherapy | Immediate implant placement† |
| Ptosis† |
†, risk factors in nipple-sparing mastectomy. BMI, body mass index.
When oncologic criteria are met, nipple sparing mastectomy (NSM) allows for the preservation of the nipple-areola complex (NAC) and entire skin envelope to optimize aesthetic results. However, NSM has a predilection for ischemic complications in certain cases due to the increased surface areas of perfusion, retraction of tissues during mastectomy and inability to resect significant amount of compromised skin intraoperatively. Rates of NAC necrosis range from around 2% to 7% in the literature and are affected by similar risk factors as mastectomy flap necrosis (67-72). Important considerations in addition to comorbidities, smoking and adjuvant therapies include incision patterns as periareolar incisions have been associated with higher rates of ischemic complications (4,67). Reconstruction type (tissue expander versus immediate implant) as well as breast size and ptosis are also important variables (4,73). In patients with macromastia and ptosis, prophylactic staged reductions prior to NSM have demonstrated the ability to decrease ischemic complications of the skin envelope (74).
Adherence to an anatomic plane separating the breast parenchyma from the subcutaneous fat superficially is crucial to preserving circulation to the overlying skin and nipple and is likely the single most important factor in preventing ischemic complications. Intraoperative assessment of mastectomy flap quality is a critical component in the management of potentially impaired skin envelope perfusion (75). Relative mastectomy flap thickness to preoperative subcutaneous tissue thickness is an important predictor of mastectomy flap quality (Figure 2). Postoperative mastectomy flap thickness less than 70% of the preoperative subcutaneous tissue thickness has been associated with ischemic complications of the skin envelope (76). Additional important factors in assessment of mastectomy flap quality include preservation of subcutaneous tissue, extent of visible dermis, skin mottling, incision edge bleeding and cautery burns (77).
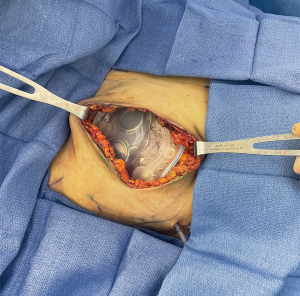
Laser-assisted fluorescent angiography, or indocyanine green (ICG) angiography, has recently become an effective surgical decision-making tool in reducing skin envelope necrosis and downstream complications threatening reconstructive failure (78,79). The vascular imaging modality is advantageous in permitting real-time visual assessments of superficial blood flow throughout a procedure. Perfusion of skin edges has been correlated with clinical outcomes by means of an absolute emission score or a relative perfusion percentage, with predictive values for skin envelope necrosis increasing stepwise as scores or perfusion percentages decrease below 8.0 or 45%, respectively (65,78,80,81).
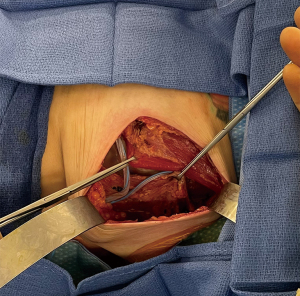
Intraoperative decision-making based on these different variables has important implications for minimizing complications associated with compromised mastectomy flap or NAC perfusion. In situations were poor mastectomy flap quality is suspected, surgeons may respond accordingly by decreasing expander volumes, resecting poorly perfused tissue in skin-sparing mastectomies or converting from immediate implant to tissue expander reconstructions. Other techniques to avoid potentially more significant complications such as implant exposure include conversion from prepectoral to total submuscular reconstruction (Figure 3) or reconstructive delay. The potential for changes in operative plans based on intraoperative factors such as number of stages, prosthesis plane alterations or total reconstructive delay must be thoroughly communicated to, and understood by, the patient preoperatively.
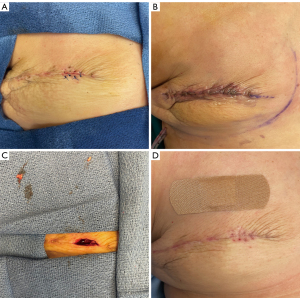
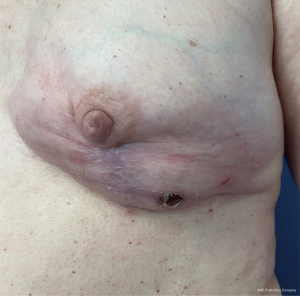
Therapeutic options after the onset of skin envelope necrosis are largely contingent on complication severity. Small areas of partial thickness defects may be successfully managed with local wound care and clinic debridement under close observation in the outpatient setting. Negative pressure wound therapy has also been shown to not only reduce skin envelope necrosis when used prophylactically but also limit complication progression when used as adjuvant therapy (82). A threshold to advance to operative debridement has not been established and management requires a case-by-case analysis. Reports for skin envelope necrosis amenable to non-operative management show a median necrotic area of 5.5 cm2 as compared to 15 cm2 for those receiving surgery (83). In addition to surgical debridement, local or free tissue transfer may be necessary for instances of skin envelope necrosis where epithelization may be inadequate, or implant exposure is likely imminent (83,84). Management strategies are particularly affected by the risk of implant exposure. In this regard, total submuscular reconstruction may allow for more conservative management or in-office debridement (Figure 4A-4D) compared to prepectoral reconstruction in which full-thickness necrosis can lead to direct prosthesis contamination or exposure (Figure 5). The latissimus dorsi muscle flap is a reliable salvage procedure for severely threatened implant reconstructions, and more recently Brown et. al. have demonstrated success with less invasive rotational thoracoepigastric and thoracoabdominal fasciocutaneous flaps (83,85). Early and expeditious intervention remains an important overarching principle for management of ischemic complications of the skin envelope in implant-based reconstruction (86).
Adjunctive treatments include topical nitroglycerin therapy as well as hyperbaric oxygen. The venous and vasodilatory effects of nitrates first implemented for angina can be also recruited in topical application of nitroglycerin for both prophylactic and acute management of skin flap necrosis of the breasts. Drug penetration enables improved perfusion and venous outflow while producing prostacyclin to inhibit thrombosis (87). Known side effects include headaches, dizziness and mild hypotension, all secondary to systemic absorption and endothelial stimulation. Retrospective studies, systematic reviews and randomized trials have cemented the efficacy of topical nitroglycerin in reducing rates of partial and full-thickness skin flap necrosis in immediate and delayed implant based breast reconstruction, both as patch therapy applied in the days preceding surgery, or in the immediate post-operative phase as part of dressing changes (65,88-91). The post-operative therapeutic period for most studies was 48 hours, whereas the maximum effect duration for a single application of topical nitroglycerin is 12 hours (92). Taking into account the medication’s relatively short half-life, it is possible that the brevity of post-operative application timelines causes therapeutic effects to fall short of ample collateral vessel dilation and angiogenesis reaching capable of supporting the remainder of skin healing. More recently, sessions with hyperbaric oxygen therapy have also shown potential for improving skin envelope necrosis in the immediate post-operative period, although larger trials will be necessary to further investigate the impact of this therapy (93,94).
Long-term complications
Capsular contracture
Fibrous encapsulation of breast implants is a physiologic process resembling that observed with other nonporous foreign body responses. This immune-mediated reaction can become exacerbated, leading to thickened and helical re-distribution of collagen fibers of the capsule and scar-like contracture of the breast-implant pocket symptomatic with pain and breast distortion (95). The etiology for this dysregulated response has not been confirmed and is likely patient specific; however, ongoing theories include subclinical implant infection with biofilm development as well as predisposition to autoimmune responses leading to chronic inflammatory states (96). The incidence of capsular contracture was previously believed to be limited to the first post-operative year; however, studies have demonstrated continued occurrence beyond 2 years as well, with observations of an added 1% risk per year and 24.6% incidence over the span of 10 years (97,98).
