
The clinical significance and application of the peri-implant phenotype in dental implant surgery: a narrative review
Introduction
Since Ochsenbein et al. first introduced the concept of the “gingival biotype” in 1969 (1), a series of studies have investigated the connection between natural teeth and periodontal soft tissues (2-4). Up to 2017, the Consensus Report of the World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions recommended the uniform use of “periodontal phenotype”, which not only describes both soft and bone tissue conditions, but also emphasizes its variability influenced by genotype, clinical treatment, and environment factors (5). Based on the gingival thickness, periodontal phenotype can be divided into thin (≤1 mm) and thick (>1 mm) types (5).
Given the instructiveness of the peri-implant phenotype in the selection of implantation treatment timing and the formulation of surgical approaches, and its role of standardized description of peri-implant tissue in clinical and scientific work, in 2020, Avila-Ortiz et al. proposed its components and definition as a description and measurement index for future studies (6). The peri-implant phenotype can be defined as the morphological and dimensional features that characterize the clinical manifestations of soft and bone tissues around an implant, namely: keratinized mucosa width (KMW), mucosal thickness (MT), supracrestal tissue height (STH), and peri-implant bone thickness (PBT) (Figure 1).

The long-term therapeutic benefits of oral implant restoration are directly related to the stability of the soft and hard tissues surrounding the implant. Only with strong peri-implant tissue support which include abundant bone tissue and suitable soft tissue can the functional and aesthetic benefits of implant repair be realized. Otherwise, in case of diseases damaging the peri-implant tissue, such as peri-implantitis, there will be a prolonged and complicated treatment process and even the risk of implant loss (7). However, there is a lack of appropriate timing and intervention measures for the improvement of the peri-implant phenotype, and inadequate consideration of the influence of peri-implant phenotype on prognosis in the formulation of implant treatment plan may result in iatrogenic variables leading to insufficient peri-implant supporting tissues even dental implant failure. Hence, this review considers the clinical significance of peri-implant phenotype components as well as their assessment and management at different stages of implant surgery, and recommends strategies for the prevention and treatment of peri-implant soft and hard tissue defects. We present this article in accordance with the Narrative Review reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1752/rc).
Methods
A literature search was conducted to identify relevant papers on peri-implant tissue management and clinical outcomes published up to November 24th, 2022 in the PubMed, Web of Science, EMBASE, and Scopus databases. The search terms included “peri-implant phenotype”, “peri-implant tissues”, “implant therapy”, “soft tissue augmentation”, “bone tissue augmentation”, “peri-implant bone thickness”, “keratinized mucosa”, “mucosal thickness”, “supracrestal tissue height”, “implant restoration”, and “clinical treatment” (Table 1). Original researches, systematic reviews describing peri-implant tissue were included in the review. W Yin and M Rong extracted data from studies that contained qualitative and quantitative analyses, and interpreted each paper in cycles to avoid the omission of potentially valuable data. Until the peri-implant phenotype was clearly defined, some studies utilized the terminology related to the natural teeth, which possibly with some relevant literature omitted during the process of reviewing.
Table 1
| Items | Specification |
|---|---|
| Date of search | November 24th, 2022 |
| Databases and other sources searched | PubMed, Web of Science, EMBASE, and Scopus |
| Search terms used | “Peri-implant phenotype”, “peri-implant tissues”, “implant therapy”, “soft tissue augmentation”, “bone tissue augmentation”, “keratinized mucosa”, “mucosal thickness”, “supracrestal tissue height”, “peri-implant bone thickness”, “implant restoration”, and “clinical treatment” |
| Timeframe | From 1969 until November 24th, 2022 |
| Inclusion and exclusion criteria | No restrictions on study type or language |
| Selection process | Search conducted by M Rong, with consensus by all authors |
Discussion
Considerations regarding the peri-implant phenotype during implant therapy
The clinical significance of the peri-implant phenotype
The KMW refers to the vertical height between the mucosal margin and the mucogingival junction. It can also be interpreted as the sum of the widths of the free gingiva and the attached gingiva. Keratinized mucosa is tough and inflexible, and constitutes the top coronal part of peri-implant soft tissue. A sufficient KMW is conducive to the formation of a stable soft tissue sealing area at the neck of the prosthesis. The prevailing view is that plaque accumulation, mucosal inflammation, soft tissue recession, bleeding on probing, and discomfort during toothbrushing are more likely to occur around implants when the KMW is insufficient (8-11). In clinical practice, however, the minimum KMW required to achieve long-term healthy, functional, and aesthetically acceptable effects in peri-implant tissues has yet to be determined, and there are conflicting findings on whether KMW is related to the aforementioned adverse effects (12,13). Current research findings indicate that KMW is predominantly classified as inadequate at <2 mm or adequate at ≥2 mm (14,15).
