Implementing sepsis bundles
Introduction
Septic shock is one of the main causes of admission and death in critically ill patients (1). Septic shock is a combination of vasodilation, microvascular failure and, sometimes, cardiac dysfunction, resulting in hypovolemia, hypotension and cell dysfunction.
As early hemodynamic assessment based on physical examination, urinary output and central venous pressure (CVP) fails to detect persistent impairment of oxygen delivery to tissues in septic shock patients, Rivers et al. tested in 2001 a new therapeutic strategy, called “early goal-directed therapy” (EGDT) in 263 septic shock patients in a single-center randomized control trial (2). This step-by-step strategy in the early phase of septic shock with low blood flow and oxygen delivery, was an aggressive hemodynamic resuscitation, including fluids, vasopressors, inotropes and blood transfusion, which aimed at normalizing within the first 6 hours of therapy the central venous oxygen saturation (ScvO2), used as a surrogate of oxygen delivery. The first step was fluid resuscitation to achieve a CVP between 8 and 12 mmHg. Secondly, physicians had to administer vasopressors to target a mean arterial pressure (MAP) between 65 and 90 mmHg. Thirdly, in case of persistently low ScvO2 despite achievement of CVP and MAP targets, patients received blood transfusions until hematocrit was above 30% and eventually inotropic agents when ScvO2 is less than 70% (2). Compared to standard care, EGDT significantly decreased the in-hospital, day-28 and day-60 mortality (2).
In 2004, the Surviving Sepsis Campaign (SSC) endorsed the EGDT protocol and published the first international guidelines for management of severe sepsis and septic shock. It was aimed at obtaining a 25% reduction in mortality over the following 5 years in patients with septic shock worldwide (3,4). These guidelines have been updated every 4 years. They cover all the aspects of the management of septic shock patients, such as hemodynamic, respiratory, metabolic, diagnosis and antimicrobial therapy management. These complex guidelines have been summarized by the SSC in sepsis bundles, which represent key elements of care regarding the diagnosis and treatment of patients with septic shock.
Sepsis bundles: definition and benefits
Definition
Bundles are defined by the Institute for Healthcare Improvement as “a group of interventions related to a disease process that, when executed together, result in better outcomes than when implemented individually” (5). Bundles aim at converting complex guidelines into meaningful changes in behavior and clinical outcomes (4). Bundles can help physicians to identify the key elements of care regarding both the diagnosis and treatment of patients with septic shock. The main goals of the bundles approach to sepsis diagnosis and management are (I) to reduce the mortality and to improve patients’ outcomes; (II) to ensure more consistent and timely application of evidence-based care and; (III) to ensure reductions in clinical practice variability. In this regard, the Institute for the SSC provides in 2004 two bundles for septic shock patients: the 6-hour resuscitation bundle and the 24-hour management bundle (http://www.ihi.org/IHI/Topics/CriticalCare/Sepsis).
Beneficial effects on survival
Many studies showed in septic shock patients that implementation of 6-hour resuscitation and 24-hour management sepsis bundles recommended in 2004 by the SSC decreased crude, in-hospital or day-28 mortality (6-17), reduced in-hospital or in-intensive care unit (ICU) length of stay (10,15,17), reduced the cost of care per patient (15) and improved the patients’ quality of life (15). The beneficial effect of sepsis bundles on mortality has been confirmed in a meta-analysis, including 50 studies from 2004 to 2014, in which the treatment of sepsis was based on 2004 or 2008 SSC guidelines (18). Implementing sepsis bundles allowed a significant decrease in mortality in the majority of studies with an overall odds ratio of 0.66 (95% confidence interval: 0.61–0.72) (18). This significant decrease in mortality corresponds to about six patients needed to be treated to save one life (11). This beneficial effect could be related to a better identification of septic shock or to a better compliance to key therapeutic elements, such as earlier administration of broad spectrum antibiotics. Several studies also showed that the extent of the decrease in mortality could depend on the number of bundle interventions completed (7,9,10,15). Nevertheless, one cannot currently define the most important bundle intervention in septic shock patients. Castellanos-Ortega et al. found that the only intervention with a positive impact on mortality was to achieve a ScvO2 ≥70% (10). Levy et al. confirmed that the measurement of ScvO2 was independently associated with a decrease in mortality, as well as the measurement of CVP (17). Other studies found that the bundles which were independently associated with decreased mortality were: performing blood cultures (11), achieving a MAP >65 mmHg and the lactate clearance (13), or to administer broad spectrum antibiotics and fluid bolus followed by vasopressors if needed (15). Finally, the beneficial effect of bundles on mortality could also depend on the timing of bundles application: the earlier the bundle is achieved, the lower is the mortality. In this regard, it has been shown that the compliance with early resuscitation bundle elements, within the first 3 hours, was associated with a lower probability of being eligible for later resuscitation and maintenance bundle elements (14). The mortality was also found to be significantly lower even if completion of the 6-hour resuscitation bundle was achieved later, at 18 hours, compared with not completing the bundle at all (19). The importance of timing has been confirmed by a meta-analysis, in which therapies delivered early, within the first 6 hours, may have been the main determinant of survival (18).