Several components of reconstructive techniques have been identified that influence capsular contracture development. In assessing infectious etiologies of capsular contracture, betadine irrigation of the implant pocket has been shown to reduce complication rates whereas introducing antibiotic irrigation or post-operative oral regimens have not (99-101). Irritation of surrounding tissues from hemoglobin deposition or subsequent evacuation procedures may explain the observed association between post-operative hematoma and contracture occurrence (97,102,103). Implant texturing is a well-described variable in the onset, and therefore prevention of, capsular contracture (104,105). The uneven surface of textured implants creates a three-dimensional capsule in which collagen fiber vectors counteract and yield reduced net-linear contractile forces as more commonly observed following encapsulation of smooth-surfaced implants (106). Long-term data suggest that silicone implants may have a greater association with capsular contracture in the setting of implant rupture, due to an insidious presentation that promotes delayed recognition and also an inability of surrounding tissues to absorb leakage, compared to saline counterparts (107-109). Higher rates of capsular contracture are also observed in patients receiving radiation therapy, a correlative utilized in support of the two-stage reconstructive approach, which may provide opportunity to negate the effects of contracture either in the expansion phase or surgically during implant exchange, although data refuting these expectations does also exist (110,111).
Non-operative avenues of managing capsular contracture are less common and limited to medical therapies targeting varying components of the inflammatory cascades. Typically used as adjuvants in the management of asthma, oral regimens with the leukotriene inhibitors zafirlukast and montelukast have also been efficacious for improvement and resolution of breast implant capsular contracture symptoms when employed during early stages of symptom development (112,113). Targeting the cyclooxygenase-2 enzyme has provided similar benefit in patients with Baker II–III capsular contracture by way of diclofenac dermal patches (114). Systemic effects, drug-drug interactions, and patient comorbidities, in particular hepatocellular and renal function for the aforementioned medications, may create further barriers when assessing suitability of medical management for patients concomitantly undergoing oncological therapies.
Capsulectomy and implant exchange remain the mainstay surgical options for management of breast implant capsular contracture. Separation and removal of the capsule during total capsulectomy has long been justified as the most complete approach for eliminating the root cause of patient symptoms and the underlying immune response. Creation of a neopocket can also be a useful technique during operative management of capsular contracture. In prepectoral cases with recurrent contracture, consideration should be given to a submuscular or dual-plane alteration. ADM has also been associated with lower capsular contracture rates (115,116) and is a useful adjunctive tool in recurrent capsular contracture cases when combined with capsulectomy, with or without plane change. Patients should be aware of the high risk of recurrent capsular contracture after revision in implant-based breast reconstruction (117).
Malposition
Implant malposition may occur both early and late in the postoperative course, and can manifest as lateral, medial, superior, and/or inferior displacement (118-120). When anatomic/shaped implants are used, rotational malposition can also occur (121). Risk factors for malposition include those related to patient anatomy, surgical technique, and implant selection (119). Patient chest wall contour is an important consideration. Pectus excavatum can predispose to medial implant displacement, while pectus carinatum may contribute to the development of lateral displacement (119,122). Inferior malposition may result from inadequate soft tissue support (30). Soft tissue quality should be closely assessed preoperatively, especially in those with a history of weight loss, pregnancy, or soft tissue atrophy (119).
Improper dissection of the implant pocket is often implicated in malposition. Overdissection in a particular dimension can lead to malposition in that same direction. Lateral overdissection is the most common and can cause lateral displacement whereas medial overdissection results in symmastia (122,123). Violation of the inframammary fold (IMF) is often responsible for inferior malposition (119).
Management strategies of malposition fall into three categories: (I) revision of the existing implant pocket, (II) creation of a new implant pocket and (III) change of implant. Implant pocket revision principally involves capsulorraphy and/or capsulotomy. ADM reinforcement of capsulorraphy may be indicated when the capsule appears thin/weak or in cases of recurrent malposition (124). In cases of symmastia, medial capsulorraphy is typically quite weak, and it is therefore advised to use ADM to buttress the repair (120). In cases of superior malposition, pocket revision may necessitate the surgeon to address a constricted lower pole, which may be secondary to complete muscular coverage or tightly sewn ADM. Any inferior pole capsulotomies should be reinforced with ADM or other support materials to prevent excessive downward implant migration (120).
Conversion from a prepectoral to subpectoral plane may prove beneficial in treating more challenging cases of symmastia. A neosubpectoral pocket can also be created in patients with a history of subpectoral implant placement. The neosubpectoral pocket is created deep to the pectoralis muscle and superficial to the intact anterior capsule. It is often considered technically easier than modifying a distorted implant pocket in revision breast surgery and also allows for precise dissection in a virgin plane to facilitate appropriate implant placement (125).
Contour deformities & rippling
Contour deformities in breast reconstruction can occur over the entire surface of the reconstruction, but are most common at the upper pole. The “stepoff” contour deformity is seen at the transition of the superior implant edge to the chest wall and is exaggerated with round implants, prepectoral positioning and in patients with low BMI. Patients at a higher risk for step-off deformity should be counseled on the aesthetic implications beforehand as well as the potential treatments. Autologous fat grafting is an excellent and well-tolerated reconstructive technique in implant-based breast reconstruction and can be utilized to treat this stepoff deformity at the time of implant exchange or at a later revision (Figure 6A,6B) (126,127). Care must be taken to avoid damage to the implant, especially in low BMI patients with prepectoral implants, as this technique can challenging if there is a thin mastectomy flap as a recipient bed. Overfilling should be avoided and multiple rounds of fat grafting may be needed. Plane change from a prepectoral to dual-plane position can also soften the upper pole stepoff, though this is less commonly needed due to the utility of autologous fat grafting.

Implant rippling is also more commonly seen in the prepectoral plane, especially with lower BMI patients who lack a robust subcutaneous soft tissue envelope after mastectomy. Implant exchange to highly cohesive form-stable implants is a useful technique due to the ability of these implants to resist deformation (128). Autologous fat grafting is also a powerful adjunctive tool to increase soft tissue thickness and camouflage contour deformities and rippling (129,130). Additional techniques include pocket modification to address a discrepancy between pocket and implant size and plane change to a subpectoral pocket to improve soft tissue coverage, when appropriate. ADM can also be utilized to bolster coverage at the time of revision (131).
Animation deformity
Animation deformity, also known as “dynamic breast deformity” or “jumping breast deformity”, is a recognized complication of subpectoral implant-based breast reconstruction with incidence reported as high as 76% following reconstruction (132,133). A subpectoral implant receiving posterior pressure from pectoralis contraction can displace, often in a superolateral direction provided by the dissection plane. The subsequent animation deformity may be cosmetically dissatisfying while also causing musculoskeletal discomfort along the pectoralis major and shoulder (134,135). The majority of grading systems utilize survey and rater-based assessments, whereas a more recent approach proposed by Fracol et al. is the first to quantify animation severity based on the degree of nipple displacement (132,133,136,137).
Stratifying risk of postoperative animation deformity, if a subpectoral reconstruction is planned, requires understanding individual patient lifestyles so that those with more at-risk hobbies, such as athletes or patients who engage in weight-training or yoga, may pursue reconstructive options with informed decision making. For patients with animation deformity, subpectoral to prepectoral conversion of the implant provides definitive treatment by eliminating distortive forces from the pectoralis muscle. Partial submuscular or dual-plane implant placement are still associated with deformity occurrence and so are not recommended over prepectoral conversion for treatment of the complication (132,133). Attempts at less invasive approaches such as fat grafting, botulinum toxin injection and nerve ablation have been reported; however, symptom relief is often temporary, inadequate, or associated with functional morbidity (138,139). The literature is consistent in demonstrating complete resolution of animation deformity symptoms as well as full recovery of pectoralis function following prepectoral conversion and resuspension of the deep muscle surface to the anterior thoracic wall (140-144).