MT refers to the horizontal thickness of the peri-implant soft tissue. The MT value varies for implants according to the site of measurement (e.g., on the buccal or lingual side, or at different coronal-root heights), with the most critical location being the labial/buccal coronal side, due to its significant role in buccal peri-implant soft tissue stability (16), peri-implant bone resorption (17,18), and the final aesthetic effect (19). There is no consensus on whether the MT value corresponds to the long-term health and stability of peri-implant soft and hard tissues; however, most studies suggest that an MT of 2 mm is ideal, as mucosa of this thickness can completely conceal the color of the metal abutment beneath it to satisfy aesthetic requirements (20,21). Therefore, MT can be divided into two categories: thin (<2 mm) and thick (≥2 mm).
The STH refers to the height of the peri-implant soft tissue above the alveolar crest. It represents the vertical distance from the alveolar crest to the gingival margin, including the vertical height of both the connective and epithelial (sulcular and junctional) tissue layers. Although the STH is not completely equivalent to the “biological width” of natural teeth, a study has shown that the periodontal phenotype of natural teeth is associated with the peri-implant STH, with patients with a thick periodontal phenotype having a greater STH (22). For equicrestal implants, the STH is the precise depth of the upper gingival cuff of the implant; for subcrestal implants, the STH is the vertical distance from the gingival margin to the highest point of the stable bone around the implant. Several studies have shown the STH to be relatively stable at approximately 3.5 mm (23,24). Based on the anatomical and restorative difference, available research evidence suggests that the STH can be classified as short (<3 mm) or tall (≥3 mm) (6). Implant sites with a short STH experience physiological changes in bone and soft tissue during the healing phase, with more peri-implant bone resorption in the early stage (25,26). However, a very tall STH is not desirable either. Peri-implant mucositis is more challenging to treat when the mucosal tunnel depth of the soft tissue level implant restoration is ≥3 mm (27). Furthermore, in patients with periodontitis, an increased STH at the implant site is associated with increased peri-implant bone resorption and an increased risk of peri-implantitis (28).
The term “peri-implant bone thickness” describes the thickness of the peri-implant alveolar bone in the horizontal section and varies at different coronal-root heights (29), with a PBT value of 0 at the fenestration and dehiscence sites. Sufficient alveolar bone is necessary for implant osseointegration, and an adequate bone thickness in the edentulous area is a prerequisite in determining the ideal implantation site. At present, a minimum of 1.5–2 mm of bone around the implant is generally accepted to be required to achieve a predictable therapeutic effect, and marginal bone resorption is further reduced when there is a PBT ≥2 mm at the time of implant placement (30-32). Both vertical and horizontal bone resorption occurs during the peri-implant soft and hard tissue remodeling phases. Insufficient PBT on the buccal or proximal side of the implant may result in a supracrestal bone defect (33), gingival recession, or loss of gingival papillae, all of which can significantly impact on the aesthetic results. PBT can be divided into two categories: thick (≥2 mm) and thin (<2 mm).
Every implantologist should be aware of the need to improve the peri-implant phenotype—a process that begins with the planning of implant placement and continues throughout the patient’s treatment—and to optimize the conditions of the peri-implant hard and soft tissue as early as possible and proactively. A comprehensive examination and assessment of the soft and bone tissues at the implant site is necessary for the formulation of an implant treatment plan. In terms of soft tissues, this includes an assessment of the KMW, supracrestal tissue, presence of scarring, frenum attachment position, and location of the gingival margin of the adjacent teeth. Bone tissue assessments are mainly performed using cone beam computed tomography, which depicts the density of the alveolar bone in the edentulous area, the morphology of bone defects, and the alveolar crest level of the adjacent teeth. If the implantologist focuses solely on the adequacy of bone mass and neglects the assessment of soft tissue, there is a high risk that the long-term success of the implant restoration will be compromised.
