
Narrative review—diagnosing and managing malignant epidural spinal cord compression: an evidence-based approach
Introduction
Malignant spinal cord compression (MSCC) is an oncological emergency that carries significant morbidity if not diagnosed and treated promptly. MSCC is a common complication of metastatic malignancies, occurring in 5–10% of all cancer patients (1) and 40% of cancer patients with known bone metastases (2). The frequency of MSCC is increasing, perhaps due to improved diagnostic accuracy, an ageing population and the fact that cancer patients are living longer due earlier detection and improved therapies (3).
It is well established that steroids and radiotherapy are the cornerstone of management (4,5) as well as surgery in very select cases (6). Only recently have phase III clinical trials examined different radiotherapy prescriptions (7-9). Additionally novel approaches to the management of cord compression are emerging as a result of improved technology in the delivery of radiotherapy (10-16), as well as the need to revaluate management in the modern era of systemic therapies that are improving overall survival (OS) and progression free survival.
It is clear that correctly diagnosing and managing MSCC is critical in the management of oncology patients. Our objective is to provide a narrative review of the latest evidence in light of these clinical trials and novel treatments to guide treatment in the modern era in addition to an overview of the pathophysiology and diagnosis of MSCC. We present this article in accordance with the Narrative Review reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3076/rc).
Methods
This paper was a narrative review to search for relevant investigations in the active management of MSCC. Studies were obtained from the following data bases: MEDLINE, PubMed, Cochrane, and Embase. A search was performed through January 7th, 2023 with following key words and synonyms: “cord compression”, “radiotherapy”, “stereotactic ablative radiotherapy (SABR)”, “stereotactic body radiotherapy (SBRT)” “surgery”, “decompression”, “steroid”, “dexamethasone”. Studies were only included if patients were 18 years and older, publication was available in full and in English. Abstracts, reviews, commentaries and editorials were excluded. Our search strategy is summarised in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of literature search | January 7th, 2023 |
| Databases and other sources searched | MEDLINE, PubMed, Cochrane, Embase |
| Search terms used | “cord compression” AND “radiotherapy” OR “stereotactic ablative radiotherapy” OR “SABR” OR “stereotactic body radiotherapy” OR “SBRT” OR “surgery” OR “decompression” OR “steroid” OR “dexamethasone” |
| Timeframe | January 1st, 1970–January 7th, 2023 |
| Inclusion and exclusion criteria | Inclusion: patients 18 years and older, publication available in full and in English |
| Exclusion: abstracts, reviews, commentaries and editorials | |
| Selection process | Articles were selected and reviewed by radiation oncology consultant and registrar |
Pathophysiology
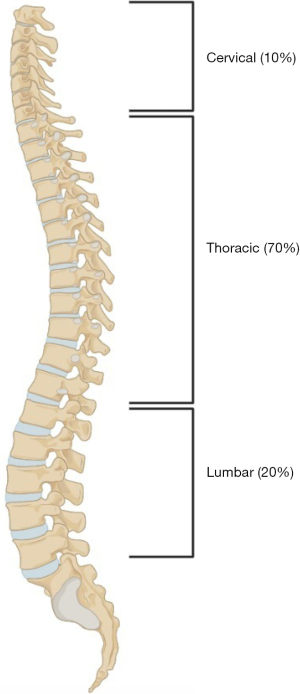
The main mechanisms of damage in MSCC are from direct cord compression by tumour in the epidural space, by bony encroachment on the spinal cord, and by epidural vein plexus compression and obstruction (17,18). In the great majority of cases, tumour reaches the epidural space by initial haematogenous spread to an adjacent vertebra. This is why the numerical distribution of metastases along the length of the spine parallels that of the MSCC location levels (see below and Figure 1) with the most frequent region being the thoracic spine, accounting for up to 70% of spinal column metastases/MSCC (19). Less commonly, MSCC occurs by direct extension of tumour through an intervertebral foramen. The latter is a characteristic spread mode of lymphomas (20) or can occur secondarily, as buttonhole spread from malignant paraaortic lymphadenopathy (21). Epidural tumour causes compressive vasogenic oedema in the spinal cord, and if for an extended period, secondary vascular injury may ensue, resulting in ischaemia and infarction of the cord.
