
Review on endobronchial therapies—current status and future
Introduction
Despite lobectomy remaining the gold standard treatment for resectable lung cancer, there are increasing indications for local treatment of lung malignancies. One of which is the rising prevalence of multifocal lung cancers, as multiple ground glass opacities with or without solid components demonstrating varying invasiveness exist in the same patient. Many of these subsolid nodules represent pre-malignant or very early stage cancers without regional spread, thus potentially eradicable with locoregional treatment rather than anatomical surgical resection. Nowadays, not uncommonly we encounter patients who have had major lung resection performed for primary lung cancers previously, and upon follow up other suspicious lung nodules in the remaining lung either enlarged or increased in solidity, leading to the need of lung parenchymal sparing strategies (1). These include sublobar resection, stereotactic body radiation therapy (SBRT), and local ablation (2). The aging population also increased the proportion of elderly patients who suffer from multiple comorbidities, poor lung function or high clinical frailty score, making them high risk for major lung resection and more suited to locoregional treatment.
As in the lung adenocarcinoma sequence, recent studies have shown that lung adenocarcinomas progress in a stepwise manner from pre-malignant lesions, to adenocarcinoma-in-situ or minimally invasive adenocarcinoma, to invasive adenocarcinoma (3,4). The extent of invasiveness can be estimated by the consolidation to tumour ratio (>0.25) and size of lesions (>2 cm) on computed tomography (CT) scans (5), thus providing a basis for biopsying and treating the most “risky” nodules in patients with multiple lung nodules.
Local therapies for early lung cancers are gaining evidence support, especially in patients with high surgical risks or those who decline surgery. The JCOG 0802 trial is a recently published phase 3 randomized controlled trial comparing segmentectomy versus lobectomy in small sized peripheral non-small cell lung carcinomas (NSCLC) less than 2 cm in diameter and >0.5 consolidation to tumour ratio. It showed that the 5-year overall survival was higher in the segmentectomy group than the lobectomy group (94.3% vs. 91.1%, P=0.0082), the forced expiratory volume in 1 second was higher in the segmentectomy group, while local relapse was lower in the lobectomy group (5.4% vs. 10.5%) (6). The CALGB 140503 investigators recently further reported non-inferiority of sublobar resection (59% wedge resection and 41% segmentectomy) with respect to disease-free survival and overall survival in stage 1 NSCLC <2 cm (7). In tumours less than 2 cm in diameter and consolidation tumour ratio 0.25 or less, the JCOG 0804 trial further suggested that wedge resection is sufficient, yielding a 5-year relapse-free survival of 99.7% (8). This is further supported by a recent randomized controlled trial by Altorki et al., reporting similar 5-year disease-free survival (63.6% vs. 64.1%) and 5-year overall survival (80.3% vs. 78.9%) between groups treated with sublobar and lobar resection, for stage I lung cancers less than 2 cm in size (9). SBRT is targeted towards patients with stage I or II NSCLC without lymph node involvement and who are medically inoperable. SBRT has a local control rate of more than 80% in multiple retrospective series (10), and disease-free survival of 26% and overall survival of 40% at 4 years in a multicentre phase II study (11). However, sublobar resection still carries surgical risks while SBRT has up to 22.3% risk of radiation pneumonitis and pneumonia. Since the early 2000s, percutaneous ablation of lung tumours have been attempted (12) following reports of efficacy of local ablation in liver cancers. The subsequent decade saw the blossom of image-guided local ablative therapies of lung tumours, first with radiofrequency ablation (RFA), later with microwave ablation (MWA) and cryoablation.
This article aims to examine the current progress and application of transbronchial therapies for peripheral lung cancers, encompassing various energy modalities (13), effective add-ons to enhance precision and efficiency, promising non-thermal ablation methods, and the relatively novel approach of transbronchial intratumoral injection of therapeutic agents.
Transbronchial microwave ablation
In contrast to the percutaneous route, transbronchial ablation has gained popularity in recent years. A Japanese group pioneered a bronchoscopy-guided cooled RFA technique for lung tumours in humans in 2010 (14,15), followed by a Chinese group using electromagnetic navigation bronchoscopy (ENB) guidance (16). More recently, a first-in-human dose escalation study involving 8 patients using an external cooled bronchoscopic RFA catheter revealed adequate ablation zone coverage in all cases, with more uniform necrosis observed at higher energy levels (17). Compared to percutaneous approach, a major advantage of bronchoscopic ablation is absence of pleural puncture, and hence fewer pleural-based complications. The Japanese group reported no pneumothorax, bronchopleural fistula nor pleural effusion in 28 cases of bronchoscopic RFA (15), while the rate of pneumothorax for percutaneous ablation ranges from 3.5–54%. Similarly, bronchoscopic ablation also eliminates the chance of needle tract seeding. Another edge of bronchoscopic ablation is its ability to reach certain regions of lung which are otherwise difficult or dangerous for percutaneous access, for instance areas near mediastinal pleura, diaphragm, lung apex, or areas shielded by scapula.
The author’s institute is one of the first to perform ENB-guided microwave ablation on patients in the hybrid operating room (18). Microwave energy is utilized rather than RFA because it directly heats tissue to lethal temperatures greater than 150 ℃ through dielectric hysteresis (19), therefore independent from electrical conductance. Since the aerated lung has a relatively high impedance among all solid organs, microwave energy more ideal as it is less susceptible to tissue impedance, being able to produce faster, larger and more predictable ablation zones than RFA (20). Selected patients had high surgical risks, either due to underlying medical comorbidities or due to inadequate respiratory reserve. Ablated nodules were less than 3 cm in size, and away from large blood vessels which may lead to under-ablation due to heat-sink effect, and from structures that may suffer from thermal injury, including the phrenic nerve, brachial plexus, heart and esophagus.
By using ENB navigation systems such as SuperDimensionTM (Covidien, Plymouth, MN, USA), along with confirmation of position through fluoroscopy and cone-beam CT (CBCT), the accuracy of navigation is significantly increased. The EmprintTM Ablation Catheter with ThermosphereTM technology (Covidien, Plymouth, MN, USA) is placed inside the lung tumor through bronchoscopy and specific tunneling tools, then ablated for up to 10 minutes at 100W per burn. To ensure that sufficient ablation margins are achieved for larger tumors, double or triple ablation may be performed either in the same position or with adjustments made to the catheter’s position.
Our institute contributed to the NAVABLATE study, which presented findings for 30 nodules that underwent transbronchial MWA. The nodules had a median size of 12.5 mm, and the technical success rate on the day of the procedure was 100%. The average ablative margin was 9.9 mm, as assessed on day 0 CBCT, and the technique’s efficiency was 100%, as evidenced by satisfactory ablation observed on CT scans taken one month after the procedure (21). The technique was found to be safe, with a device-related adverse event rate of only 3.3%, consisting of mild hemoptysis. There were no serious adverse events reported during the 30-day follow-up period (21).