Importance of compliance to bundles
It must be stressed that the beneficial effects of sepsis bundles are tightly coupled to the compliance with the sepsis bundles (6,8,10,12-18). Levy et al. have recently demonstrated in a multicenter study conducted in 218 ICUs in the United States, South America and Europe from January 2005 to June 2012, including 29,470 patients, that the overall mortality was lower in sites with high-compliance to resuscitation bundles compared with sites with low-compliance (17). An increase in compliance with the sepsis bundles was associated with a 25% relative risk reduction in mortality rate (17). Nevertheless, the application of sepsis bundles seems to be quite low in most studies, confirming the difficulty of transferring evidence to the clinical practice (7,20-23). In this regard, the total bundles compliance is around 5% (9,14), the compliance with the 6-hour resuscitation bundles varies from 0% (7,10) to 10% (11-13,15) and the compliance with the 24-hour management sepsis bundles is around 15% (8,12). In a large multicenter study conducted in 2005 in 59 medical-surgical ICUs throughout Spain, Ferrer et al. also reported a very low compliance with resuscitation (5%) and with management (11%) sepsis bundles (8). Only one study conducted between 2004 and 2005 in two university hospitals in England found a high compliance of 52% with the 6-hour resuscitation bundles and a compliance of 30% with the 24-hour management bundles (6). It is important to note that, in this latter study (6), the 6-hour bundles did not include the optimization of ScvO2, which is the most frequent uncompleted bundle in the other studies. Moreover, the compliance with sepsis bundles differs worldwide. It seems to be higher in United States than in Europe (17,24), whereas a larger percentage of the South American septic shock patients were in low-compliance sites (17). Interestingly, there is a growing evidence that dedicated performance improvement initiatives can significantly improve the compliance with each item of the sepsis bundles, as well as the compliance with the entire 6-hour resuscitation and 24-hour management sepsis bundles (7-10,12-18). Performance improvement initiatives may vary from multifaceted educational programs to interventions specifically aimed at inducing a variation in standard sepsis care (18). Importantly, there is a greater increase in compliance with sepsis bundles over time in hospitals that continue to participate to dedicated programs (17). By contrast, one can observe a decrease in compliance with sepsis bundles at the end of the educational effort (8), even though mortality seems to remain stable, with respect to the mortality reached with the increase in compliance with bundles (8). Finally, such multifaceted intervention allows improving the compliance with sepsis bundles not only in developed countries but also in emergent countries such as Brazil with an increase in compliance with sepsis bundles from 13% to 62% (15).
Sepsis bundles: limits and pitfalls
Sepsis bundles before multicenter randomized clinical trials on EGDT
In 2012, the Institute for the SSC revised a first time the sepsis bundles in conjunction with the 2012 full guidelines (1). The 24-hour management bundle was dropped, since new robust data emerged about glucose control, corticosteroids administration or recombinant activated protein C administration. This latter was withdrawn from the market since it showed no consistent benefit in survival (25). The resuscitation 6-hour bundle has been modified into two bundles. The first part, named “the severe sepsis 3-hour resuscitation bundle”, contains all the therapeutic goals to be completed within 3 hours of the time of presentation with septic shock: (I) to measure lactate level; (II) to obtain blood cultures prior to administration of antibiotics; (III) to administer broad spectrum antibiotics; and (IV) to administer 30 mL/kg crystalloids for hypotension or lactate ≥4 mmol/L. The second part, named “the 6-hour septic shock bundle” contains all the therapeutic goals to be completed within 6 hours of the time of presentation with septic shock: to apply vasopressors (for hypotension that does not respond to initial fluid resuscitation) to maintain a MAP ≥65 mmHg, to measure CVP and ScvO2 when arterial hypotension persists despite volume resuscitation or initial lactate ≥4 mmol/L, and to re-measure lactate if initial lactate was elevated. In other words, these new sepsis bundles still endorsed EGDT, as proposed in 2001 by Rivers and colleagues (2).