Implant rupture
The clinical presentation, risk factors, and management of implant rupture vary by implant type. Saline implant rupture is easy to detect by clinical exam alone due to an acute decrease in breast volume (145). Deflation in the immediate postoperative course typically indicates iatrogenic etiology, such as intraoperative puncture of the implant wall, or an improperly closed valve. Delayed presentation of saline implant deflation may represent a valve defect, external physical trauma, or simply the development of implant wall defects over time. Pinhole defects in the implant can occur secondary to mechanical friction and wear of implant shell folding. Underinflation of implants has been identified as a major cause of deflation due to a greater tendency for shell folding to occur (146,147). Once saline implant deflation is detected, earlier replacement is often preferable to preserve the size of the original implant pocket.
Comparatively, silicone implant rupture is more difficult to detect and typically not apparent on physical exam. In the majority of cases, rupture is intracapsular (silicone gel is contained within the breast capsule) but may also be extracapsular (silicone gel extends beyond the capsule) or involve migrated gel (gel moves beyond the breast) (148). Extracapsular silicone may cause local tissue inflammation and granuloma formation that is sometimes palpable on physical examination (149). Silicone implant rupture is often “silent” and detected on routine mammography or ultrasound. Currently the US Food and Drug Administration (FDA) recommends ultrasound or magnetic resonance imaging (MRI) for asymptomatic patients at 5–6 years postoperatively, then every 2–3 years thereafter. MRI is recommended at any time postoperatively for symptomatic patients or those with equivocal ultrasound findings (148). MRI is more sensitive and specific (sensitivity 83%, specificity 98%) than ultrasound (sensitivity 69%, specificity 73%) (150,151). It is recommended that ruptured silicone implants be removed to prevent the possibility of silicone gel causing inflammation and other local and systemic reactions (152-155). Capsulectomy is typically performed with device removal to order to adequately remove silicone gel from the breast pocket.
BIA-ALCL
BIA-ALCL is a rare T-cell lymphoma developing from an immune mediated response against breast implant surfaces. Disease incidence is low and studies are underway to better delineate pathogenesis, which may be linked to predisposition to overactivation of JAK1 and STAT3 signaling pathways, allowing for oncogenic transformation of immune cells in a chronic inflammatory state (156,157). The timeline for BIA-ALCL development is insidious and clinical presentation is typically 7–10 years after implant placement. Patients most commonly report unilateral breast swelling, secondary to peri-prosthetic fluid collection or, less commonly, a peri-prosthetic mass, in the absence of systemic symptoms concerning for infectious etiologies (158). Aspiration or guided biopsy and cytological and immunobiological analysis of the late-onset seroma demonstrating anaplastic monoclonal CD30+ T-cell morphology is diagnostic for BIA-ALCL (159).
Occurrence of BIA-ALCL has been reported primarily in the setting of textured implants at rates consistently below 1%, with reports of smooth implant association very infrequent in patients having previously undergone implant exchange following a textured device (160). In 2021, FDA issued a box warning and informed consent checklists for all breast implant devices (148). However, a much stronger association between textured implants and BIA-ALCL is observed, which remains the sole modifiable risk factor for disease occurrence (106,161,162). Whereas the now recalled Allergan BIOCELL has the highest involvement in disease incidence, the independent association with textured devices is consistent regardless of manufacturer (162).
Surgical management is recommended for localized BIA-ALCL, with patients undergoing implant removal and en bloc capsulectomy with resection of any residual mass. No adjuvant chemo or radiation therapy is indicated at this stage, as prognosis is good with 91% survival at 5 years (163). Disseminated disease is much less common, and consensus guidelines recommend approaching Stage II and more advanced cases by way of non-operative oncological management identical to patients presenting with de novo systemic anaplastic lymphoma (159).
Breast implant illness (BII)
BII, also referred to as “silicone adjuvant syndrome” or “autoimmune syndrome induced by adjuvants (ASIA syndrome)” describes a prodrome of symptoms following breast implant placement ranging from cognitive fog, fatigue, malaise, arthralgias, myositis and alopecia to a clinical picture more closely overlapping with rheumatoid arthritis, Sjögren syndrome or scleroderma. Currently, little has been established in terms of diagnostic criteria. The timeline of symptom onset relative to implant placement may span 2–13 years. Characteristic findings on imaging modalities are absent, and aberrant serologies are numerous and inconsistent between analyzed patient populations (164). The currently amorphous standing of BII has resulted in little observational data available to reliably determine incidence rates in patients receiving breast implants, whether for augmentative or reconstructive indications.
Silicone containing implants have long been speculated as the primary factor responsible for pathogenesis; however, multiple barriers exist in assessing this theory by means of the current literature. The vast majority of studies lack a saline implant control group for accurate comparison, instead opting to compare prevalence of autoimmune symptoms and diagnoses against national norms (165-167). Coroneos et al. produced one of the largest observational data sets, with opposing findings at 5-year interim analysis and 7-year final follow up, the latter concluding a significant association between autoimmune illness and silicone implants. Further assessment of the pooled data yields substantial heterogeneity, for example, given no standardization for outcome reporting measures (167,168). Still, a higher prevalence of autoimmune disease is observed after large and more controlled investigations, indicating that an association between implants in general or silicone implants in particular cannot be refuted (169,170).
Systematic review of outcomes following breast implant explantation demonstrates a wide 17–84% margin for symptom improvement without a commonality to identify the subset of patients most likely to benefit from surgery. Certain studies have observed greater symptom resolution amongst patients without diagnosis of autoimmune disease, perhaps supporting a psychosomatic etiology, as well as recurrence of symptoms 1 year following explantation (164,171). Nonetheless the decision to pursue explantation belongs entirely to the patient. Given the obscurity surrounding BII etiology, plastic surgeons may pursue surgical management by empirically removing all implant-related tissues via en bloc explantation or explantation with total capsulectomy. Symptom correlation with implant capsules, and moreover symptom relief with capsulectomy, has not been demonstrated (172). Depending on further discussion with patients, volume restoration can be achieved by autologous fat grafting or free tissue transfer, either immediately or as a staged approach (167,172).