MSCC cancer histologies
The most common malignancies metastasising to the spine and causing MSCC are epithelial, most frequently lung (24%), breast (21%) and prostate (20%), which account for >50% of cases (22). Other cancers causing MSCC include renal cell cancer, myeloma, lymphoma, gastrointestinal tract malignancies and melanoma. The spectrum of tumours causing MSCC in the paediatric population is different, with sarcomas, neuroblastomas, and neuroectodermal tumours being more common (23,24). There is no known primary tumour in 10–20% of cases presenting with MSCC (25).
Symptoms and signs
The overwhelmingly most common initial symptom of MSCC is pain. This occurs predominantly at the site of the lesion, but may also have other characteristics, such as a radicular nature due to nerve infiltration or compression by tumour or collapsed bone. In one study, pain was the first symptom in 94% of cases (26). Other presenting symptoms are weakness (<10%), sensory changes and ataxia. Another study found that at diagnosis, 97% of patients had pain, 74% weakness, 53% sensory changes, 52% autonomic alterations and 4% ataxia (27). Pre-treatment ambulatory status is an important predictor for OS: 6-month OS is as low as 30% in non-ambulatory patients compared to 71% in ambulatory patients (28). Once a neurological sign is present, paraplegia develops within 48 hours in 22% patients, or 7–10 days in 65% patients (29). New back or neck pain in a cancer patient should raise the suspicion for metastatic disease at that site; some of these cases will have MSCC. This relates in particular to the thoracic spine, as it is the commonest site of bone metastases and the least common site for non-cancer pathologies.
Back pain antedated the development of other neurological symptoms by approximately 7 weeks in one study (30). Pathophysiologically, there are three main types of pain attributable to MSCC (31). (I) Local pain. This pain is often described as gnawing or aching, is worse nocturnally, may improve with activity and worsen with recumbency. It may be responsive to non-steroidal anti-inflammatory drugs (NSAIDs) and simple analgesics. It is caused by inflammation from tumour growth and periosteal stretching. (II) Mechanical pain. This pain varies with position and activity, is often refractory to NSAIDs and is exacerbated by movement and axial loading. It is due to spinal instability or pathological fracture and strain on the surrounding soft tissues of the spine. (III) Radicular pain. This pain is typically described as sharp, shooting or stabbing, is often associated with dysaesthesia and is worse with the Valsalva manoeuvre. It is due to nerve root compression/infiltration by tumour or collapsed vertebra and/or pathological fracture. There are various mechanisms of pain production in MSCC (32,33) (Figure 2).

Sensory changes may be ascending, and are often associated with a sensory level; loss may be patchy and bilateral. Sensory loss occurs in a similar distribution to the motor changes, but is less often bilateral (34).
In terms of autonomic manifestations, bladder changes are the most common, with retention +/− overflow incontinence, although bladder and bowel dysfunction is rare in isolation (34). This should be distinguished from the lower motor neuron changes associated with cauda equina syndrome, which lead to urinary incontinence, also in the presence of back pain. Another autonomic consequence of MSCC is reduced anal tone.