Sepsis bundles after multicenter randomized clinical trials on EGDT
Three recent multicenter randomized studies (ProCESS, ARISE and ProMISe) have shown that EGDT using ScvO2 did not reduce all-cause mortality (26-28), duration of organ support or in hospital length of stay (26,28). A recent meta-analysis confirmed that EGDT was not superior to usual care in patients with septic shock (29), whereas a previous meta-analysis, including the ProCESS study but not the ARISE and the ProMISe studies, showed that EGDT significantly reduced overall mortality in patients with septic shock, especially when initiated early (30). However, compared to the study by Rivers et al. (2), patients of the ProCESS, ARISE and ProMISe studies were fluid resuscitated before randomization, such that the average baseline ScvO2 was already higher than 70% (the target of the EGDT arm). Thus, what is called “usual care” in these three studies could be already considered as EGDT (31,32), as attested by the very low mortality in the usual care arm in these studies (26,28) compared to the Rivers’ study (2). Such a fact certainly accounted for the absence of superiority of the EGDT over the control arms in these studies (26-28). Taken together, these recent data somewhat question the real advantages of sepsis bundles.
Implementation of the bundles is aimed at tracking changes in practice and reporting how often these evidence-based interventions are used. Thus, as stated by the leadership of the SSC, “both the guidelines and the performance improvement indicators will evolve as new evidence that improves our understanding of how best to care for patients with severe sepsis and septic shock becomes available.” That is why following the publication of ProCESS, ARISE and ProMISe studies the sepsis bundles were again revised in 2015 (Table 1). Compared to sepsis bundles from 2012, “the severe sepsis 3-hour resuscitation bundle” was unchanged, unlike “the 6-hour septic shock bundle”. The bundle “to measure CVP and ScvO2 when persistent arterial hypotension despite volume resuscitation or initial lactate ≥4 mmol/L” was updated as follows: “in the event of persistent hypotension after initial fluid administration (MAP <65 mmHg) or if initial lactate was ≥4 mmol/L, re-assess volume status and tissue perfusion and document findings.” For this purpose, “either repeat focused exam (after initial fluid resuscitation) by licensed independent practitioner including vital signs, cardiopulmonary, capillary refill, pulse, and skin findings” or document findings with two of the following: “measure CVP, measure ScvO2, bedside cardiovascular ultrasound or dynamic assessment of fluid responsiveness with passive leg raise or fluid challenge” (http://www.survivingsepsis.org/Bundles).

Full table
Limits and pitfalls of the current sepsis bundles
Nevertheless, as for the previous bundles with controversial validity (33,34), these new bundles are still quite general and may have some limits and pitfalls (Table 2). Firstly, the measurement of ScvO2 is no longer a first line therapeutic goal. Nevertheless, it must be stressed that the results of the three recent multicenter studies (26-28) and of the meta-analysis (29) clearly cannot rule out the strategy of increasing oxygen delivery and targeting ScvO2 >70% when ScvO2 is lower than 70% as this was the case in the majority of the patients of the Rivers et al. study (2). Moreover, bundles provide no value of ScvO2 to achieve.

Full table
Secondly, the bundles recommend to measure CVP in order to assess volume status. However, is it currently widely demonstrated and admitted that CVP does not reflect intravascular volume and that cardiac filling pressures such as CVP are not appropriate to predict fluid responsiveness (35,36). In this regard, fluid responsiveness can be assessed by a passive leg raising (PLR) (37), as proposed by the most recent bundles. It is important to note that the method for performing PLR is crucial, because it fundamentally affects its hemodynamic effects and its reliability (38). Five rules have to be followed. First, PLR should start from the semi-recumbent and not the supine position. Second, the PLR effects must be assessed on continuous cardiac output measurement and not on blood pressure measurement. Third, the technique used to measure cardiac output during PLR must be enough sensitive to track short-term and transient changes of cardiac output. Fourth, cardiac output must be measured not only before and during PLR but also after PLR in order to check that it returns to its baseline value. Fifth, some precautions must be taken to avoid some confounding factors resulting in adrenergic stimulation, which can induce a mistaken interpretation of cardiac output changes (38).