Conclusions
Consideration of patient comorbidities and modifiable risk factors, individualized decision-making, and intraoperative flexibility with surgical techniques are critical tenets in limiting complications following implant-based breast reconstruction. Certain complications, however, will be unavoidable and the focus therefore must include preoperative informed and shared decision-making as well as postoperative early and aggressive treatment. The literature demonstrates an abundance of both reproducible and anecdotal therapeutic options, ranging from pharmacological to operative, for many of the short and long-term complications after implant-based breast reconstruction. This updated review provides evidence-supported management options, of which familiarity is needed to adequately guide patient education and decision-making throughout the reconstructive timeline.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Oscar J. Manrique) for the series “Breast Reconstruction” published on Annals of Translational Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1384/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1384/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1384/coif). The series “Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Society of Plastic Surgeons. Plastic Surgery Statistics Report: ASPS National Clearinghouse of Plastic Surgery Procedural Statistics. 2020. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf
- DeLong MR, Tandon VJ, Farajzadeh M, et al. Systematic Review of the Impact of Acellular Dermal Matrix on Aesthetics and Patient Satisfaction in Tissue Expander-to-Implant Breast Reconstructions. Plast Reconstr Surg 2019;144:967e-74e. [Crossref] [PubMed]
- Djohan R, Gage E, Gatherwright J, et al. Patient satisfaction following nipple-sparing mastectomy and immediate breast reconstruction: an 8-year outcome study. Plast Reconstr Surg 2010;125:818-29. [Crossref] [PubMed]
- Frey JD, Choi M, Salibian AA, et al. Comparison of Outcomes with Tissue Expander, Immediate Implant, and Autologous Breast Reconstruction in Greater Than 1000 Nipple-Sparing Mastectomies. Plast Reconstr Surg 2017;139:1300-10. [Crossref] [PubMed]
- Haddock NT, Kadakia Y, Liu Y, et al. Prepectoral versus Subpectoral Tissue Expander Breast Reconstruction: A Historically Controlled, Propensity Score-Matched Comparison of Perioperative Outcomes. Plast Reconstr Surg 2021;148:1-9. [Crossref] [PubMed]
- Karp NS, Salibian AA. Splitting the Difference: Using Synthetic and Biologic Mesh to Decrease Cost in Prepectoral Immediate Implant Breast Reconstruction. Plast Reconstr Surg 2021;147:580-4. [Crossref] [PubMed]
- Kim JYS, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg 2012;129:28-41. [Crossref] [PubMed]
- Safran T, Al-Halabi B, Viezel-Mathieu A, et al. Direct-to-Implant, Prepectoral Breast Reconstruction: A Single-Surgeon Experience with 201 Consecutive Patients. Plast Reconstr Surg 2020;145:686e-96e. [Crossref] [PubMed]
- Salibian AA, Bekisz JM, Kussie HC, et al. Do We Need Support in Prepectoral Breast Reconstruction? Comparing Outcomes with and without ADM. Plast Reconstr Surg Glob Open 2021;9:e3745. [Crossref] [PubMed]
- Bennett KG, Qi J, Kim HM, et al. Comparison of 2-Year Complication Rates Among Common Techniques for Postmastectomy Breast Reconstruction. JAMA Surg 2018;153:901-8. [Crossref] [PubMed]
- Lee CN, Ubel PA, Deal AM, et al. How Informed Is the Decision About Breast Reconstruction After Mastectomy?: A Prospective, Cross-sectional Study. Ann Surg 2016;264:1103-9. [Crossref] [PubMed]
- Frey JD, Salibian AA, Choi M, et al. Putting Together the Pieces: Development and Validation of a Risk-Assessment Model for Nipple-Sparing Mastectomy. Plast Reconstr Surg 2020;145:273e-83e. [Crossref] [PubMed]
- Naoum GE, Ho AY, Shui A, et al. Risk of Developing Breast Reconstruction Complications: A Machine-Learning Nomogram for Individualized Risk Estimation with and without Postmastectomy Radiation Therapy. Plast Reconstr Surg 2022;149:1e-12e. [Crossref] [PubMed]
- Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg 2010;125:429-36. [Crossref] [PubMed]
- Collins JB, Verheyden CN. Incidence of breast hematoma after placement of breast prostheses. Plast Reconstr Surg 2012;129:413e-20e. [Crossref] [PubMed]
- Ho G, Nguyen TJ, Shahabi A, et al. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg 2012;68:346-56. [Crossref] [PubMed]
- Sbitany H, Serletti JM. Acellular dermis-assisted prosthetic breast reconstruction: a systematic and critical review of efficacy and associated morbidity. Plast Reconstr Surg 2011;128:1162-9. [Crossref] [PubMed]
- Seth AK, Hirsch EM, Kim JY, et al. Hematoma after mastectomy with immediate reconstruction: an analysis of risk factors in 883 patients. Ann Plast Surg 2013;71:20-3. [Crossref] [PubMed]
- Smith JM, Broyles JM, Guo Y, et al. Human acellular dermis increases surgical site infection and overall complication profile when compared with submuscular breast reconstruction: An updated meta-analysis incorporating new products J Plast Reconstr Aesthet Surg 2018;71:1547-56. [Crossref] [PubMed]
- Grippaudo FR, Renzi L, Costantino B, et al. Late unilateral hematoma after breast reconstruction with implants: case report and literature review. Aesthet Surg J 2013;33:830-4. [Crossref] [PubMed]
- Khan SM, Smeulders MJ, Van der Horst CM. Wound drainage after plastic and reconstructive surgery of the breast. Cochrane Database Syst Rev 2015;2015:CD007258. [Crossref] [PubMed]
- Bekisz JM, Salibian AA, Frey JD, et al. Picking the Right Plane: A Comparison of Total Submuscular, Dual-Plane, and Prepectoral Implant-Based Breast Reconstruction. Plast Reconstr Surg 2022;150:737e-46e. [Crossref] [PubMed]
- Manrique OJ, Banuelos J, Abu-Ghname A, et al. Surgical Outcomes of Prepectoral Versus Subpectoral Implant-based Breast Reconstruction in Young Women. Plast Reconstr Surg Glob Open 2019;7:e2119. [Crossref] [PubMed]
- Mikhaylov Y, Weinstein B, Schrank TP, et al. Ketorolac and Hematoma Incidence in Postmastectomy Implant-Based Breast Reconstruction. Ann Plast Surg 2018;80:472-4. [Crossref] [PubMed]
- Weissler JM, Banuelos J, Jacobson SR, et al. Intravenous Tranexamic Acid in Implant-Based Breast Reconstruction Safely Reduces Hematoma without Thromboembolic Events. Plast Reconstr Surg 2020;146:238-45. [Crossref] [PubMed]
- Rohrich RJ, Cho MJ. The Role of Tranexamic Acid in Plastic Surgery: Review and Technical Considerations. Plast Reconstr Surg 2018;141:507-15. [Crossref] [PubMed]
- Lalani T. Breast Implant Infections: An Update. Infect Dis Clin North Am 2018;32:877-84. [Crossref] [PubMed]
- Jianhui Z, Chenggang Y, Binglun L, et al. Autologous fat graft and bone marrow-derived mesenchymal stem cells assisted fat graft for treatment of Parry-Romberg syndrome. Ann Plast Surg 2014;73:S99-103. [Crossref] [PubMed]
- Long C, Sue GR, Chattopadhyay A, et al. Critical Evaluation of Risk Factors of Infection Following 2-Stage Implant-Based Breast Reconstruction. Plast Reconstr Surg Glob Open 2017;5:e1386. [Crossref] [PubMed]
- Nahabedian MY, Spear SL. Acellular dermal matrix for secondary procedures following prosthetic breast reconstruction. Aesthet Surg J 2011;31:38S-50S. [Crossref] [PubMed]
- Reish RG, Damjanovic B, Austen WG Jr, et al. Infection following implant-based reconstruction in 1952 consecutive breast reconstructions: salvage rates and predictors of success. Plast Reconstr Surg 2013;131:1223-30. [Crossref] [PubMed]
- Zhao X, Wu X, Dong J, et al. A Meta-analysis of Postoperative Complications of Tissue Expander/Implant Breast Reconstruction Using Acellular Dermal Matrix. Aesthetic Plast Surg 2015;39:892-901. [Crossref] [PubMed]
- Gahm J, Ljung Konstantinidou A, Lagergren J, et al. Effectiveness of Single vs Multiple Doses of Prophylactic Intravenous Antibiotics in Implant-Based Breast Reconstruction: A Randomized Clinical Trial. JAMA Netw Open 2022;5:e2231583. [Crossref] [PubMed]
- Hai Y, Chong W, Lazar MA. Extended Prophylactic Antibiotics for Mastectomy with Immediate Breast Reconstruction: A Meta-analysis. Plast Reconstr Surg Glob Open 2020;8:e2613. [Crossref] [PubMed]
- Yeo H, Lee D, Kim JS, et al. Strategy for salvaging infected breast implants: lessons from the recovery of seven consecutive patients. Arch Plast Surg 2021;48:165-74. [Crossref] [PubMed]
- Cohen JB, Carroll C, Tenenbaum MM, et al. Breast Implant-Associated Infections: The Role of the National Surgical Quality Improvement Program and the Local Microbiome. Plast Reconstr Surg 2015;136:921-9. [Crossref] [PubMed]
- Halvorson EG, Disa JJ, Mehrara BJ, et al. Outcome following removal of infected tissue expanders in breast reconstruction: a 10-year experience. Ann Plast Surg 2007;59:131-6. [Crossref] [PubMed]
- Song JH, Kim YS, Jung BK, et al. Salvage of Infected Breast Implants. Arch Plast Surg 2017;44:516-22. [Crossref] [PubMed]
- Spear SL, Seruya M. Management of the infected or exposed breast prosthesis: a single surgeon's 15-year experience with 69 patients. Plast Reconstr Surg 2010;125:1074-84.