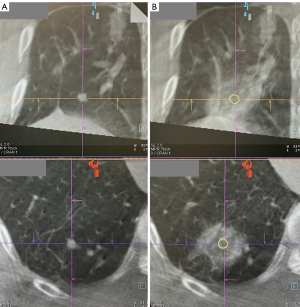
Since the beginning of 2019, the author’s institute has treated 122 cases using a transbronchial MWA approach with a technical success rate of 100% (22) (Figure 1). Similar to the percutaneous approach, patients had a short median length of stay of only 1 day. Pneumothorax requiring chest drainage occurred in only 3.4% of cases, and there were 2 cases of bronchopleural fistula that were successfully treated with an endobronchial valve (23). In 8.9% of cases, patients experienced post-ablation reaction and fever, while minor hemoptysis or hemorrhage occurred in 4.4% of cases, pleural effusion in 1.7%, and chest or pleural space infection in 2.6%. Updated mid-term data since the early results published in 2021 (18) showed that 5 cases (4.0%) had local ablation site recurrence over a median follow-up period of 507 days. In contrast, a Chinese group using different instruments for transbronchial MWA treated 13 patients and reported a complete ablation rate of 78.6%, a 2-year local control rate of 71.4%, and a median progression-free survival of 33 months (24,25). Despite the success of reported ENB ablation series, ENB itself carries inherent error margins that can at times be larger than the target nodule. The accuracy of ENB can be affected by nearby interference, for example the position of CBCT or certain electronic devices close to the field. Therefore, in practice another modality of imaging, either CBCT or EBUS, should be used in adjunct with ENB to provide double confirmation of catheter location before ablating nodule.
The ablation workflow is still in need of improvement, and further enhancements are currently being developed to streamline the process and reduce radiation dosage. In our case series, the average number of CBCT scans per nodule ablation was 7.1 (18), although a minimum of four scans—pre-procedure, needle position confirmation, ablation catheter position confirmation, and post-ablation—are theoretically required. The extra scans required are mostly in the navigation stage, and this is mainly due to the CT-to-body divergence, caused by the discrepancy between the static pre-operative planning CT scan and the dynamic breathing lung. This may necessitate needle renavigation or redeployment. The IllumisiteTM platform is the first navigation system available that corrects for CT-to-body divergence. This digital tomosynthesis-based navigation correction is achieved using fluoroscopic navigation technology, which creates a 3D image to enhance the visibility of the nodule and to update its position. Throughout the procedure, the platform provides real-time confirmation that the catheter is aligned with the nodule, and this alignment can be maintained even after the locatable guide (LG) is removed, thanks to the position sensor coils embedded in the tip of the extended working channel (EWC) (Figure 2A,2B). This continuous positional data allows for multidirectional sampling and more thorough biopsy, as demonstrated in biopsy series that have utilized this platform (26,27).
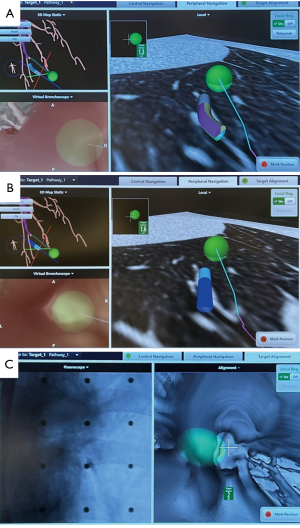
The use of the IllumisiteTM platform can greatly simplify the workflow of transbronchial microwave lung ablation, thanks to its improved navigation accuracy. Previous models without extended working channel (EWC) positional electronics often experienced off-centre needle deployment after the locatable guide (LG) was removed, as the EWC would return to its original curvature. However, with the continuous positional data provided by the IllumisiteTM EWC, the CrossCountryTM needle can be advanced directly at the green ball in the most accurate view. Unlike previous platforms, this technology also provides the exact distance between the centre of the nodule and the tip of the EWC, allowing for precise placement of the ablation catheter tip (Figure 2C). By advancing the EWC 1 cm beyond the centre of the lesion and placing the ablation catheter flush to the EWC, the centre of ablation coincides with the centre of the lesion, reducing the need for multiple CBCT scans. The first-ever ENB microwave ablation using the IllumisiteTM fluoroscopic navigation platform was successfully performed in mid-2022 (28,29).
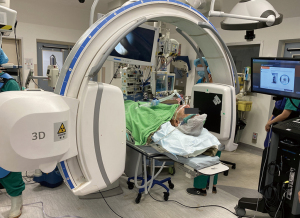
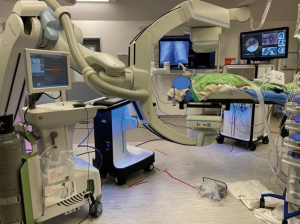
Mobile C-arm machines can provide high-quality intra-operative reconstructed CT images, making transbronchial ablation more accessible to institutes without built-in hybrid theatres. Examples of these machines are the Cios Spin® by Siemens Healthineers (30,31) (as shown in Figure 3), the O-armTM O2 imaging system by Medtronic (32), OEC 3D by General Electric (33), and RFD 3D by Ziehm Imaging (34). A case series utilizing the Cios Spin® found an 80% navigation success rate and an 80% diagnostic yield for biopsy in 10 cases (35). While mobile C-arms are comparable in ease-of-use to floor-mounted CBCT machines and can identify most lung lesions, they may not be able to visualize sub-centimeter pure ground glass nodules. To expand the use of mobile C-arms for lung nodule ablation, further tools for marking, image overlay, and segmentation capabilities are necessary to ablate nodules that are difficult to visualize on fluoroscopy (35). To address this issue, IonTM by Intuitive has recently integrated the Cios Spin® within its own robotic bronchoscopic platform, allowing for automatic image transfer from the mobile C-arm machine to the IonTM bronchoscope during procedures (36). This may be the first step towards streamlining workflow and improving efficiency by integrating imaging from mobile units into navigation and ablation platforms. We would like to stress the importance of high resolution 3D imaging for determining the ablation success, and mobile systems currently available suffer from lower resolution than floor-mounted systems. While augmented fluoroscopy can help operators reach the lesion, confirmation of catheter location by 3D imaging is still necessary before ablation.