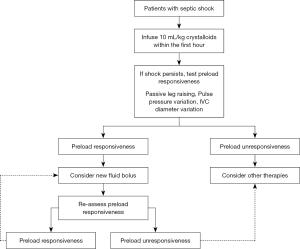
Thirdly, bundles recommend to administer 30 mL/kg crystalloids for hypotension or lactate ≥4 mmol/L within 3 hours of the time of presentation with septic shock. Nevertheless, it is now well established that a positive fluid balance (39-41) is an independent factor of mortality in septic shock patients: the higher the positive fluid balance, the poorer the prognosis. It would be more reasonable to recommend administration of around 10 mL/kg crystalloids for 1 hour and then, in case of persistent shock, to assess fluid responsiveness by performing PLR or by using other dynamic indices such as pulse pressure variation or inferior vena cava (IVC) diameter variation, when applicable (Figure 1).
Finally, bundles recommend to administer vasopressors only within 6 hours when MAP remained <65 mmHg despite initial fluid resuscitation. Nevertheless, the timing of vasopressors (usually norepinephrine) administration within these 6 hours is critical, as a delayed initiation of vasopressors in septic shock patients is associated with increased mortality (42). It was indeed shown that survivors at day-28 received norepinephrine significantly earlier and for a shorter period than non-survivors. There was also a relationship between the delay of norepinephrine initiation and mortality: for each hour delay in norepinephrine initiation (within the first 6 hours), the mortality rate increased by 5.3% (42). Indeed, delayed administration of vasopressors could lead to prolonged hypotension in the context of severely depressed vascular tone since fluid infusion alone cannot restore vascular tone. In this regard, it has been shown that not only the degree but also the duration of hypotension is associated with increased mortality (43,44). Moreover, norepinephrine might have some positive effects when administered early. Indeed, by increasing the systemic venous return and cardiac preload, norepinephrine can increase cardiac output in patients with preload reserve (45,46). This increase in cardiac preload is the result of an increase in mean systemic pressure (47,48) due to venous blood redistribution from unstressed to stressed volume. Finally, early administration of norepinephrine in severely hypotensive patients improves tissue oxygenation, as assessed at the level of the thenar eminence muscles using a near-infrared spectroscopy device (49). In a clinical study in severely hypotensive patients, early correction of hypotension with norepinephrine resulted in an improved muscle tissue oxygenation and in microcirculatory reserve capacities (49). These beneficial effects on tissue oxygenation can be related to the increase in cardiac output, as described above, and/or to the increase in MAP, which allows the increase in the tissue perfusion pressure and thus improves microvascular blood flow in pressure-dependent vascular beds. In this regard, a correlation was shown between sublingual microvascular perfusion indices and MAP in the first 6 hours of management of septic shock (50). Finally, early initiation of vasopressors may prevent the harmful fluid overload. In this regard, an experimental study in a murine model of endotoxinic shock showed that the early use of norepinephrine associated with volume expansion resulted in a decrease in the amount of fluid infused (51). These results have been retrospectively confirmed in septic shock patients (42). Patients where norepinephrine was administered within the first 2 hours of resuscitation received less fluid than patients with delayed norepinephrine administration (42).
Conclusions
Beyond the sepsis bundles, the prognosis of septic shock is tightly linked to the earliness of both appropriate antibiotic therapy and early aggressive hemodynamic resuscitation. This includes administration of fluids and of vasopressors in order to target a MAP >65 mmHg, which remain the cornerstone of the management of septic shock patients. Nevertheless, the implementation of sepsis bundles results in a decrease in mortality and to better outcomes in septic shock patients. These benefits mainly depend on the compliance with the sepsis bundles, highlighting the importance of dedicated educational programs.
Acknowledgements
None.