- Jordan SW, Khavanin N, Kim JYS. Seroma in Prosthetic Breast Reconstruction. Plast Reconstr Surg 2016;137:1104-16. [Crossref] [PubMed]
- Hashemi E, Kaviani A, Najafi M, et al. Seroma formation after surgery for breast cancer. World J Surg Oncol 2004;2:44. [Crossref] [PubMed]
- Porter KA, O'Connor S, Rimm E, et al. Electrocautery as a factor in seroma formation following mastectomy. Am J Surg 1998;176:8-11. [Crossref] [PubMed]
- Srivastava V, Basu S, Shukla VK. Seroma formation after breast cancer surgery: what we have learned in the last two decades. J Breast Cancer 2012;15:373-80. [Crossref] [PubMed]
- Carruthers CA, Dearth CL, Reing JE, et al. Histologic characterization of acellular dermal matrices in a porcine model of tissue expander breast reconstruction. Tissue Eng Part A 2015;21:35-44. [Crossref] [PubMed]
- Constantine RS, Constantine FC, Rohrich RJ. The ever-changing role of biofilms in plastic surgery. Plast Reconstr Surg 2014;133:865e-72e. [Crossref] [PubMed]
- Lembo F, Cecchino LR, Parisi D, et al. Reduction of seroma and improvement of quality of life after early drain removal in immediate breast reconstruction with tissue expander. Preliminary report from a randomized controlled study. J Plast Reconstr Aesthet Surg 2021;74:2565-72. [Crossref] [PubMed]
- Safran T, Vorstenbosch J, Viezel-Mathieu A, et al. Topical Tranexamic Acid in Breast Reconstruction: A Double-Blind, Randomized Controlled Trial. Plast Reconstr Surg 2023;e010322. [Crossref] [PubMed]
- Oni G, Chow W, Ramakrishnan V, et al. Plastic Surgeon-Led Ultrasound. Plast Reconstr Surg 2018;141:300e-9e. [Crossref] [PubMed]
- Liu F, Henn D, Shrefren K, et al. Advances in Tissue Expander Technology Enable Early Targeted Intervention in Prepectoral Breast Reconstruction. Plast Reconstr Surg Glob Open 2021;9:e3781. [Crossref] [PubMed]
- Zeidler KR, Capizzi PJ, Pittman TA. Sientra AlloX2 Short-Term Case Study, Surgical Pearls, and Roundtable Discussion. Plast Reconstr Surg 2018;141:29S-39S. [Crossref] [PubMed]
- Momeni A, Li AY, Tsai J, et al. The Impact of Device Innovation on Clinical Outcomes in Expander-based Breast Reconstruction. Plast Reconstr Surg Glob Open 2019;7:e2524. [Crossref] [PubMed]
- Franck P, Chadab T, Poveromo L, et al. Prepectoral Dual-Port Tissue Expander Placement: Can This Eliminate Suction Drain Use? Ann Plast Surg 2020;85:S60-2. [Crossref] [PubMed]
- Patel H, Kulber D, Ray E. Toward Drainless Breast Reconstruction: A Pilot Study. Plast Reconstr Surg Glob Open 2022;10:e4560. [Crossref] [PubMed]
- Daniel RK, Kerrigan CL. Skin flaps: an anatomical and hemodynamic approach. Clin Plast Surg 1979;6:181-200.
- Takei T, Mills I, Arai K, et al. Molecular basis for tissue expansion: clinical implications for the surgeon. Plast Reconstr Surg 1998;102:247-58. [Crossref] [PubMed]
- Oleck NC, Gu C, Pyfer BJ, et al. Defining Mastectomy Skin Flap Necrosis: A Systematic Review of the Literature and a Call for Standardization. Plast Reconstr Surg 2022;149:858e-66e. [Crossref] [PubMed]
- Pagliara D, Schiavone L, Garganese G, et al. Predicting Mastectomy Skin Flap Necrosis: A Systematic Review of Preoperative and Intraoperative Assessment Techniques. Clin Breast Cancer 2023;23:249-54. [Crossref] [PubMed]
- Coon D, Tuffaha S, Christensen J, et al. Plastic surgery and smoking: a prospective analysis of incidence, compliance, and complications. Plast Reconstr Surg 2013;131:385-91. [Crossref] [PubMed]
- Huo J, Smith BD, Giordano SH, et al. A comparison of patient-centered economic and clinical outcomes of post-mastectomy breast reconstruction between obese and non-obese patients. Breast 2016;30:118-24. [Crossref] [PubMed]
- Ibrahim AMS, Shuster M, Koolen PGL, et al. Analysis of the National Surgical Quality Improvement Program database in 19,100 patients undergoing implant-based breast reconstruction: complication rates with acellular dermal matrix. Plast Reconstr Surg 2013;132:1057-66. [Crossref] [PubMed]
- McCarthy CM, Mehrara BJ, Riedel E, et al. Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg 2008;121:1886-92. [Crossref] [PubMed]
- Kempton SJ, Burish NM, Rao VK. Electronic cigarettes: have you asked your patients about vaping? Plast Reconstr Surg 2014;133:907e. [Crossref] [PubMed]
- Gradishar WJ, Anderson BO, Balassanian R, et al. Breast Cancer, Version 4.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:310-20. [Crossref] [PubMed]
- Stone HB, Coleman CN, Anscher MS, et al. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol 2003;4:529-36. [Crossref] [PubMed]
- Abedi N, Ho AL, Knox A, et al. Predictors of Mastectomy Flap Necrosis in Patients Undergoing Immediate Breast Reconstruction: A Review of 718 Patients. Ann Plast Surg 2016;76:629-34. [Crossref] [PubMed]
- Mlodinow AS, Fine NA, Khavanin N, et al. Risk factors for mastectomy flap necrosis following immediate tissue expander breast reconstruction. J Plast Surg Hand Surg 2014;48:322-6. [Crossref] [PubMed]
- Colwell AS, Tessler O, Lin AM, et al. Breast reconstruction following nipple-sparing mastectomy: predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg 2014;133:496-506. [Crossref] [PubMed]