The size of the ablation zone is a significant factor in determining the range of early lung cancers that can be treated using bronchoscopic ablation. It is important to note that the typical post-ablative morphology of concentric ground glass opacities (GGO) on CT includes an outer rim of denser GGO that retains viability (37). This means that the ablation size seen on a CT scan overestimates the true coagulation necrosis area by 4.1 mm (38). Therefore, to ensure adequate tumor kill, multiple authors recommend a margin of at least 5 mm on CT scan assessment (38-40). The maximal size of a lung nodule for the EmprintTM ablation catheter, taking into account a 5 mm margin on both sides, is 2.5 cm for a maximal single ablation. This can be extrapolated to 3 cm if double or triple ablation is planned. In our experience with MWA, the mean minimal ablation margin is 5.51 mm, and the size of the ablation zone is not significantly associated with the presence of emphysema, solidity of lesion, or the presence of a ≥3 mm diameter blood vessel within 5 mm of the lesion. Further studies are required to investigate the factors influencing the actual size of the ablation zone. In addition to improving the design of ablation coils to produce a wider ablation zone, metallic nanoparticles such as nanoshells, comprising gold as the metallic component and silica as the dielectric material (41), could be injected into the tumorous region to enhance the effect of microwave heating as metals reflect microwave.
Robotic-assisted bronchoscopy (RAB)
RAB was originally developed with the aim of improving the accuracy and efficacy of lung nodule biopsy. In comparison to conventional bronchoscopy and other types of guided bronchoscopy, such as virtual bronchoscopy (VB), radial endobronchial ultrasound (r-EBUS), and electromagnetic navigation bronchoscopy (ENB), RAB offers certain advantages, including direct visualization of the airways and greater depth of navigation, up to the 9th generation of airways (42). At present, there are two major players in the RAB market, namely the Auris Health MonarchTM platform by Ethicon (43) and the Ion platform by Intuitive (44). The MonarchTM RAB system utilizes a telescopic mother-daughter configuration with a 6 mm outer sheath and a 4.4 mm inner scope, utilizing electromagnetic navigation and peripheral vision for guiding navigation. The inner bronchoscope has a 4-way steering control, providing greater maneuverability compared to conventional bronchoscopy. Additionally, the RAB system provides structural support while the inner scope is advanced, reducing the risk of scope prolapse in proximal airways. The IonTM RAB system consists of an articulating catheter with a 3.5 mm outer diameter and a thin 1.8 mm removal visual probe, which employs fiberoptic shape-sensing for peripheral navigation. Shape-sensing technology allows for the reconstruction and display of the entire shape of a thin, flexible optical fiber with ultra-high speed feedback, providing operators with an unprecedented 3-dimensional perspective on the location of the tip of the catheter. Moreover, this technology enables adjustment and realignment when passing relatively rigid instruments through the bronchoscope. There are other new players joining the market. The Galaxy SystemTM by Noah Medical (45) provides a single-use bronchoscope with 4.0 mm outer diameter and 2.1 mm working channel, and uses proprietary digital tomosynthesis (TiLT+ TechnologyTM) via augmented fluoroscopy to confirm tool-in-lesion (46). Their recent animal MATCH study had up to 95% tool-in-lesion rate. Vision during biopsy can be maintained (47).
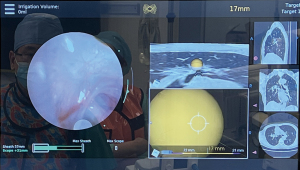
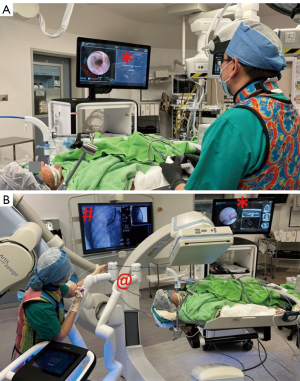
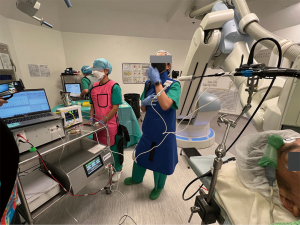
RAB has demonstrated excellent navigation and diagnostic yield in cadaveric and early human trials, with success rates ranging from 88–97% and diagnostic yield of 77–97% (48-54), particularly when combined with radial endobronchial ultrasound (r-EBUS). In lesions without bronchus sign, RAB was reported to have a diagnostic yield of 54%, which was higher than the previously reported yield of 31–44% using non-robotic systems (55). The latest interim results from a multicenter observational real-world robotic bronchoscopy biopsy study (TARGET study) reported navigational success in 97.5% and nodule localization by radial EBUS in 91%, although diagnostic yield data has yet to be published (56,57). The improved accuracy of RAB can be attributed to its ability to maneuver and control the distal end of the scope, as well as to aim instruments with greater precision through multiple active articulation points within the scope (58,59) (Figure 4). RAB combined with cone-beam CT in the hybrid operating room streamlines the navigation process and further enhances accuracy in ablation catheter placement (Figure 5). Currently, the prospective multicenter single-arm POWER study using the Neuwave Flex microwave ablation system and Auris Monarch platform is underway (clinicaltrials.gov NCT05299606) (Figures 6,7).

Other ablative energies and adjuncts
Cryoablation has been utilized for lung nodules in a percutaneous manner since the mid-2000s. Pressurized carbon dioxide or argon gas expansion can cause temperatures to drop to −20 to −40 ℃ (60). Cryoablation offers several advantages such as larger ablation zone sizes, independence from impedance, preservation of bronchovascular structures, no effect from tissue charring, and good visibility of the iceball on CT (61-63). However, the primary drawback is the lengthy time per freeze-thaw-freeze cycle, which takes around 25 minutes per ablation, in contrast to 10 minutes for MWA (64). Currently, there are no commercially available transbronchial cryoablation models on the market, although early studies in in-vivo porcine models have shown satisfactory coagulative necrotic zones and safety (65,66).
Bronchoscopic thermal vapor ablation (BTVA) is a well-established technique for minimally invasive lung volume reduction in severe emphysema (67). In 2021, it was applied to lung cancer treatment for the first time in humans. The single-arm treat-and-resect feasibility study demonstrated that 330 Cal thermal vapor energy delivered to target segments containing the lung cancer resulted in large, uniform ablation zones in four out of five patients (68). Upon lobectomy five days after vapor ablation, two patients exhibited complete or near-complete necrosis of target lesions, and no major procedure-related complications were observed. However, non-uniform ablation zones resulted in significant remaining viable tumor cells in one case. While BTVA may not be sufficient as a standalone treatment modality for cancer, it may be used as an adjunct to microwave ablation (MWA). This approach involves the transbronchial ablation of cancerous nodules using established technology, followed by BTVA to capture the margins, satellite nodules, and spread-through-air-space surrounding the nodule, potentially lowering the local recurrence rate.