Diagnosis
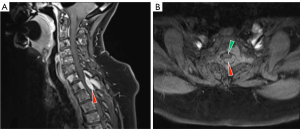
The investigation of choice in cases of suspected MSCC is magnetic resonance imaging (MRI) (35) (Figure 3). MRI has excellent spatial and soft tissue resolution. Sites of MSCC along the spinal cord parallel those of bony metastases (Figure 1), with the most frequent region being the thoracic spine, accounting for up to 70% of spinal column metastases/MSCC (19). The entire spine should be evaluated as in up to 30% of cases, MSCC occurs at multiple spinal levels (36,37). This mandates imaging of the entire spine during evaluation for possible MSCC. In patients in whom MRI is contraindicated, contrast myelography is diagnostic (35). Although it will only reveal one level at complete cerebrospinal fluid (CSF) blocks, it should be combined with a computed tomography (CT) scan at the level of the lesion to define its border (35). CT scans through the lesion may also distinguish bony cord compression from soft tissue compression, and indicate the extent of bony erosion/sclerosis. In terms of radiotherapy treatment, MRI led to an alteration in radiotherapy fields in 50% of cases, and in 20%, a major change (38). Other investigations should be aimed at determining the primary site of origin, e.g., tumour markers, CT chest, abdomen and pelvis, nuclear bone scans, fluorodeoxyglucose (FDG)-positron emission tomography (PET) and other investigations as clinically indicated.
Survival and prognostication
OS after experiencing MSCC generally remains poor. Randomised clinical trials consistently report a median OS of 3–4 months, whether treated with radiotherapy alone or with surgical management and radiotherapy (6,7,9,39,40). Reported ranges of OS from trials can be short weeks to over a year. Prognostication is an important component of assessment as the goals of treatment and management, particularly radiotherapy prescriptions (i.e., short vs. long courses) can be guided by prognosis.
Rades et al. (41) published a validated scoring system after a retrospective review of more than 1,800 patients and have identified six prognostic factors. A score out of a maximum of 45 points can be given with more points being favourable. These factors include histology, presence of visceral metastases, presence of additional sites of bony metastases, ambulatory status prior to treatment, interval from initial cancer diagnosis to MSCC, and time to developing motor deficits prior to treatment. Three categories of patients based off points have been identified with statically significant differences in 6-month OS. Poor prognosis (20–30 points), intermediate (31–35 points), and favourable (36–45 points) were found to have a 6-month OS of 14%, 55%, and 79% respectively. Additionally subgroup analysis from a phase 3 randomised clinical trial performed by Maranzano et al. (40), found pre-treatment ambulatory status and histology as statistically significant factors in median OS. This trial examined the use of 1×8 vs. 2×8 Gy in the setting of MSCC for patients expected to live less than 6 months. Patients who were ambulant prior to commencement of treatment demonstrated a median OS of 5 vs. 2 months in non-ambulant patients. Histology was also associated with prognosis as patients with favourable histologies (defined by the authors as breast, prostate, seminoma, myeloma, and lymphoma) as demonstrating median OS of 9.5 vs. 3 months for unfavourable histologies. This classification in histology is also consistent to the findings of by Rades et al. (41), with breast, prostate, and myeloma/lymphoma being attributed with higher points in their scoring system.
MSCC: management
The cornerstones of MSCC management are steroids and radiotherapy. Ancillary approaches, which will not be discussed further here, include systemic therapy, physiotherapy and rehabilitation, bladder/bowel/skin care and involvement of the palliative care team.
Steroids
Steroids reduce inflammation and thereby cancer-associated pain and swelling and may be oncolytic, especially for haematological cancers. There is also some evidence for steroid-induced oncolysis in breast cancer (38). Steroids should be given in high doses, e.g., dexamethasone 4 mg t.d.s. to q.i.d., with proton pump inhibitor cover. Such doses reduce cord oedema, which can improve neurological function in the short-term, but higher doses do not appear to be more efficacious (42-44). However, if no risk factors for peptic ulcer are present the effectiveness of adding a proton pump inhibitor is unknown.
Steroids can contribute robustly to outcome: one randomised controlled phase III trial compared dexamethasone plus radiotherapy, with radiotherapy alone. In the steroid plus radiotherapy arm, 81% were ambulatory after radiotherapy, compared to 63% in the radiotherapy alone arm (45). Graham et al. (42) performed a small prospective trial comparing an initial bolus of 96 mg of intravenous (IV) dexamethasone to 16 mg in in patients with MSCC. High dose dexamethasone demonstrated a higher incidence of toxicities without improvement in ambulatory status. Care should be taken with steroid use in diabetics, whose control may be strongly affected by steroids. However, the short term steroid benefits almost always outweigh their potential side-effects.