The choice of modality to enhance the peri-implant phenotype depends on the position of the implant (maxillary or mandibular, anterior or posterior) and the conditions of the preoperative soft and hard tissue. Bone augmentation surgical options to improve the PBT can be selected based on a comprehensive review by other experts (34). Research on improvement of the peri-implant soft tissue phenotype has focused mainly on KMW and MT augmentation, which can be performed at any stage before the final restoration (35). Although several soft tissue replacement materials can be used to repair defects around natural teeth and implants, autologous grafts (using free gingival and subepithelial connective tissue removed from the palate) remain the gold standard for peri-implant soft tissue augmentation (36). To increase the KMW, an apically positioned flap combined with a free gingival graft is recommended as it achieves a greater KMW with less postoperative contraction (37). However, the result is not aesthetically pleasing because the newly formed keratinized tissue above the graft maintains the color and texture characteristics of the mucosal donor sites, which may not correspond with those of the surrounding tissues. As a result, this technique is mostly used in non-aesthetic regions. MT augmentation usually enhances the aesthetic results by preventing the metallic color of implants from being visible through the gingival margin or by increasing the fullness of the labial soft tissue to some extent (21). The preferred method of MT augmentation is a bilaminar technique combined with a connective tissue graft (CTG) or other substitutes such as acellular dermal matrix, xenogenic collagen matrix, enamel matrix derivative and platelet-rich fibrin (38,39). Besides the most intuitive method by using a graduated periodontal probe preoperatively and intraoperatively, transgingival probing, cone beam computed tomography alone or combine with an intraoral digital scan (40) and ultrasound (41) are included to accurately evaluating peri-implant soft tissue changes. Also, since soft tissue grafting is a delicate surgical procedure with numerous complications, the application of microsurgical techniques has increased the survival rate of grafts and provided a practical basis for the further development of new procedures (42).
Whether or not bone and soft tissue augmentation achieves the desired result depends on the timing of treatment. We believe that obtaining a successful dental implant requires the selection of the most appropriate soft and hard tissue augmentation materials and surgical methods in the course of treatment based on a comprehensive assessment of the peri-implant phenotype, in order to improve the peri-implant supporting tissues and ensure the function, aesthetics and stability of a dental implant. Therefore, the next part of this review will discuss how the peri-implant phenotype is affected by treatment at different implantation stages, and some clinical cases treated by the authors were also provided (Figures 2-11).







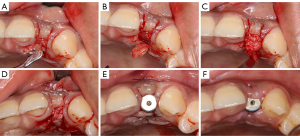


Pre-implantation procedures
In the development of a treatment plan, the KMW and supracrestal tissue of the edentulous area should be evaluated first. When there is a severe soft tissue defect at the implant site, its repair should be a priority before bone mass evaluation (Figure 2). Soft tissue augmentation performed prior to implant placement can effectively reduce the exposure risk of the tissue grafts and membrane in the subsequent implant-related procedures (43). Only after improvement of the soft tissue conditions can the implant therapy obtain a relatively favorable prognosis.
If the KMW and supracrestal tissue are in fair condition, but a severe bone defect is present in the alveolar ridge of the edentulous area, a suitable bone augmentation procedure should be selected to reconstruct the alveolar ridge contour according to the patient’s actual situation, in order to create better pre-surgical conditions for implant placement (Figure 3). Horizontal bone augmentation effectively widens the PBT after implant placement, whereas vertical bone augmentation reduces the bone tissue height difference between the peri-implant and the natural tooth, which avoids an excessive STH after implantation while reducing the risk of inflammation (28).
First-stage surgical procedures
The most fundamental requirement before implantation is the selection of an appropriate three-dimensional location for implant placement that can achieve a favorable peri-implant phenotype. Implant sites have a significant impact on bone remodeling, and their incorrect placement may have a detrimental effect on peri-implant soft tissues. Labially placed implants lead to resorption of the labial bone plate and increase the risk of gingival recession three-fold compared to normally placed implants (44,45). The height of the gingival papillae is affected by deviation at the mesiodistal implant site; when the distance between the implant and the natural adjacent teeth is less than 1.5 mm or the distance between two adjacent implants is less than 3 mm, the gingival papillae tend to not fill the “black triangle” (46). However, considering the bone remodeling process during STH establishment, the implantation depth can be increased at sites with thin crestal mucosa to limit the negative consequences of crestal bone resorption (25). When the height of mucosa is too tall, some submucosal connective tissue can be removed to prevent clinical complications from an excessive STH (28).