Pulsed electric field (PEF)
PEF is a high-voltage, high-frequency, non-thermal ablative modality that increase the transmembrane potential of a cell above critical threshold, leading to disruption of membrane integrity and creation of microscopic pores in cell membranes (electroporation). Electric pulses in the nanosecond domain, in contrast to millisecond or microsecond domain, are able to penetrate into cells and organelles creating millions of 1nm-wide pores in plasma membrane and organelle membranes (69). Cells die via osmotic swelling and apoptosis without denaturing cellular and stromal proteins, releasing intact tumour-associated antigens into surrounding tissue. These antigens are processed by antigen-presenting cells, triggering immunogenic cell death with release of HMBG1, promoting tumour-specific T-cell response and recruiting T cells to tumours. Similar immune-mediated abscopal effects, where localized treatment causing shrinking of tumour at a distance from treated volume, occurs after radiotherapy too, and have been demonstrated in animal models of melanoma (70) and colorectal tumours (71).
In mice models, nanosecond pulsed electric field induced hepatocellular carcinoma cell death, increased its phagocytosis by human macrophage cell by eliciting a host immune response against tumour cells (72). Safety of transbronchial delivery of pulsed electric fields in lungs have been reported in swine models, revealing well-demarcated treatment effect and structurally intact major airways and vessels, without disruption of pleura (73). There is also postulation that lesions treated with PEF may have increased PD1 expression allowing synergistic treatment with anti-PD1 checkpoint blockade (74,75).
Recently, the treat-and-resect INCITE ES study reported the potential of PEF to induce anti-tumoral immune response after PEF (Aliya System, Galvanize Therapeutics) had been applied to lung cancers either percutaneously or transbronchially (72) (Figure 8). Tertiary lymphoid structures (TLS), which is a strong prognostic biomarker associated with patient survival benefits in NCSLC (76,77), have been demonstrated in higher maturity (52% vs. 17%) and higher density than control in resected lung cancers which have previously been treated with PEF within 21 days. These TLS occur both within the tumour at the periphery of cell depletion zone (absence of tumour cellularity due directly to PEF application), and also proximal to the PEF delivery zone (72). The application of PEF did not impact surgical field or increase adhesions, and was not associated with serious adverse events in the 30 patients treated. Importantly, sensitive structures including bronchi, pleural and vessels larger than arterioles were not disrupted by PEF. Cytokine trends also demonstrated a downregulation of IL-6 (related to angiogenesis and metastatic potential), initial upregulation and subsequent downregulation of interferon-gamma (related to innate immune response), and upregulation of BCA-1/CXCL13 (crucial for generating TLS) (72).
Photodynamic therapy (PDT)
PDT involves systemic administration of a photosensitizer approximately 40–50 hours before direct illumination of tissue with visible light. Upon absorption of light at appropriate wavelength, the photosensitizer becomes excited and generates reactive oxygen species, leading to the destruction of tumour cells (78). The most commonly used photosensitizers porfimer sodium (first generation) and talaporfin sodium (second generation) preferentially binds to intracellular membranes in cancer cells, leading to tumour-specific apoptosis and microvascular damage to tumour bed. The advantage of PDT over thermal ablation is that it generates no heat and leads to less fibrosis and scaring than other forms of localized necrosis (79). The integrity of organ is preserved as the biological effect is photochemical instead of thermal, leaving minimal effect on collagen connective tissue. Sunlight precautions for several weeks is required after treatment to avoid photosensitivity, which is usually mild. Multiple sessions of PDT is also possible within the photosensitive period.
Since 1995, PDT has been approved across many countries for the ablation of obstructing endobronchial tumours with excellent results (79). The delivery of PDT in earlier days require rigid bronchoscopes, but with the advent of flexible bronchoscopes and navigation techniques, deeper peripheral lung lesions can also be potentially treated via interstitial PDT, or intratumoral delivery of light. Percutaneous interstitial PDT usually involves placement of multiple optical fiber diffusers to illuminate the target tumour, and the number and location of these diffusers depend on tumour size (80). A Japanese pilot study utilizing CT-guided percutaneous insertion of up to 6 diffusers per patient showed 78% partial response and no progressive disease at 4 weeks for NSCLC (78). A more recent study 1 complete response, 3 stable disease and 1 progressive disease at 6 months follow up after percutaneous PDT for 5 patients (81). However, this is limited by the risk of pneumothorax, thus transbronchial PDT has been developed. Case reports of treating NSCLC with ENB placement of PDT diffusing fiber reported feasibility and no complications, and subsequent lobectomy showed tumour necrosis with no viable remaining tumour (82). Another phase 1 study utilizes a new laser probe, the composite-type optical fiberscope which allows accurate laser irradiation with simultaneous visualization, to perform endobronchial PDT for 7 patients with peripheral lung cancers. At 6 months post-treatment, complete response was found in 3 cases and stable disease in 4 cases (83). A major difference between central PDT and peripheral PDT is that the former usually requires follow up bronchoscopy to clean necrotic tissue out of large airways, while it is not mandatory in the latter. Nevertheless, transbronchial PDT needs to overcome the limitation of small treatment zone, of approximately 12 mm diameter with a single laser fiber. An ingenious method described infusion of high refractory index lipiodol into a segmental bronchial tree via bronchoscope in a swine model (84). The lipiodol acts as a light diffuser allowing PDT light to be transmitted through airways instead of the conventional straight line of light, thus penetrating more lung tissue.
Future research will have to overcome PDT limitations, namely short half life of photosensitizer in plasma, fair tissue penetration, and moderate tumour specificity. Although PDT alone may be insufficient for tumour kill, it demonstrates synergistic effect when used with subsequent high dose radiotherapy providing up to 97% complete response rates. PDT may also prime the effect of immune checkpoint inhibitors in PD-1 axis, as brisk inflammatory reaction is demonstrated locally after PDT, inducing release of tumour antigen and proinflammatory mediators.
Endobronchial intratumoral injection of anti-tumour agents
Neoadjuvant inoculation of immunotherapy before surgical resection has been proposed to yield multiple benefits. Off-target toxicity of immune mediated medication is minimized as the agent is injected locally, and a much lower dose is required to generate a comparable local and systemic anti-tumour effect as compared to systemic immunotherapy (85). Therefore intratumoral delivery avoids problems with dose-limiting toxicity and allows the use of medications with poor systemic safety profiles. A higher concentration of immunotherapeutic drug within an antigen-rich tumour microenvironment provides superior T-cell priming (86). Within the tumour, the rich pool of antigens is able to stimulate a polyclonal anti-tumour response leading to a broad attack against tumour heterogeneity. To date, there are only a handful of completed intratumoral immunotherapy trials, and most used ex situ dendritic cell vaccines, and proof of concept has been achieved in soft tissue sarcomas showing enhanced T lymphocyte response and some clinical response (87).