- De Vita R, Zoccali G, Buccheri EM, et al. Outcome Evaluation after 2023 Nipple-Sparing Mastectomies: Our Experience. Plast Reconstr Surg 2017;139:335e-47e. [Crossref] [PubMed]
- Endara M, Chen D, Verma K, et al. Breast reconstruction following nipple-sparing mastectomy: a systematic review of the literature with pooled analysis. Plast Reconstr Surg 2013;132:1043-54. [Crossref] [PubMed]
- Mastroianni M, Lin AM, Smith BL, et al. Nipple Loss following Nipple-Sparing Mastectomy. Plast Reconstr Surg 2016;138:24e-30e. [Crossref] [PubMed]
- Orzalesi L, Casella D, Santi C, et al. Nipple sparing mastectomy: Surgical and oncological outcomes from a national multicentric registry with 913 patients (1006 cases) over a six year period. Breast 2016;25:75-81. [Crossref] [PubMed]
- Spear SL, Shuck J, Hannan L, et al. Evaluating long-term outcomes following nipple-sparing mastectomy and reconstruction in the irradiated breast. Plast Reconstr Surg 2014;133:605e-14e. [Crossref] [PubMed]
- Frey JD, Salibian AA, Karp NS, et al. The Impact of Mastectomy Weight on Reconstructive Trends and Outcomes in Nipple-Sparing Mastectomy: Progressively Greater Complications with Larger Breast Size. Plast Reconstr Surg 2018;141:795e-804e. [Crossref] [PubMed]
- Salibian AA, Frey JD, Karp NS, et al. Does Staged Breast Reduction before Nipple-Sparing Mastectomy Decrease Complications? A Matched Cohort Study between Staged and Nonstaged Techniques. Plast Reconstr Surg 2019;144:1023-32. [Crossref] [PubMed]
- Salibian AA, Frey JD, Choi M, et al. Optimizing the Mastectomy Flap to Improve Aesthetic Outcomes. Aesthet Surg J 2020;40:S1-S12. [Crossref] [PubMed]
- Frey JD, Salibian AA, Choi M, et al. Mastectomy Flap Thickness and Complications in Nipple-Sparing Mastectomy: Objective Evaluation using Magnetic Resonance Imaging. Plast Reconstr Surg Glob Open 2017;5:e1439. [Crossref] [PubMed]
- Frey JD, Salibian AA, Bekisz JM, et al. What Is in a Number? Evaluating a Risk Assessment Tool in Immediate Breast Reconstruction. Plast Reconstr Surg Glob Open 2019;7:e2585. [Crossref] [PubMed]
- Komorowska-Timek E, Gurtner GC. Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast Reconstr Surg 2010;125:1065-73. [Crossref] [PubMed]
- Lauritzen E, Damsgaard TE. Use of Indocyanine Green Angiography decreases the risk of complications in autologous- and implant-based breast reconstruction: A systematic review and meta-analysis. J Plast Reconstr Aesthet Surg 2021;74:1703-17. [Crossref] [PubMed]
- Moyer HR, Losken A. Predicting mastectomy skin flap necrosis with indocyanine green angiography: the gray area defined. Plast Reconstr Surg 2012;129:1043-8. [Crossref] [PubMed]
- Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg 2012;129:778e-88e. [Crossref] [PubMed]
- Chicco M, Huang TC, Cheng HT. Negative-Pressure Wound Therapy in the Prevention and Management of Complications From Prosthetic Breast Reconstruction: A Systematic Review and Meta-analysis. Ann Plast Surg 2021;87:478-83. [Crossref] [PubMed]
- Sue GR, Long C, Lee GK. Management of Mastectomy Skin Necrosis in Implant Based Breast Reconstruction. Ann Plast Surg 2017;78:S208-11. [Crossref] [PubMed]
- Robertson SA, Jeevaratnam JA, Agrawal A, et al. Mastectomy skin flap necrosis: challenges and solutions. Breast Cancer (Dove Med Press) 2017;9:141-52. [Crossref] [PubMed]
- Brown CA, Losken A. Fold Flaps to the Rescue in Postmastectomy Breast Reconstruction. Plast Reconstr Surg 2023;151:35-8. [Crossref] [PubMed]
- Frey JD, Salibian AA, Choi M, et al. The Importance of Tissue Perfusion in Reconstructive Breast Surgery. Plast Reconstr Surg 2019;144:21S-9S. [Crossref] [PubMed]
- Erba M, Jungreis CA, Horton JA. Nitropaste for prevention and relief of vascular spasm. AJNR Am J Neuroradiol 1989;10:155-6.
- Gdalevitch P, Van Laeken N, Bahng S, et al. Effects of nitroglycerin ointment on mastectomy flap necrosis in immediate breast reconstruction: a randomized controlled trial. Plast Reconstr Surg 2015;135:1530-9. [Crossref] [PubMed]
- Kutun S, Ay AA, Ulucanlar H, et al. Is transdermal nitroglycerin application effective in preventing and healing flap ischaemia after modified radical mastectomy? S Afr J Surg 2010;48:119-21.
- Turin SY, Li DD, Vaca EE, et al. Nitroglycerin Ointment for Reducing the Rate of Mastectomy Flap Necrosis in Immediate Implant-Based Breast Reconstruction. Plast Reconstr Surg 2018;142:264e-70e. [Crossref] [PubMed]
- Wang P, Gu L, Qin Z, et al. Efficacy and safety of topical nitroglycerin in the prevention of mastectomy flap necrosis: a systematic review and meta-analysis. Sci Rep 2020;10:6753. [Crossref] [PubMed]
- Berry SM, Barish CF, Bhandari R, et al. Nitroglycerin 0.4% ointment vs placebo in the treatment of pain resulting from chronic anal fissure: a randomized, double-blind, placebo-controlled study. BMC Gastroenterol 2013;13:106. [Crossref] [PubMed]
- Rajpal N, Walters ET, Elmarsafi T, et al. Use of hyperbaric oxygen therapy for tissue ischemia after breast reconstruction. Undersea Hyperb Med 2019;46:461-5.