If only mild to moderate bone defects are present in the edentulous area, an ideal implant site and initial stability with a favorable soft tissue phenotype can be obtained by performing a bone augmentation procedure during first-stage surgery (Figure 4). Bone augmentation procedure based on the biological principle of guided bone regeneration is one of the most frequently utilized techniques in this scenario. Soft tissue augmentation can be performed simultaneously with implant placement in sites with an insufficient KMW or thin supracrestal tissue but sufficient bone mass to avoid the influence on the blood supply of soft tissue grafts in the presence of bone grafts (Figure 5) (47).
The final restorative effects that can be attained are directly related to the peri-implant phenotype of the aesthetic zone. Many implantologists have encountered cases of gingival recession and soft-tissue contour collapse following immediate implant surgery. According to a recent systematic review, immediate implantation with simultaneous CTG to increase the MT enhances soft tissue stability, decreases gingival recession at the labial midpoint, and decreases the degree of bleeding on probing to a degree (48). Moreover, studies have reported that under immediate implantation with simultaneous soft and hard tissue grafting in the aesthetic area, the soft tissue pink and white aesthetic score and marginal bone loss were significantly improved (49,50). Although there is a risk of resorption due to exposure of the facial bone wall in immediate implant placement combined with flapped bone grafting, careful selection of the incision, the use of a coronally positioned flap, and an adequate postoperative bone volume can ensure the stability of the soft tissue in the aesthetic area (51). Patients with an MT of less than 2 mm or a labial plate thickness of less than 0.5 mm can receive a CTG during initial implant surgery to increase the labial MT and improve their clinical prognosis (Figure 6). For patients who are not suitable for immediate implantation and restoration, customized healing abutments can be used to maintain the gingival contour and support the regenerative area on the labial side (Figure 7).
Procedures during the bone healing stage
Soft tissue augmentation of the implant area can also be performed during osseointegration after implant placement. For patients with mucosal wounds that had healed well at 1 month after first-stage surgery (without concurrent bone augmentation), the use of a partial-thickness flap was found to achieve satisfactory aesthetic results (43). The condition of the soft tissue can be intuitively and accurately assessed at this stage, and soft tissue grafts can also achieve good stability up to healing for patients without bone augmentation. Although soft tissue surgery does not affect the subperiosteal implant or the bone graft material, an increasing number of implant sites require concurrent bone augmentation, which in the majority of cases impairs soft tissue healing following bone grafting (52). Therefore, the procedures used for soft tissue augmentation during the bone healing stage should be selected carefully.
During the process of healing and reconstruction after the first-stage surgery, the gingival soft tissue is relatively brittle. As a result, it can tear easily if it undergoes secondary surgical trauma prematurely, which makes achieving a satisfactory recovery challenging. Therefore, the timing of soft tissue augmentation surgery after implant placement should be appropriately delayed to avoid clinical complications.
Second-stage surgical procedures
In general, bone augmentation is not required for mild bone defects discovered in the neck of the implant during second-stage surgery. If the KMW and MT are sufficient and the peri-implant soft tissues are in good health, the subsequent restoration can proceed normally and without extra treatment. However, if the KMW or soft tissue fullness in the peri-implantation aesthetic region is insufficient, appropriate soft tissue augmentation can be performed to improve the peri-implant soft tissue phenotype without additional bone grafting. Such augmentation during second-stage surgery eliminates the need for further treatment and makes it easier to obtain patient compliance. For implant sites with submerged healing, the STH is often measured and improved during the second-stage surgery, and can be adjusted by using healing abutments and provisional prostheses (53). Implant sites located in the aesthetic area were found to undergo soft tissue grafting during the healing period after first-stage surgery, and the soft tissue volume around implants located on the labial side was observed to significantly improve during 1 year of follow-up (54). Furthermore, a systematic review demonstrated that using an apically positioned partial-thickness flap/vestibuloplasty combined with different tissue grafts for soft tissue augmentation concurrently at the second stage of surgery can provide favorable clinical outcomes (55).