Other agents that may be beneficial when injected intratumorally include gene therapy, immunostimulatory antibodies and small molecular immune modulators. Transfection of IL-12 gene via intratumoural electroporation has been shown to produce systemic antitumour effects in melanoma, leading to a 33% overall response and half of patients showing regression of untreated lesions in a metastatic setting (88,89). Locally administered immunostimulatory antibodies avoids issues with tissue penetration of systemic administration, and can be coupled with target binding sites on tumour infiltrating lymphocytes easily. Multiple clinical trials are ongoing investigating antibodies like EX40 agonistic antibody, MDX-447, anti-ATLA-4 antibodies (86).
Combination injection of small molecules like STING (stimulator of interferon genes) agonist together with checkpoint inhibitors shows anti-tumour activity in PD-1-naïve triple negative breast cancer and melanoma (90). Similarly, PD-1 blockage in combination with intratumoral delivery of TLR7 and TLR 9 promoted M1 polarization of macrophages, cytotoxic T cell and NK cell activities in pre-clinical models (91). Other pre-clinical trials showed that neoadjuvant oncolytic virotherapy sensitizes tumor to checkpoint inhibitors. Clinically, an oncolytic virus called HF10 has been injected locally together with ipilimumab producing 41% overall response rate in patients with metastatic melanoma (92).
The use of chimeric antigen receptors (CAR)-T cells have been met with great success in the treatment of hematological B cell malignancies. However, the application of systemic CAR T cells in solid tumours is met with greater difficulty including lack of tumour-specific antigen, inefficient infiltration of CAR T cells, antigen heterogeneity and immunosuppressive tumour microenvironment (93). Recent study has proposed that local ablative therapies like microwave ablation lead to release of tumour antigens and cytokines which in combination with CAR T cells can effectively stimulate systemic anti-tumour immune response via tumour microenvironment remodelling (94). In the study, AXL which is highly expressed in NSCLC but not in normal tissues is the target for CAR T cells, and combination therapy increases the mitochondrial oxidative metabolism of tumour-infiltrating CAR T cells in immunocompetent mice.
Intratumoral therapy to lung cancers is still faced with biochemical and technical obstacles. While systemic immunotherapy is dosed by patient weight, it is uncertain whether intratumoral drugs should be dosed according to local lesion size, systemic tumour burden, or to patient weight. Multiple injections with steerable needle may need to be specifically designed for endobronchial injection, and mechanisms should be in place to prevent backflow of the pharmaceutical agent. Alternatively, multiple needle delivery and incorporation of fast mechanical advancement into tumour may be required.
Other novel technologies to simplify transbronchial biopsy workflow
During endobronchial biopsy with navigation, extra imaging is often required in order to confirm tip of catheter/needle in lesion, usually by means of EBUS or CBCT. This confirmation is necessary to ensure higher yield, as navigation alone by ENB or virtual bronchoscopy may not be entirely accurate, especially when lesions are small. However, these additional steps can be labour intensive and time consuming. In 2022, the notion of intraoperative molecular imaging has been proven clinically safe and feasible. For example, intravenous injection of folate receptor-targeted fluorescent tracer which preferentially binds to malignant tumours allows surgeons to visualize non-palpable lung lesions during thoracoscopic surgery in 83% of patients, and shows compatible margin clearance as final pathology (95). Another group used a similar highly specific tumour cell-labelling fluorescence molecule VGT-309, which demonstrated high tumour-to-background ratio and visual localization of non-palpable tumours (96). Taking this concept a step further, future bronchoscopes with fluorescence reading capability will be able to identify such tumours visually within airways, thus providing a second confirmation of on-target biopsy.
Conclusions
The demand for parenchymal-sparing local therapies is on the rise due to the increasing prevalence of multifocal lung cancers and the growing population of patients who are ineligible for surgery. The latest evidence on the effectiveness of lung cancer screening has resulted in the discovery of more pre-malignant or early-stage lung cancers, which has shifted the focus from treatment to prevention. Transbronchial therapy is a crucial tool in the local treatment of lung cancers, with microwave ablation being the most promising option based on early and mid-term results. Adjuncts to improve the efficiency and precision of transbronchial ablation include mobile C-arm platforms, software to correct for CT-to-body divergence, metal-containing nanoparticles, and robotic bronchoscopy. Other forms of energy, such as steam vapor therapy, pulsed electric field and photodynamic therapy, are currently undergoing intensive investigation. The future of transbronchial therapies appears to extend beyond ablation and towards intratumoral injection of novel agents, such as immunomodulating agents, gene therapies, chimeric antigen receptor T cells, or their combinations which work synergistically to produce abscopal effects.
Acknowledgments
Funding: This work was supported by
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Translational Medicine for the series “Lung Cancer Management—The Next Decade”. The article has undergone external peer review.
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1430/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1430/coif). The series “Lung Cancer Management—The Next Decade” was commissioned by the editorial office without any funding or sponsorship. C.S.H.N. served as the unpaid Guest Editor of the series and serves as the Editor-in-Chief of Annals of Translational Medicine from January 2022 to December 2023. He is also a consultant for Johnson and Johnson, Medtronic USA and Siemens Healthineer. R.W.H.L. is a consultant for Medtronic USA and Siemens Healthineer. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leventakos K, Peikert T, Midthun DE, et al. Management of Multifocal Lung Cancer: Results of a Survey. J Thorac Oncol 2017;12:1398-402. [Crossref] [PubMed]
- Chan JWY, Lau R, Chang A, et al. 96P Transbronchial microwave ablation: Important role in the battle of lung preservation for multifocal lung primaries or metastases. Ann Oncol 2022;33:S76-7.