- Spruijt NE, Hoekstra LT, Wilmink J, et al. Hyperbaric oxygen treatment for mastectomy flap ischaemia: A case series of 50 breasts. Diving Hyperb Med 2021;51:2-9. [Crossref] [PubMed]
- Moyer KE, Ehrlich HP. Capsular contracture after breast reconstruction: collagen fiber orientation and organization. Plast Reconstr Surg 2013;131:680-5. [Crossref] [PubMed]
- Araco A, Caruso R, Araco F, et al. Capsular contractures: a systematic review. Plast Reconstr Surg 2009;124:1808-19. [Crossref] [PubMed]
- Handel N, Cordray T, Gutierrez J, et al. A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plast Reconstr Surg 2006;117:757-67; discussion 768-72. [Crossref] [PubMed]
- Spear SL, Murphy DK, Allergan Silicone Breast Implant US.. Natrelle round silicone breast implants: Core Study results at 10 years. Plast Reconstr Surg 2014;133:1354-61. [Crossref] [PubMed]
- Mirzabeigi MN, Sbitany H, Jandali S, et al. The role of postoperative antibiotics in reducing biofilm-related capsular contracture in augmentation mammaplasty. Plast Reconstr Surg 2011;128:34e-5e. [Crossref] [PubMed]
- Pfeiffer P, Jørgensen S, Kristiansen TB, et al. Protective effect of topical antibiotics in breast augmentation. Plast Reconstr Surg 2009;124:629-34. [Crossref] [PubMed]
- Wiener TC. The role of betadine irrigation in breast augmentation. Plast Reconstr Surg 2007;119:12-5. [Crossref] [PubMed]
- Araco A, Gravante G, Araco F, et al. A retrospective analysis of 3,000 primary aesthetic breast augmentations: postoperative complications and associated factors. Aesthetic Plast Surg 2007;31:532-9. [Crossref] [PubMed]
- Codner MA, Mejia JD, Locke MB, et al. A 15-year experience with primary breast augmentation. Plast Reconstr Surg 2011;127:1300-10. [Crossref] [PubMed]
- Calobrace MB, Stevens WG, Capizzi PJ, et al. Risk Factor Analysis for Capsular Contracture: A 10-Year Sientra Study Using Round, Smooth, and Textured Implants for Breast Augmentation. Plast Reconstr Surg 2018;141:20S-8S. [Crossref] [PubMed]
- Liu X, Zhou L, Pan F, et al. Comparison of the postoperative incidence rate of capsular contracture among different breast implants: a cumulative meta-analysis. PLoS One 2015;10:e0116071. [Crossref] [PubMed]
- McGuire P, Reisman NR, Murphy DK. Risk Factor Analysis for Capsular Contracture, Malposition, and Late Seroma in Subjects Receiving Natrelle 410 Form-Stable Silicone Breast Implants. Plast Reconstr Surg 2017;139:1-9. [Crossref] [PubMed]
- Hölmich LR, Friis S, Fryzek JP, et al. Incidence of silicone breast implant rupture. Arch Surg 2003;138:801-6. [Crossref] [PubMed]
- Macadam SA, Ho AL, Cook EF Jr, et al. Patient satisfaction and health-related quality of life following breast reconstruction: patient-reported outcomes among saline and silicone implant recipients. Plast Reconstr Surg 2010;125:761-71. [Crossref] [PubMed]
- Rohrich RJ, Reece EM. Breast augmentation today: saline versus silicone--what are the facts? Plast Reconstr Surg 2008;121:669-72. [Crossref] [PubMed]
- Lardi AM, Ho-Asjoe M, Junge K, et al. Capsular contracture in implant based breast reconstruction-the effect of porcine acellular dermal matrix. Gland Surg 2017;6:49-56. [Crossref] [PubMed]
- Spear SL, Seruya M, Rao SS, et al. Two-stage prosthetic breast reconstruction using AlloDerm including outcomes of different timings of radiotherapy. Plast Reconstr Surg 2012;130:1-9. [Crossref] [PubMed]
- Huang CK, Handel N. Effects of Singulair (montelukast) treatment for capsular contracture. Aesthet Surg J 2010;30:404-8. [Crossref] [PubMed]
- Reid RR, Greve SD, Casas LA. The effect of zafirlukast (Accolate) on early capsular contracture in the primary augmentation patient: a pilot study. Aesthet Surg J 2005;25:26-30. [Crossref] [PubMed]
- Le Louarn C, Buis J, Auclair E. Flector tissugel used to treat capsular contracture after breast augmentation surgery. Aesthetic Plast Surg 2008;32:453-8. [Crossref] [PubMed]
- Cheng A, Lakhiani C, Saint-Cyr M. Treatment of capsular contracture using complete implant coverage by acellular dermal matrix: a novel technique. Plast Reconstr Surg 2013;132:519-29. [Crossref] [PubMed]
- Hester TR Jr, Ghazi BH, Moyer HR, et al. Use of dermal matrix to prevent capsular contracture in aesthetic breast surgery. Plast Reconstr Surg 2012;130:126S-36S. [Crossref] [PubMed]
- Maxwell GP, Van Natta BW, Bengtson BP, et al. Ten-year results from the Natrelle 410 anatomical form-stable silicone breast implant core study. Aesthet Surg J 2015;35:145-55. [Crossref] [PubMed]
- Chasan PE. Breast capsulorrhaphy revisited: a simple technique for complex problems. Plast Reconstr Surg 2005;115:296-301; discussion 302-3.
- Chopra K, Gowda AU, Kwon E, et al. Techniques to Repair Implant Malposition after Breast Augmentation: A Review. Aesthet Surg J 2016;36:660-71. [Crossref] [PubMed]
- Colwell AS. Correction of Suboptimal Results in Implant-Based Breast Reconstruction. Aesthet Surg J 2020;40:S38-44. [Crossref] [PubMed]
- Adams WP Jr. Breast deformity caused by anatomical or teardrop implant rotation. Plast Reconstr Surg 2003;111:2110-1 author relply 2111-2.
- Spear SL, Bogue DP, Thomassen JM. Synmastia after breast augmentation. Plast Reconstr Surg 2006;118:168S-171S; discussion 172S-174S. [Crossref] [PubMed]
- Spear SL, Dayan JH, Bogue D, et al. The "neosubpectoral" pocket for the correction of symmastia. Plast Reconstr Surg 2009;124:695-703. [Crossref] [PubMed]
- Spear SL, Sher SR, Al-Attar A, et al. Applications of acellular dermal matrix in revision breast reconstruction surgery. Plast Reconstr Surg 2014;133:1-10. [Crossref] [PubMed]
- Maxwell GP, Gabriel A. The neopectoral pocket in revisionary breast surgery. Aesthet Surg J 2008;28:463-7. [Crossref] [PubMed]
- Kaoutzanis C, Xin M, Ballard TN, et al. Autologous Fat Grafting After Breast Reconstruction in Postmastectomy Patients: Complications, Biopsy Rates, and Locoregional Cancer Recurrence Rates. Ann Plast Surg 2016;76:270-5. [Crossref] [PubMed]
- Sbitany H, Lee KR. Optimizing Outcomes in 2-Stage Prepectoral Breast Reconstruction Utilizing Round Form-Stable Implants. Plast Reconstr Surg 2019;144:43S-50S. [Crossref] [PubMed]
- Panettiere P, Marchetti L, Accorsi D. Soft cohesive silicone gel breast prostheses: a comparative prospective study of aesthetic results versus lower cohesivity silicone gel prostheses. J Plast Reconstr Aesthet Surg 2007;60:482-9. [Crossref] [PubMed]
- Kanchwala SK, Glatt BS, Conant EF, et al. Autologous fat grafting to the reconstructed breast: the management of acquired contour deformities. Plast Reconstr Surg 2009;124:409-18. [Crossref] [PubMed]
- Maxwell GP, Gabriel A. Bioengineered Breast: Concept, Technique, and Preliminary Results. Plast Reconstr Surg 2016;137:415-21. [Crossref] [PubMed]
- Maxwell GP, Gabriel A. Acellular dermal matrix for reoperative breast augmentation. Plast Reconstr Surg 2014;134:932-8. [Crossref] [PubMed]
- Dyrberg DL, Gunnarsson GL, Bille C, et al. A simple clinical assessment of breast animation deformity following direct-to-implant breast reconstruction. Arch Plast Surg 2019;46:535-43. [Crossref] [PubMed]
- Nigro LC, Blanchet NP. Animation Deformity in Postmastectomy Implant-Based Reconstruction. Plast Reconstr Surg Glob Open 2017;5:e1407. [Crossref] [PubMed]
- Becker H, Fregosi N. The Impact of Animation Deformity on Quality of Life in Post-Mastectomy Reconstruction Patients. Aesthet Surg J 2017;37:531-6. [Crossref] [PubMed]