Surgery
Surgery has been advocated in selected patients with MSCC. Historically, surgery has usually comprised posterior laminectomy. Although this achieves a degree of decompression and can provide a histological diagnosis, non-randomised data and one small clinical trial showed that it was inferior to radiotherapy alone (46-48) and it could exacerbate spinal instability. Since the 1980s, anterior/circumferential decompression surgery has been advocated. The techniques involved are not available in all centres and few patients are fit enough for it. There is one randomised trial comparing circumferential decompressive surgery plus radiotherapy (30 Gy in 10 fractions over 2 weeks), with radiotherapy alone (6). The primary endpoint for the trial was the ability to walk; secondary endpoints were urinary continence, muscle power, functional status, the requirement for steroids and opiates and OS. The trial showed that those treated with surgery plus radiation retained the ability to walk for longer, as well as regaining walking ability more frequently, than those who received radiotherapy alone. The benefits in the surgery + radiotherapy arm were limited to those <65 years and the radiotherapy alone arm performed poorly. The trial has been criticised however. It was a small study with fit patients (only 10–15% of presenting cases met their resectability criteria). Additionally, patients with pathological fractures and spinal instability, for which radiotherapy would not be of benefit, were included in the randomisation. Seventy percent of cases from this multi-institutional trial were from the one institution and accrual of the 101 reported cases took 10 years. Overall, randomised definitive data to guide selection of surgery over radiotherapy remains limited although some recommendations have been put forward (49). Surgery is indicated where there are features such as a patient with a single level cord compression, the patient is of at least relatively good performance status, with spinal instability or known radioresistant tumour type, or surgery is required to obtain a histological diagnosis (49).
Conventional external beam radiotherapy (cEBRT)
Radiotherapy has been the mainstay of treatment for the vast majority of MSCC. The aims of radiotherapy are pain relief, decompression and preservation of neurological function. Most MSCC patients have multiple sites of metastatic disease or are of too poor a performance status for anterior-approach (circumferential) major surgery. Assessment of spinal instability is an important consideration prior to any irradiation. This can be assessed with the Spinal Instability Neoplastic Score (SINS), with scores >7 warranting a surgical opinion for surgical stabilization (50).
When utilizing cEBRT, the available prospective data suggests that the choice of dose fractionation between longer and shorter courses of radiotherapy should be tailored to prognosis. Shorter courses of treatment include 1×8 Gy and more commonly 5×4 Gy. Longer courses commonly include 10×3 Gy, or sometimes 20×2 Gy. A non-randomized prospective trial (SCORE-1) examined short vs. long course radiotherapy in 265 patients (8). The primary endpoint was local control at one year, with secondary endpoints of motor function and OS. There was significantly more local control with longer courses of radiotherapy (81% vs. 65%, P=0.005), but no statistically significant improvement in motor function or OS. In patients with more favourable prognosis that require more durable local control, longer courses of radiotherapy should be considered. However, this can make re-irradiation within tolerance more difficult in patients should patients recur in the future. A phase III randomised controlled trial (SCORE-2) comparing 5×4 and 10×3 Gy for poor to intermediate prognosis patients examined ambulatory status at 1 month as the primary endpoint (9). No significant difference was found, or any significant differences in 6-month local control (71.8% vs. 74%) or 6-month OS (42.3% vs. 37.8%). The largest phase III trial to date (SCORAD) was a multi-centre international trial examining a single 1×8 vs. 5×4 Gy in 686 poor prognosis patients (7). The primary endpoint was non-inferiority of ambulatory status at 8 weeks, with secondary endpoints of ambulatory status at 1, 4, and 12 weeks, as well as OS. Although the primary endpoint was not reached, non-inferiority was demonstrated at all other pre-specified time points, with no difference in OS between a single and multi-fraction group. Furthermore, both regimens resulted in improved pain, without any significant difference in pain reduction between treatment regimens. Previous randomised trials have also demonstrated improvement in pain as well as decrease in narcotic use with utilisation of 8 Gy × 1 regimens (39,40). Quality of life (QOL) was formally assessed in the SCORAD trial with ambulatory status. Significant differences in QOL where seen in many domains for responders vs. non-responders with similar scores irrespective of fractionation (7). Although the primary endpoint was not met, this trial suggests there is a role for a single 8 Gy fraction in select patients with limited prognosis since logistics of undergoing multiple fractions may be burdensome and of little benefit.