- Jung W, Cho S, Yum S, et al. Stepwise Disease Progression Model of Subsolid Lung Adenocarcinoma with Cystic Airspaces. Ann Surg Oncol 2020;27:4394-403. [Crossref] [PubMed]
- Inamura K. Clinicopathological Characteristics and Mutations Driving Development of Early Lung Adenocarcinoma: Tumor Initiation and Progression. Int J Mol Sci 2018;19:1259. [Crossref] [PubMed]
- Suzuki K, Kusumoto M, Watanabe S, et al. Radiologic classification of small adenocarcinoma of the lung: radiologic-pathologic correlation and its prognostic impact. Ann Thorac Surg 2006;81:413-9. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- WCLC 2022 | Sub-lobar resection ‘new standard of care’ for small-sized NSCLC | oncology.medicinematters.com. cited 2022 Nov 19. Available online: https://oncology.medicinematters.com/wclc-2022/non-small-cell-lung-cancer/calgb-140503-sub-lobar-resection-small-sized-nsclc/23353762
- Suzuki K, Watanabe SI, Wakabayashi M, et al. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg 2022;163:289-301.e2. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Abreu CE, Ferreira PP, de Moraes FY, et al. Stereotactic body radiotherapy in lung cancer: an update. J Bras Pneumol 2015;41:376-87. [Crossref] [PubMed]
- Timmerman RD, Hu C, Michalski JM, et al. Long-term Results of Stereotactic Body Radiation Therapy in Medically Inoperable Stage I Non-Small Cell Lung Cancer. JAMA Oncol 2018;4:1287-8. [Crossref] [PubMed]
- Dupuy DE, Zagoria RJ, Akerley W, et al. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol 2000;174:57-9. [Crossref] [PubMed]
- Fantin A, Manera M, Patruno V, et al. Endoscopic Technologies for Peripheral Pulmonary Lesions: From Diagnosis to Therapy. Life (Basel) 2023;13:254. [Crossref] [PubMed]
- Tanabe T, Koizumi T, Tsushima K, et al. Comparative study of three different catheters for CT imaging-bronchoscopy-guided radiofrequency ablation as a potential and novel interventional therapy for lung cancer. Chest 2010;137:890-7. [Crossref] [PubMed]
- Koizumi T, Tsushima K, Tanabe T, et al. Bronchoscopy-Guided Cooled Radiofrequency Ablation as a Novel Intervention Therapy for Peripheral Lung Cancer. Respiration 2015;90:47-55. [Crossref] [PubMed]
- Xie F, Zheng X, Xiao B, et al. Navigation Bronchoscopy-Guided Radiofrequency Ablation for Nonsurgical Peripheral Pulmonary Tumors. Respiration 2017;94:293-8. [Crossref] [PubMed]
- Steinfort DP, Antippa P, Rangamuwa K, et al. Safety and Feasibility of a Novel Externally Cooled Bronchoscopic Radiofrequency Ablation Catheter for Ablation of Peripheral Lung Tumours: A First-In-Human Dose Escalation Study. Respiration 2023;102:211-9. [Crossref] [PubMed]
- Chan JWY, Lau RWH, Ngai JCL, et al. Transbronchial microwave ablation of lung nodules with electromagnetic navigation bronchoscopy guidance-a novel technique and initial experience with 30 cases. Transl Lung Cancer Res 2021;10:1608-22. [Crossref] [PubMed]
- Lubner MG, Brace CL, Hinshaw JL, et al. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol 2010;21:S192-203. [Crossref] [PubMed]
- Brace CL, Hinshaw JL, Laeseke PF, et al. Pulmonary thermal ablation: comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model. Radiology 2009;251:705-11. [Crossref] [PubMed]
- Medtronic Announces NAVABLATE Study Results Released in Late-Breaking Podium Presentation at European Respiratory Society International Congress 2021 - Sep 7, 2021. cited 2022 Nov 12. Available online: https://news.medtronic.com/2021-09-07-Medtronic-Announces-NAVABLATE-Study-Results-Released-in-Late-Breaking-Podium-Presentation-at-European-Respiratory-Society-International-Congress-2021
- Chan J, Yu P, Lau R, et al. P02.02 Transbronchial Microwave Ablation of Lung Nodules in the Hybrid Operating Room – Mid-Term Follow Up of a Novel Technique. J Thorac Oncol 2021;16:S977.
- Mak KL, Chan JWY, Lau RWH, et al. Management of bronchopleural fistula with endobronchial valve in hybrid operating room following transbronchial microwave ablation. Interact Cardiovasc Thorac Surg 2021;33:992-4. [Crossref] [PubMed]
- Xie F, Chen J, Jiang Y, et al. Microwave ablation via a flexible catheter for the treatment of nonsurgical peripheral lung cancer: A pilot study. Thorac Cancer 2022;13:1014-20. [Crossref] [PubMed]
- Bao F, Yu F, Wang R, et al. Electromagnetic bronchoscopy guided microwave ablation for early stage lung cancer presenting as ground glass nodule. Transl Lung Cancer Res 2021;10:3759-70. [Crossref] [PubMed]
- Avasarala SK, Roller L, Katsis J, et al. Sight Unseen: Diagnostic Yield and Safety Outcomes of a Novel Multimodality Navigation Bronchoscopy Platform with Real-Time Target Acquisition. Respiration 2022;101:166-73. [Crossref] [PubMed]
- Dunn BK, Blaj M, Stahl J, et al. Evaluation of Electromagnetic Navigational Bronchoscopy Using Tomosynthesis-Assisted Visualization, Intraprocedural Positional Correction and Continuous Guidance for Evaluation of Peripheral Pulmonary Nodules. J Bronchology Interv Pulmonol 2023;30:16-23. [Crossref] [PubMed]
- Medtronic News - Business & regional news . cited 2022 Nov 19. Available online: https://news.medtronic.com/Medtronic-announces-first-in-world-lung-ablation-procedure-with-ILLUMISITE-fluoroscopic-navigation-platform?cmpid=SOC_LI_read-pr_lungablationarticle_FY23&sf167320117=1
- Chan JWY, Chang ATC, Siu ICH, et al. Electromagnetic navigation bronchoscopy transbronchial lung nodule ablation with Illumisite(TM) platform corrects CT-to-body divergence with tomosynthesis and improves ablation workflow: a case report. AME Case Rep 2023;7:13. [Crossref] [PubMed]
- Cios spin® by Siemens Healthineers. Available online: https://www.siemens-healthineers.com/surgical-c-arms-and-navigation/mobile-c-arms/cios-spin.