In cases where the MT of the maxillary distal extension absence area is favorable, harvesting a soft tissue graft from the implant site during second-stage surgery is a less invasive procedure that avoids opening up a second operative zone, thus reducing the postoperative pain and improving the experience of the patient (Figure 8). The palatal side of the maxillary alveolar ridge is covered with abundant keratinized mucosa and submucosal connective tissue. It provides a simple, effective, and minimally invasive option for increasing the labial MT using the roll flap technique (56) at a maxillary implant site during second-stage surgery. However, due to the limited availability of connective tissue, this approach can only be used to treat mild MT deficiencies (Figure 9).
The soft tissue phenotype can also be improved through the use of specific surgical incisions and small flap transposition designs during second-stage surgery. For example, peri-implant soft tissues can be preserved by making U-shaped incisions (Figure 10), T-shaped incisions, or I-shaped incisions (57), or by using the split-finger technique (58), in combination with a healing abutment that pushes the soft tissue to the labial-buccal side or into the gingival papillae area.
Procedures after restoration
Before the final restoration placement, gingival shaping using provisional restoration contributes to the creation of a healthy peri-implant soft tissue contour (53). By changing the gingival margin profile of a provisional restoration, the gingival margin can be repositioned and the STH optimized. The convexity of the profile of the provisional restoration below the gingival margin can partially compensate for the collapse of the soft-tissue profile, thereby improving the aesthetic effect. The transitional restoration period is ideal for the evaluation of the peri-implant soft tissue phenotype, and any issues caused by insufficient soft tissue volume should be promptly managed to prevent further deterioration.
Soft tissue augmentation is not recommended after the final restoration has been set (43). Not only does it have a poor postoperative prognosis, but it also demands high technical accuracy, the removal of the prosthesis, and increased time and financial investment by the patient (43). Generally, soft tissue surgery following restoration is performed only to treat peri-implant mucosal recession or peri-implantitis (Figure 11) (21). Peri-implantitis occurs after the final restoration. The available evidence suggests that augmentative procedures combined with resective therapy for the reconstructive treatment of peri-implantitis provides reliable outcomes, however, the advantages of the type of bone graft materials and the application of barrier membrane are not clearly defined (59). In addition, either apically positioned flap alone or in combination with free gingival graft or a xenogeneic collagen matrix in the surgical treatment of peri-implantitis can improve soft tissue phenotype (60). Compared to mucosal recession around natural teeth, it is more difficult to restore complete coverage of a peri-implant mucosal recession. A small mucosal recession (1–2 mm) in the aesthetic region around an implant can be addressed with an apically repositioned flap combined with a CTG; however, the procedure has no predictable therapeutic effect for more severe cases of recession (61). To date, there have been no histological studies on the nature of soft-tissue graft attachment on implant surfaces.
Conclusions
The peri-implant phenotype characterize soft and hard tissue conditions around the implant site. PBT can be improved before implant surgery or concurrently with the first-stage surgery. The optimal time to enhance the KMW and MT is before the final restoration, while STH treatment is dependent on the supracrestal MT and implant depth of the patient, which can also be adjusted by superstructures of implants at the second-stage surgery. To develop a favorable peri-implant phenotype and ensure the long-term success of implant restoration, implantologists should engage in careful planning before implant therapy, considering appropriate treatments at different stages of therapy to reduce iatrogenic variables.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1752/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1752/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1752/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ochsenbein C, Ross S. A reevaluation of osseous surgery. Dent Clin North Am 1969;13:87-102. [Crossref] [PubMed]