Footnote
Conflicts of Interest: X Monnet and JL Teboul are members of Medical Advisory board of Pulsion/Maquet. M Jozwiak has no conflicts of interest to declare.
References
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165-228. [Crossref] [PubMed]
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368-77. [Crossref] [PubMed]
- Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 2004;32:858-73. [Crossref] [PubMed]
- Levy MM, Pronovost PJ, Dellinger RP, et al. Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome. Crit Care Med 2004;32:S595-7. [Crossref] [PubMed]
- Dellinger RP, Vincent JL. The Surviving Sepsis Campaign sepsis change bundles and clinical practice. Crit Care 2005;9:653-4. [Crossref] [PubMed]
- Gao F, Melody T, Daniels DF, et al. The impact of compliance with 6-hour and 24-hour sepsis bundles on hospital mortality in patients with severe sepsis: a prospective observational study. Crit Care 2005;9:R764-70. [Crossref] [PubMed]
- Nguyen HB, Corbett SW, Steele R, et al. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med 2007;35:1105-12. [Crossref] [PubMed]
- Ferrer R, Artigas A, Levy MM, et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA 2008;299:2294-303. [Crossref] [PubMed]
- Girardis M, Rinaldi L, Donno L, et al. Effects on management and outcome of severe sepsis and septic shock patients admitted to the intensive care unit after implementation of a sepsis program: a pilot study. Crit Care 2009;13:R143. [Crossref] [PubMed]
- Castellanos-Ortega A, Suberviola B, García-Astudillo LA, et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow-up quasi-experimental study. Crit Care Med 2010;38:1036-43. [Crossref] [PubMed]
- Cardoso T, Carneiro AH, Ribeiro O, et al. Reducing mortality in severe sepsis with the implementation of a core 6-hour bundle: results from the Portuguese community-acquired sepsis study (SACiUCI study). Crit Care 2010;14:R83. [Crossref] [PubMed]
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010;36:222-31. [Crossref] [PubMed]
- Nguyen HB, Kuan WS, Batech M, et al. Outcome effectiveness of the severe sepsis resuscitation bundle with addition of lactate clearance as a bundle item: a multi-national evaluation. Crit Care 2011;15:R229. [Crossref] [PubMed]
- Miller RR 3rd, Dong L, Nelson NC, et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med 2013;188:77-82. [Crossref] [PubMed]
- Noritomi DT, Ranzani OT, Monteiro MB, et al. Implementation of a multifaceted sepsis education program in an emerging country setting: clinical outcomes and cost-effectiveness in a long-term follow-up study. Intensive Care Med 2014;40:182-91. [Crossref] [PubMed]
- van Zanten AR, Brinkman S, Arbous MS, et al. Guideline bundles adherence and mortality in severe sepsis and septic shock. Crit Care Med 2014;42:1890-8. [Crossref] [PubMed]
- Levy MM, Rhodes A, Phillips GS, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med 2015;43:3-12. [Crossref] [PubMed]
- Damiani E, Donati A, Serafini G, et al. Effect of performance improvement programs on compliance with sepsis bundles and mortality: a systematic review and meta-analysis of observational studies. PLoS One 2015;10:e0125827. [Crossref] [PubMed]
- Coba V, Whitmill M, Mooney R, et al. Resuscitation bundle compliance in severe sepsis and septic shock: improves survival, is better late than never. J Intensive Care Med 2011;26:304-13. [Crossref] [PubMed]
- Kortgen A, Niederprüm P, Bauer M. Implementation of an evidence-based "standard operating procedure" and outcome in septic shock. Crit Care Med 2006;34:943-9. [Crossref] [PubMed]
- Trzeciak S, Dellinger RP, Abate NL, et al. Translating research to clinical practice: a 1-year experience with implementing early goal-directed therapy for septic shock in the emergency department. Chest 2006;129:225-32. [Crossref] [PubMed]
- Shapiro NI, Howell MD, Talmor D, et al. Implementation and outcomes of the Multiple Urgent Sepsis Therapies (MUST) protocol. Crit Care Med 2006;34:1025-32. [Crossref] [PubMed]
- Karlsson S, Varpula M, Ruokonen E, et al. Incidence, treatment, and outcome of severe sepsis in ICU-treated adults in Finland: the Finnsepsis study. Intensive Care Med 2007;33:435-43. [Crossref] [PubMed]