- Chen J, Xie F, Zheng X, et al. Mobile 3-dimensional (3D) C-arm system-assisted transbronchial biopsy and ablation for ground-glass opacity pulmonary nodules: a case report. Transl Lung Cancer Res 2021;10:3312-9. [Crossref] [PubMed]
- Cho RJ, Senitko M, Wong J, et al. Feasibility of Using the O-Arm Imaging System During ENB-rEBUS-guided Peripheral Lung Biopsy: A Dual-center Experience. J Bronchology Interv Pulmonol 2021;28:248-54. [Crossref] [PubMed]
- OEC 3D | GE HealthCare (United States). cited 2023 Jun 3. Available online: https://www.gehealthcare.com/products/surgical-imaging/oec-3d
- Ziehm Vision RFD 3D - Ziehm Imaging | EN. cited 2023 Jun 3. Available online: https://www.ziehm.com/en/products/c-arms-with-flat-panel-detector/ziehm-vision-rfd-3d.html
- Chan JWY, Lau RWH, Chu CM, et al. Expanding the scope of electromagnetic navigation bronchoscopy-guided transbronchial biopsy and ablation with mobile 3D C-arm Machine Cios Spin(®)-feasibility and challenges. Transl Lung Cancer Res 2021;10:4043-6. [Crossref] [PubMed]
- “ion cios spin” | Search | LinkedIn. cited 2022 Nov 19. Available online: https://www.linkedin.com/search/results/all/?keywords=ionciosspin&origin=GLOBAL_SEARCH_HEADER&sid=9cG
- Chheang S, Abtin F, Guteirrez A, et al. Imaging Features following Thermal Ablation of Lung Malignancies. Semin Intervent Radiol 2013;30:157-68. [Crossref] [PubMed]
- Yamamoto A, Nakamura K, Matsuoka T, et al. Radiofrequency ablation in a porcine lung model: correlation between CT and histopathologic findings. AJR Am J Roentgenol 2005;185:1299-306. [Crossref] [PubMed]
- Anderson EM, Lees WR, Gillams AR. Early indicators of treatment success after percutaneous radiofrequency of pulmonary tumors. Cardiovasc Intervent Radiol 2009;32:478-83. [Crossref] [PubMed]
- Wolf FJ, Grand DJ, Machan JT, et al. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology 2008;247:871-9. [Crossref] [PubMed]
- Choi CKK, Chiu YTE, Zhuo X, et al. Dopamine-Mediated Assembly of Citrate-Capped Plasmonic Nanoparticles into Stable Core-Shell Nanoworms for Intracellular Applications. ACS Nano 2019;13:5864-84. [Crossref] [PubMed]
- Chen AC, Gillespie CT. Robotic Endoscopic Airway Challenge: REACH Assessment. Ann Thorac Surg 2018;106:293-7. [Crossref] [PubMed]
Monarch Platform by Auris Health - Intuitive | Robotic-Assisted Bronchoscopy | Ion Platform . cited 2022 Nov 12 . Available online: https://www.intuitive.com/en-us/products-and-services/ion
- Pioneering the next generation in medical robotics - Noah Medical . cited 2023 Jun 4. Available online: https://www.noahmed.com/
- Diddams MJ, Lee HJ. Robotic Bronchoscopy: Review of Three Systems. Life (Basel) 2023;13:354. [Crossref] [PubMed]
- Rojas-Solano JR, Ugalde-Gamboa L, Machuzak M. Robotic Bronchoscopy for Diagnosis of Suspected Lung Cancer: A Feasibility Study. J Bronchology Interv Pulmonol 2018;25:168-75. [Crossref] [PubMed]
- Chen AC, Pastis NJ, Machuzak MS, et al. Accuracy of a Robotic Endoscopic System in Cadaver Models with Simulated Tumor Targets: ACCESS Study. Respiration 2020;99:56-61. [Crossref] [PubMed]
- Chaddha U, Kovacs SP, Manley C, et al. Robot-assisted bronchoscopy for pulmonary lesion diagnosis: results from the initial multicenter experience. BMC Pulm Med 2019;19:243. [Crossref] [PubMed]
- Agrawal A, Hogarth DK, Murgu S. Robotic bronchoscopy for pulmonary lesions: a review of existing technologies and clinical data. J Thorac Dis 2020;12:3279-86. [Crossref] [PubMed]
- Kalchiem-Dekel O, Connolly JG, Lin IH, et al. Shape-Sensing Robotic-Assisted Bronchoscopy in the Diagnosis of Pulmonary Parenchymal Lesions. Chest 2022;161:572-82. [Crossref] [PubMed]
- Benn BS, Romero AO, Lum M, et al. Robotic-Assisted Navigation Bronchoscopy as a Paradigm Shift in Peripheral Lung Access. Lung 2021;199:177-86. [Crossref] [PubMed]
- Fielding DIK, Bashirzadeh F, Son JH, et al. First Human Use of a New Robotic-Assisted Fiber Optic Sensing Navigation System for Small Peripheral Pulmonary Nodules. Respiration 2019;98:142-50. [Crossref] [PubMed]
- Seijo LM, de Torres JP, Lozano MD, et al. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a Bronchus sign on CT imaging: results from a prospective study. Chest 2010;138:1316-21. [Crossref] [PubMed]
- Transbronchial Biopsy Assisted by Robot Guidance in the Evaluation of Tumors of the Lung - Full Text View - ClinicalTrials.gov . cited 2022 Nov 12. Available online: https://clinicaltrials.gov/ct2/show/NCT04182815
- Murgu S, Sterman D, Yasufuku K, et al. Demographic and Lesion Characteristics in the First 443 Subjects Enrolled in a Multicenter Observational Real-World Robotic Bronchoscopy Study: Interim results from TARGET. Presented in AABIP 5th Annual Conference in Aug 11-13, 2022.
- Ghosn M, Elsakka AS, Ridouani F, et al. Augmented fluoroscopy guided transbronchial pulmonary microwave ablation using a steerable sheath. Transl Lung Cancer Res 2022;11:150-64. [Crossref] [PubMed]
- Pritchett M, Reisenauer J, Kern R, et al. Image-Guided Transbronchial Microwave Ablation of Peripheral Primary Lung Tumors with a Flexible Probe: First in US Experience. Chest 2020;158:A1452-3.