- Barootchi S, Tavelli L, Di Gianfilippo R, et al. Soft tissue phenotype modification predicts gingival margin long-term (10-year) stability: Longitudinal analysis of six randomized clinical trials. J Clin Periodontol 2022;49:672-83. [Crossref] [PubMed]
- Sculean A, Gruber R, Bosshardt DD. Soft tissue wound healing around teeth and dental implants. J Clin Periodontol 2014;41:S6-22. [Crossref] [PubMed]
- Barootchi S, Tavelli L, Zucchelli G, et al. Gingival phenotype modification therapies on natural teeth: A network meta-analysis. J Periodontol 2020;91:1386-99. [Crossref] [PubMed]
- Jepsen S, Caton JG, Albandar JM, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 2018;89:S237-48. [Crossref] [PubMed]
- Avila-Ortiz G, Gonzalez-Martin O, Couso-Queiruga E, et al. The peri-implant phenotype. J Periodontol 2020;91:283-8. [Crossref] [PubMed]
- Froum SJ. Treatment of advanced peri-implantitis with regenerative therapy: a review of the rationale, technique, and outcomes. Front Oral Maxillofac Med 2022;4:37. [Crossref]
- Brito C, Tenenbaum HC, Wong BK, et al. Is keratinized mucosa indispensable to maintain peri-implant health? A systematic review of the literature. J Biomed Mater Res B Appl Biomater 2014;102:643-50. [Crossref] [PubMed]
- Souza AB, Tormena M, Matarazzo F, et al. The influence of peri-implant keratinized mucosa on brushing discomfort and peri-implant tissue health. Clin Oral Implants Res 2016;27:650-5. [Crossref] [PubMed]
- Lin GH, Chan HL, Wang HL. The significance of keratinized mucosa on implant health: a systematic review. J Periodontol 2013;84:1755-67. [Crossref] [PubMed]
- Isler SC, Uraz A, Kaymaz O, et al. An Evaluation of the Relationship Between Peri-implant Soft Tissue Biotype and the Severity of Peri-implantitis: A Cross-Sectional Study. Int J Oral Maxillofac Implants 2019;34:187-96. [Crossref] [PubMed]
- Wennström JL, Derks J. Is there a need for keratinized mucosa around implants to maintain health and tissue stability? Clin Oral Implants Res 2012;23:136-46. [Crossref] [PubMed]
- Berglundh T, Armitage G, Araujo MG, et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 2018;89:S313-8. [Crossref] [PubMed]
- Bouri A Jr, Bissada N, Al-Zahrani MS, et al. Width of keratinized gingiva and the health status of the supporting tissues around dental implants. Int J Oral Maxillofac Implants 2008;23:323-6. [PubMed]
- Gobbato L, Avila-Ortiz G, Sohrabi K, et al. The effect of keratinized mucosa width on peri-implant health: a systematic review. Int J Oral Maxillofac Implants 2013;28:1536-45. [Crossref] [PubMed]
- Zigdon H, Machtei EE. The dimensions of keratinized mucosa around implants affect clinical and immunological parameters. Clin Oral Implants Res 2008;19:387-92. [Crossref] [PubMed]
- Akcalı A, Trullenque-Eriksson A, Sun C, et al. What is the effect of soft tissue thickness on crestal bone loss around dental implants? A systematic review. Clin Oral Implants Res 2017;28:1046-53. [Crossref] [PubMed]
- Thoma DS, Naenni N, Figuero E, et al. Effects of soft tissue augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clin Oral Implants Res 2018;29:32-49. [Crossref] [PubMed]
- Hosseini M, Worsaae N, Gotfredsen K. Tissue changes at implant sites in the anterior maxilla with and without connective tissue grafting: A five-year prospective study. Clin Oral Implants Res 2020;31:18-28. [Crossref] [PubMed]
- Lops D, Stellini E, Sbricoli L, et al. Influence of abutment material on peri-implant soft tissues in anterior areas with thin gingival biotype: a multicentric prospective study. Clin Oral Implants Res 2017;28:1263-8. [Crossref] [PubMed]
- Thoma DS, Mühlemann S, Jung RE. Critical soft-tissue dimensions with dental implants and treatment concepts. Periodontol 2000 2014;66:106-18. [Crossref] [PubMed]