- Levy MM, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 2012;12:919-24. [Crossref] [PubMed]
- Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med 2012;366:2055-64. [Crossref] [PubMed]
- ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014;371:1496-506. [Crossref] [PubMed]
- ProCESS Investigators, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014;370:1683-93. [Crossref] [PubMed]
- Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015;372:1301-11. [Crossref] [PubMed]
- Angus DC, Barnato AE, Bell D, et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med 2015;41:1549-60. [Crossref] [PubMed]
- Gu WJ, Wang F, Bakker J, et al. The effect of goal-directed therapy on mortality in patients with sepsis - earlier is better: a meta-analysis of randomized controlled trials. Crit Care 2014;18:570. [Crossref] [PubMed]
- Levy MM. Early goal-directed therapy: what do we do now? Crit Care 2014;18:705. [Crossref] [PubMed]
- Nguyen HB, Jaehne AK, Jayaprakash N, et al. Early goal-directed therapy in severe sepsis and septic shock: insights and comparisons to ProCESS, ProMISe, and ARISE. Crit Care 2016;20:160. [Crossref] [PubMed]
- Moreno R, Rhodes A. Evidence should not be viewed in isolation. Crit Care Med 2010;38:S528-33. [Crossref] [PubMed]
- Marik PE. Surviving sepsis: going beyond the guidelines. Ann Intensive Care 2011;1:17. [Crossref] [PubMed]
- Osman D, Ridel C, Ray P, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med 2007;35:64-8. [Crossref] [PubMed]
- Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care 2011;1:1. [Crossref] [PubMed]
- Monnet X, Rienzo M, Osman D, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med 2006;34:1402-7. [Crossref] [PubMed]
- Monnet X, Teboul JL. Passive leg raising: five rules, not a drop of fluid! Crit Care 2015;19:18. [Crossref] [PubMed]
- Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 2006;34:344-53. [Crossref] [PubMed]
- Boyd JH, Forbes J, Nakada TA, et al. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011;39:259-65. [Crossref] [PubMed]
- Acheampong A, Vincent JL. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care 2015;19:251. [Crossref] [PubMed]
- Bai X, Yu W, Ji W, et al. Early versus delayed administration of norepinephrine in patients with septic shock. Crit Care 2014;18:532. [Crossref] [PubMed]
- Varpula M, Tallgren M, Saukkonen K, et al. Hemodynamic variables related to outcome in septic shock. Intensive Care Med 2005;31:1066-71. [Crossref] [PubMed]
- Dünser MW, Takala J, Ulmer H, et al. Arterial blood pressure during early sepsis and outcome. Intensive Care Med 2009;35:1225-33. [Crossref] [PubMed]
- Hamzaoui O, Georger JF, Monnet X, et al. Early administration of norepinephrine increases cardiac preload and cardiac output in septic patients with life-threatening hypotension. Crit Care 2010;14:R142. [Crossref] [PubMed]
- Monnet X, Jabot J, Maizel J, et al. Norepinephrine increases cardiac preload and reduces preload dependency assessed by passive leg raising in septic shock patients. Crit Care Med 2011;39:689-94. [Crossref] [PubMed]
- Persichini R, Silva S, Teboul JL, et al. Effects of norepinephrine on mean systemic pressure and venous return in human septic shock. Crit Care Med 2012;40:3146-53. [Crossref] [PubMed]
- Maas JJ, Pinsky MR, de Wilde RB, et al. Cardiac output response to norepinephrine in postoperative cardiac surgery patients: interpretation with venous return and cardiac function curves. Crit Care Med 2013;41:143-50. [Crossref] [PubMed]
- Georger JF, Hamzaoui O, Chaari A, et al. Restoring arterial pressure with norepinephrine improves muscle tissue oxygenation assessed by near-infrared spectroscopy in severely hypotensive septic patients. Intensive Care Med 2010;36:1882-9. [Crossref] [PubMed]
- Trzeciak S, Dellinger RP, Parrillo JE, et al. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med 2007;49:88-98, 98.e1-2.
- Sennoun N, Montemont C, Gibot S, et al. Comparative effects of early versus delayed use of norepinephrine in resuscitated endotoxic shock. Crit Care Med 2007;35:1736-40. [Crossref] [PubMed]