- Strasser EJ. Results of subglandular versus subpectoral augmentation over time: one surgeon's observations. Aesthet Surg J 2006;26:45-50. [Crossref] [PubMed]
- Alnaif N, Safran T, Viezel-Mathieu A, et al. Treatment of breast animation deformity: A systematic review. J Plast Reconstr Aesthet Surg 2019;72:781-8. [Crossref] [PubMed]
- Fracol M, Feld LN, Chiu WK, et al. An overview of animation deformity in prosthetic breast reconstruction. Gland Surg 2019;8:95-101. [Crossref] [PubMed]
- Eck DL, Nguyen DC, Barnes LL, et al. Treatment of Breast Animation Deformity in Implant-Based Reconstruction with Selective Nerve Ablation. Aesthetic Plast Surg 2018;42:1472-5. [Crossref] [PubMed]
- Figus A, Mazzocchi M, Dessy LA, et al. Treatment of muscular contraction deformities with botulinum toxin type A after latissimus dorsi flap and sub-pectoral implant breast reconstruction. J Plast Reconstr Aesthet Surg 2009;62:869-75. [Crossref] [PubMed]
- Gabriel A, Sigalove S, Sigalove NM, et al. Prepectoral Revision Breast Reconstruction for Treatment of Implant-Associated Animation Deformity: A Review of 102 Reconstructions. Aesthet Surg J 2018;38:519-26. [Crossref] [PubMed]
- Hammond DC, Schmitt WP, O'Connor EA. Treatment of breast animation deformity in implant-based reconstruction with pocket change to the subcutaneous position. Plast Reconstr Surg 2015;135:1540-4. [Crossref] [PubMed]
- Holland MC, Lentz R, Sbitany H. Surgical Correction of Breast Animation Deformity with Implant Pocket Conversion to a Prepectoral Plane. Plast Reconstr Surg 2020;145:632-42. [Crossref] [PubMed]
- Lesavoy MA, Trussler AP, Dickinson BP. Difficulties with subpectoral augmentation mammaplasty and its correction: the role of subglandular site change in revision aesthetic breast surgery. Plast Reconstr Surg 2010;125:363-71. [Crossref] [PubMed]
- Shikhman A, Erz L, Brown M, et al. Prepectoral Conversion of Subpectoral Implants for Animation Deformity after Breast Reconstruction: Technique and Experience. Plast Reconstr Surg Glob Open 2022;10:e4132. [Crossref] [PubMed]
- Gutowski KA, Mesna GT, Cunningham BL. Saline-filled breast implants: a Plastic Surgery Educational Foundation multicenter outcomes study. Plast Reconstr Surg 1997;100:1019-27. [Crossref] [PubMed]
- Baek WY, Lew DH, Lee DW. A retrospective analysis of ruptured breast implants. Arch Plast Surg 2014;41:734-9. [Crossref] [PubMed]
- Lantieri LA, Roudot-Thoraval F, Collins ED, et al. Influence of underfilling on breast implant deflation. Plast Reconstr Surg 1997;100:1740-4; discussion 1745. [Crossref] [PubMed]
- US FDA. FDA Strengthens Safety Requirements and Updates Study Results for Breast Implants 2021. Available online: https://www.fda.gov/news-events/press-announcements/fda-strengthens-safety-requirements-and-updates-study-results-breast-implants
- Hölmich LR, Lipworth L, McLaughlin JK, et al. Breast implant rupture and connective tissue disease: a review of the literature. Plast Reconstr Surg 2007;120:62S-9S. [Crossref] [PubMed]
- Lacerda Macedo AC, Carvalho G, Uggioni MLR, et al. Accuracy of Ultrasonography in Breast Implant Rupture Diagnosis: Systematic Review and Meta-Analysis. Plast Reconstr Surg 2021;148:939-47. [Crossref] [PubMed]
- Rietjens M, Villa G, Toesca A, et al. Appropriate use of magnetic resonance imaging and ultrasound to detect early silicone gel breast implant rupture in postmastectomy reconstruction. Plast Reconstr Surg 2014;134:13e-20e. [Crossref] [PubMed]
- Austad ED. Breast implant-related silicone granulomas: the literature and the litigation. Plast Reconstr Surg 2002;109:1724-30; discussion 1731-2. [Crossref] [PubMed]
- Cojocaru M, Chicoş B. ASIA or Shoenfeld's syndrome--an autoimmune syndrome induced by adjuvants. Rom J Intern Med 2013;51:131-4.
- Hölmich LR, Vejborg IM, Conrad C, et al. Untreated silicone breast implant rupture. Plast Reconstr Surg 2004;114:204-14; discussion 215-6. [Crossref] [PubMed]
- Nesher G, Soriano A, Shlomai G, et al. Severe ASIA syndrome associated with lymph node, thoracic, and pulmonary silicone infiltration following breast implant rupture: experience with four cases. Lupus 2015;24:463-8. [Crossref] [PubMed]
- Blombery P, Thompson E, Ryland GL, et al. Frequent activating STAT3 mutations and novel recurrent genomic abnormalities detected in breast implant-associated anaplastic large cell lymphoma. Oncotarget 2018;9:36126-36. [Crossref] [PubMed]
- Oishi N, Brody GS, Ketterling RP, et al. Genetic subtyping of breast implant-associated anaplastic large cell lymphoma. Blood 2018;132:544-7. [Crossref] [PubMed]
- Ebner PJ, Liu A, Gould DJ, et al. Breast implant-associated anaplastic large cell lymphoma, a systematic review and in-depth evaluation of the current understanding. J Surg Oncol 2019;120:573-7. [Crossref] [PubMed]
- Clemens MW, Jacobsen ED, Horwitz SM. 2019 NCCN Consensus Guidelines on the Diagnosis and Treatment of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Aesthet Surg J 2019;39:S3-S13. [Crossref] [PubMed]
- Nelson JA, Dabic S, Mehrara BJ, et al. Breast Implant-associated Anaplastic Large Cell Lymphoma Incidence: Determining an Accurate Risk. Ann Surg 2020;272:403-9. [Crossref] [PubMed]
- Clemens MW, McGuire PA. Discussion: A Prospective Approach to Inform and Treat 1340 Patients at Risk for BIA-ALCL. Plast Reconstr Surg 2019;144:57-9. [Crossref] [PubMed]
- Doren EL, Miranda RN, Selber JC, et al. U.S. Epidemiology of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Plast Reconstr Surg 2017;139:1042-50. [Crossref] [PubMed]
- Clemens MW, Medeiros LJ, Butler CE, et al. Complete Surgical Excision Is Essential for the Management of Patients With Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J Clin Oncol 2016;34:160-8. [Crossref] [PubMed]
- Colaris MJL, de Boer M, van der Hulst RR, et al. Two hundreds cases of ASIA syndrome following silicone implants: a comparative study of 30 years and a review of current literature. Immunol Res 2017;65:120-8. [Crossref] [PubMed]
- Brinton LA, Buckley LM, Dvorkina O, et al. Risk of connective tissue disorders among breast implant patients. Am J Epidemiol 2004;160:619-27. [Crossref] [PubMed]
- Fryzek JP, Holmich L, McLaughlin JK, et al. A nationwide study of connective tissue disease and other rheumatic conditions among Danish women with long-term cosmetic breast implantation. Ann Epidemiol 2007;17:374-9. [Crossref] [PubMed]
- Kaplan J, Rohrich R. Breast implant illness: a topic in review. Gland Surg 2021;10:430-43. [Crossref] [PubMed]
- Coroneos CJ, Selber JC, Offodile AC 2nd, et al. US FDA Breast Implant Postapproval Studies: Long-term Outcomes in 99,993 Patients. Ann Surg 2019;269:30-6. [Crossref] [PubMed]
- Balk EM, Earley A, Avendano EA, et al. Long-Term Health Outcomes in Women With Silicone Gel Breast Implants: A Systematic Review. Ann Intern Med 2016;164:164-75. [Crossref] [PubMed]
- Singh N, Picha GJ, Hardas B, et al. Five-Year Safety Data for More than 55,000 Subjects following Breast Implantation: Comparison of Rare Adverse Event Rates with Silicone Implants versus National Norms and Saline Implants. Plast Reconstr Surg 2017;140:666-79. [Crossref] [PubMed]
- Peters W, Smith D, Fornasier V, et al. An outcome analysis of 100 women after explantation of silicone gel breast implants. Ann Plast Surg 1997;39:9-19. [Crossref] [PubMed]
- Rohrich RJ, Bellamy JL, Alleyne B. Assessing Long-Term Outcomes in Breast Implant Illness: The Missing Link? A Systematic Review. Plast Reconstr Surg 2022;149:638e-45e. [Crossref] [PubMed]