- Romeo E, Lops D, Rossi A, et al. Surgical and prosthetic management of interproximal region with single-implant restorations: 1-year prospective study. J Periodontol 2008;79:1048-55. [Crossref] [PubMed]
- Tomasi C, Tessarolo F, Caola I, et al. Morphogenesis of peri-implant mucosa revisited: an experimental study in humans. Clin Oral Implants Res 2014;25:997-1003. [Crossref] [PubMed]
- Askar H, Wang IC, Tavelli L, et al. Effect of Implant Vertical Position, Design, and Surgical Characteristics on Mucosal Vertical Dimension: A Meta-Analysis of Animal Studies. Int J Oral Maxillofac Implants 2020;35:461-78. [Crossref] [PubMed]
- Linkevicius T, Puisys A, Steigmann M, et al. Influence of Vertical Soft Tissue Thickness on Crestal Bone Changes Around Implants with Platform Switching: A Comparative Clinical Study. Clin Implant Dent Relat Res 2015;17:1228-36. [Crossref] [PubMed]
- Berglundh T, Lindhe J. Dimension of the periimplant mucosa. Biological width revisited. J Clin Periodontol 1996;23:971-3. [Crossref] [PubMed]
- Chan D, Pelekos G, Ho D, et al. The depth of the implant mucosal tunnel modifies the development and resolution of experimental peri-implant mucositis: A case-control study. J Clin Periodontol 2019;46:248-55. [Crossref] [PubMed]
- Zhang Z, Shi D, Meng H, et al. Influence of vertical soft tissue thickness on occurrence of peri-implantitis in patients with periodontitis: a prospective cohort study. Clin Implant Dent Relat Res 2020;22:292-300. [Crossref] [PubMed]
- Vera C, De Kok IJ, Chen W, et al. Evaluation of post-implant buccal bone resorption using cone beam computed tomography: a clinical pilot study. Int J Oral Maxillofac Implants 2012;27:1249-57. [PubMed]
- Merheb J, Quirynen M, Teughels W. Critical buccal bone dimensions along implants. Periodontol 2000 2014;66:97-105. [Crossref] [PubMed]
- Monje A, Chappuis V, Monje F, et al. The Critical Peri-implant Buccal Bone Wall Thickness Revisited: An Experimental Study in the Beagle Dog. Int J Oral Maxillofac Implants 2019;34:1328-36. [Crossref] [PubMed]
- Aizcorbe-Vicente J, Peñarrocha-Oltra D, Canullo L, et al. Influence of Facial Bone Thickness After Implant Placement into the Healed Ridges on the Remodeled Facial Bone and Considering Soft Tissue Recession: A Systematic Review. Int J Oral Maxillofac Implants 2020;35:107-19. [Crossref] [PubMed]
- Nohra J, Dagher M, Matni G, et al. Effect of Primary Stability and Soft- and Hard-Tissue Thickness on Marginal Bone Loss: A Prospective Pilot Study. Implant Dent 2018;27:542-6. [Crossref] [PubMed]
- Chiapasco M, Casentini P. Horizontal bone-augmentation procedures in implant dentistry: prosthetically guided regeneration. Periodontol 2000 2018;77:213-40. [Crossref] [PubMed]
- Lin CY, Chen Z, Pan WL, et al. Impact of timing on soft tissue augmentation during implant treatment: A systematic review and meta-analysis. Clin Oral Implants Res 2018;29:508-21. [Crossref] [PubMed]
- Zucchelli G, Tavelli L, McGuire MK, et al. Autogenous soft tissue grafting for periodontal and peri-implant plastic surgical reconstruction. J Periodontol 2020;91:9-16. [Crossref] [PubMed]
- Oh SL, Masri RM, Williams DA, et al. Free gingival grafts for implants exhibiting lack of keratinized mucosa: a prospective controlled randomized clinical study. J Clin Periodontol 2017;44:195-203. [Crossref] [PubMed]
- Tavelli L, Barootchi S, Avila-Ortiz G, et al. Peri-implant soft tissue phenotype modification and its impact on peri-implant health: A systematic review and network meta-analysis. J Periodontol 2021;92:21-44. [Crossref] [PubMed]
- Jankovic S, Aleksic Z, Milinkovic I, et al. The coronally advanced flap in combination with platelet-rich fibrin (PRF) and enamel matrix derivative in the treatment of gingival recession: a comparative study. Eur J Esthet Dent 2010;5:260-73. [PubMed]
- Ferry K, AlQallaf H, Blanchard S, et al. Evaluation of the accuracy of soft tissue thickness measurements with three different methodologies: An in vitro study. J Periodontol 2022;93:1468-75. [Crossref] [PubMed]