- Erinjeri JP, Clark TW. Cryoablation: mechanism of action and devices. J Vasc Interv Radiol 2010;21:S187-91. [Crossref] [PubMed]
- Das SK, Huang YY, Li B, et al. Comparing cryoablation and microwave ablation for the treatment of patients with stage IIIB/IV non-small cell lung cancer. Oncol Lett 2020;19:1031-41. [Crossref] [PubMed]
- Maiwand MO. The role of cryosurgery in palliation of tracheo-bronchial carcinoma. Eur J Cardiothorac Surg 1999;15:764-8. [Crossref] [PubMed]
- Inoue M, Nakatsuka S, Yashiro H, et al. Percutaneous cryoablation of lung tumors: feasibility and safety. J Vasc Interv Radiol 2012;23:295-302; quiz 305. [Crossref] [PubMed]
- Hinshaw JL, Littrup PJ, Durick N, et al. Optimizing the protocol for pulmonary cryoablation: a comparison of a dual- and triple-freeze protocol. Cardiovasc Intervent Radiol 2010;33:1180-5. [Crossref] [PubMed]
- Zheng X, Yuan H, Gu C, et al. Transbronchial lung parenchyma cryoablation with a novel flexible cryoprobe in an in vivo porcine model. Diagn Interv Imaging 2022;103:49-57. [Crossref] [PubMed]
- Kohno M, Hashimoto R, Oiwa K, et al. Initial experience with transbronchial cryoablation as a novel local treatment for malignant peripheral lung lesions. BMJ Open Respir Res 2018;5:e000315. [Crossref] [PubMed]
- Harris K, Puchalski J, Sterman D. Recent Advances in Bronchoscopic Treatment of Peripheral Lung Cancers. Chest 2017;151:674-85. [Crossref] [PubMed]
- Steinfort DP, Christie M, Antippa P, et al. Bronchoscopic Thermal Vapour Ablation for Localized Cancer Lesions of the Lung: A Clinical Feasibility Treat-and-Resect Study. Respiration 2021;100:432-42. [Crossref] [PubMed]
- Nuccitelli R. Application of Pulsed Electric Fields to Cancer Therapy. Bioelectricity 2019;1:30-4. [Crossref] [PubMed]
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373-7. [Crossref] [PubMed]
- Rodriguez-Ruiz ME, Rodriguez I, Garasa S, et al. Abscopal Effects of Radiotherapy Are Enhanced by Combined Immunostimulatory mAbs and Are Dependent on CD8 T Cells and Crosspriming. Cancer Res 2016;76:5994-6005. [Crossref] [PubMed]
- Iding J, VanderLaan P, Jimenez M, et al. Tertiary Lymphoid Structures (TLS) Observed in Non-Small Cell Lung Cancer (NSCLC) Tumors Treated with Pulsed Electric Fields. J Immunother Cancer 2022;10:A732-A734 abstract.
- O’Brien T, Krimsky W, Neal R. The Safety of Transbronchial and Percutaneous Delivery of Pulsed Electric Fields in Lung. ATS 2022. San Francisco, CA.
- Silvestrini M, Pastori C, Tamakloe S, et al. Synergy of Local Treatment with Pulsed Electric Fields and Anti-PD1 Checkpoint Blockade. IASLC 2022. Vienna, AT.
- Silvestrini M, Pastori C, O’Brien T, et al. Immunogenicity of Pulsed Electric Fields is Enhanced with the Inclusion of Checkpoint Inhibitor Therapy. SIO 2022. San Francisco, CA.
- Schumacher TN, Thommen DS. Tertiary lymphoid structures in cancer. Science 2022;375:eabf9419. [Crossref] [PubMed]
- Sautès-Fridman C, Petitprez F, Calderaro J, et al. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer 2019;19:307-25. [Crossref] [PubMed]
- Usuda J, Kato H, Okunaka T, et al. Photodynamic therapy (PDT) for lung cancers. J Thorac Oncol 2006;1:489-93.
- Okunaka T, Kato H, Tsutsui H, et al. Photodynamic therapy for peripheral lung cancer. Lung Cancer 2004;43:77-82. [Crossref] [PubMed]
- Moghissi K. Role of bronchoscopic photodynamic therapy in lung cancer management. Curr Opin Pulm Med 2004;10:256-60. [Crossref] [PubMed]
- Wilson BC, Patterson MS. The physics, biophysics and technology of photodynamic therapy. Phys Med Biol 2008;53:R61-109. [Crossref] [PubMed]
- Bansal S, Bechara R, Patel J, et al. Safety and feasibility study of photodynamic therapy for ablation of peripheral lung cancer. Chest 2020;157:A239.
- Allison RR, Bansal S. Photodynamic therapy for peripheral lung cancer. Photodiagnosis Photodyn Ther 2022;38:102825. [Crossref] [PubMed]
- Usuda J, Inoue T, Tsuchida T, et al. Clinical trial of photodynamic therapy for peripheral-type lung cancers using a new laser device in a pilot study. Photodiagnosis Photodyn Ther 2020;30:101698. [Crossref] [PubMed]
- Chang H, Liao KS, Hsieh YS. Bronchoscopic light delivery method for peripheral lung cancer photodynamic therapy. J Thorac Dis 2020;12:3611-21. [Crossref] [PubMed]
- Marabelle A, Tselikas L, de Baere T, et al. Intratumoral immunotherapy: using the tumor as the remedy. Ann Oncol 2017;28:xii33-43. [Crossref] [PubMed]
- Hong WX, Haebe S, Lee AS, et al. Intratumoral Immunotherapy for Early-stage Solid Tumors. Clin Cancer Res 2020;26:3091-9. [Crossref] [PubMed]
- Raj S, Bui MM, Springett G, et al. Long-Term Clinical Responses of Neoadjuvant Dendritic Cell Infusions and Radiation in Soft Tissue Sarcoma. Sarcoma 2015;2015:614736. [Crossref] [PubMed]
- Daud A, Algazi AP, Ashworth MT, et al. Systemic antitumor effect and clinical response in a phase 2 trial of intratumoral electroporation of plasmid interleukin-12 in patients with advanced melanoma. J Clin Oncol 2014;32:9025.
- Daud AI, DeConti RC, Andrews S, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol 2008;26:5896-903. [Crossref] [PubMed]
- Meric-Bernstam F, Sweis RF, Kasper S, et al. Combination of the STING Agonist MIW815 (ADU-S100) and PD-1 Inhibitor Spartalizumab in Advanced/Metastatic Solid Tumors or Lymphomas: An Open-Label, Multicenter, Phase Ib Study. Clin Cancer Res 2023;29:110-21. [Crossref] [PubMed]
- Sato-Kaneko F, Yao S, Ahmadi A, et al. Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight 2017;2:e93397. [Crossref] [PubMed]
- Andtbacka RHI, Ross MI, Agarwala SS, et al. Final results of a phase II multicenter trial of HF10, a replication-competent HSV-1 oncolytic virus, and ipilimumab combination treatment in patients with stage IIIB-IV unresectable or metastatic melanoma. J Clin Oncol 2017;35:9510.
- Qin Y, Xu G, Enhancing CAR. T-cell therapies against solid tumors: Mechanisms and reversion of resistance. Front Immunol 2022;13:1053120. [Crossref] [PubMed]
- Cao B, Liu M, Wang L, et al. Remodelling of tumour microenvironment by microwave ablation potentiates immunotherapy of AXL-specific CAR T cells against non-small cell lung cancer. Nat Commun 2022;13:6203. [Crossref] [PubMed]
- Kennedy GT, Azari FS, Bernstein E, et al. Targeted Intraoperative Molecular Imaging for Localizing Nonpalpable Tumors and Quantifying Resection Margin Distances. JAMA Surg 2021;156:1043-50. [Crossref] [PubMed]
- Kennedy GT, Holt DE, Azari FS, et al. A Cathepsin-Targeted Quenched Activity-Based Probe Facilitates Enhanced Detection of Human Tumors during Resection. Clin Cancer Res 2022;28:3729-41. [Crossref] [PubMed]