- Sonmez G, Kamburoglu K, Gulsahi A. Accuracy of high-resolution ultrasound (US) for gingival soft tissue thickness mesurement in edentulous patients prior to implant placement. Dentomaxillofac Radiol 2021;50:20200309. [Crossref] [PubMed]
- Mingdeng R, Yanhong H, Haibin L, et al. Application of periodontal microsurgery in the augmentation of attached gingiva around an implant. Hua Xi Kou Qiang Yi Xue Za Zhi 2018;36:71-5. [PubMed]
- Thoma DS, Gil A, Hämmerle CHF, et al. Management and prevention of soft tissue complications in implant dentistry. Periodontol 2000 2022;88:116-29. [Crossref] [PubMed]
- Lin GH, Chan HL, Wang HL. Effects of currently available surgical and restorative interventions on reducing midfacial mucosal recession of immediately placed single-tooth implants: a systematic review. J Periodontol 2014;85:92-102. [Crossref] [PubMed]
- Chen ST, Buser D. Esthetic outcomes following immediate and early implant placement in the anterior maxilla--a systematic review. Int J Oral Maxillofac Implants 2014;29:186-215. [Crossref] [PubMed]
- Ramanauskaite A, Roccuzzo A, Schwarz F. A systematic review on the influence of the horizontal distance between two adjacent implants inserted in the anterior maxilla on the inter-implant mucosa fill. Clin Oral Implants Res 2018;29:62-70. [Crossref] [PubMed]
- Zuhr O, Baumer D, Hurzeler M. The addition of soft tissue replacement grafts in plastic periodontal and implant surgery: critical elements in design and execution. J Clin Periodontol 2014;41:S123-42. [Crossref] [PubMed]
- Seyssens L, De Lat L, Cosyn J. Immediate implant placement with or without connective tissue graft: A systematic review and meta-analysis. J Clin Periodontol 2021;48:284-301. [Crossref] [PubMed]
- Zuiderveld EG, Meijer HJA, den Hartog L, et al. Effect of connective tissue grafting on peri-implant tissue in single immediate implant sites: A RCT. J Clin Periodontol 2018;45:253-64. [Crossref] [PubMed]
- Cosyn J, Eghbali A, Hermans A, et al. A 5-year prospective study on single immediate implants in the aesthetic zone. J Clin Periodontol 2016;43:702-9. [Crossref] [PubMed]
- Li S, Su Z, Mo A. Clinical outcomes of immediate implantation and provisionalization combined with guided bone regeneration for a single anterior maxillary tooth with a thin facial bone phenotype. J Prev Treat Stomatol Dis 2022;30:556-63.
- Kofina V, Demirer M, Erdal BS, et al. Bone grafting history affects soft tissue healing following implant placement. J Periodontol 2021;92:234-43. [Crossref] [PubMed]
- González-Martín O, Lee E, Weisgold A, et al. Contour Management of Implant Restorations for Optimal Emergence Profiles: Guidelines for Immediate and Delayed Provisional Restorations. Int J Periodontics Restorative Dent 2020;40:61-70. [Crossref] [PubMed]
- Schneider D, Grunder U, Ender A, et al. Volume gain and stability of peri-implant tissue following bone and soft tissue augmentation: 1-year results from a prospective cohort study. Clin Oral Implants Res 2011;22:28-37. [Crossref] [PubMed]
- Bassetti RG, Stähli A, Bassetti MA, et al. Soft tissue augmentation procedures at second-stage surgery: a systematic review. Clin Oral Investig 2016;20:1369-87. [Crossref] [PubMed]
- Scharf DR, Tarnow DP. Modified roll technique for localized alveolar ridge augmentation. Int J Periodontics Restorative Dent 1992;12:415-25. [PubMed]
- Lee EK, Herr Y, Kwon YH, et al. I-shaped incisions for papilla reconstruction in second stage implant surgery. J Periodontal Implant Sci 2010;40:139-43. [Crossref] [PubMed]
- Misch CE, Al-Shammari KF, Wang HL. Creation of interimplant papillae through a split-finger technique. Implant Dent 2004;13:20-7. [Crossref] [PubMed]
- Schwarz F, Jepsen S, Obreja K, et al. Surgical therapy of peri-implantitis. Periodontol 2000 2022;88:145-81. [Crossref] [PubMed]
- Solonko M, Regidor E, Ortiz-Vigon A, et al. Efficacy of keratinized mucosal augmentation with a collagen matrix concomitant to the surgical treatment of peri-implantitis: A dual-center randomized clinical trial. Clin Oral Implants Res 2022;33:105-19. [Crossref] [PubMed]
- Fickl S. Peri-implant mucosal recession: Clinical significance and therapeutic opportunities. Quintessence Int 2015;46:671-6. [PubMed]
(English Language Editor: J. Reylonds)