Hybrid therapy: separation surgery and stereotactic ablative radiotherapy (SABR)
In patients who are surgical candidates an emerging treatment paradigm is the combination of ‘separation surgery’ followed by SABR. The traditional goal of surgery is for aggressive debulking followed by adjuvant conventional radiotherapy. ‘Separation surgery’ is inherently less cytoreductive, as the aim is instead to simply create a circumferential space between tumour (generally 3 mm) and cord so a target is available to safely deliver ablative radiotherapy while respecting cord tolerance (10). SABR confers several advantages over cEBRT and is being increasingly utilized in the treatment of spinal metastases, but still requires more extensive prospective evaluation in the setting of MSCC. A large retrospective study of 186 patients examining the use of adjuvant SABR for MSCC reported 1 year local progression rates of 4% and 9% with 3×9 and 1×24 Gy regimens respectively (11). However, 55.4% of patients died in less than a year, with a median survival of 5.6 months (11). Ideal dose fractionation is debatable, but more hypofractionated regimens may produce better outcomes (11). A small prospective phase II trial enlisting 35 patients who received adjuvant SABR for spinal metastases that included MSCC patients demonstrated a 1-year local control of 90% as per protocol, with a 5×6 Gy regimen, although 19/35 of patients died before a year. Importantly the outcomes with SABR appear to overcome radioresistance in both the MSCC and non-MSCC spinal metastasis setting, as these results have been found independent of histology (11-13,51). Currently there are no clinical trials which compare adjuvant SABR and adjuvant cEBRT in MSCC, but if safe to deliver, adjuvant SABR should be advocated for in favourable prognosis patients in the management of MSCC, as current data supports improved local control.
SABR alone
SABR alone is an established treatment choice in the treatment of non-MSCC spinal metastases. A recent large multicenter international phase III trial compared 2×12 Gy SABR to 4×5 Gy conventional radiotherapy in 229 patients, assessing complete pain relief as the primary endpoint. A statistically significant improvement of complete pain relief was achieved in the SABR group at both 3 and 6 months, even adjusting for radioresistant tumours (51). However, there is a paucity of data in utilising SABR alone in the treatment of MSCC. A prospective phase 2 trial comprising 62 patients to treat MSCC with SABR alone was performed by Ryu et al. (14). In this study, a single fraction of SABR was delivered, with doses ranging from 12–20 Gy with a median dose of 16 Gy. Patients were followed for a median of 11.5 months. Patients demonstrated favourable neurological outcomes: 94% retained ambulatory status and 63% of patients with deficits improved to regain intact neurology. There was a response in 80% of tumours, with a complete response in 27%. The caveat of interpreting neurology in this study is that patients with limb power of less the 4/5 were excluded and treated with surgical decompression. Of note however is that this study examined radioresistant tumours exclusively. Lee et al. (15) performed a retrospective study comprising 35 patients which examined the use of single fraction SABR in the management of radiologically high grade MSCC, with doses of 16–18 Gy. Patients were followed for a median of 14 months with 67% preserving or improving neurology. OS was statistically significantly improved for patients whose neurological function improved or remained the same, vs. those who deteriorated neurologically. The findings were independent of clinical radiosensitivity. One of the concerns with SABR alone is not being able to adequately cover the target due to spinal cord constraints. A phase 1 safety trial with 32 patients examining single fraction SABR at a dose of 18 or 24 Gy examined relaxed spinal cord constraints, ranging from 10–16 Gy. One-year local control was found to be 89% and no radiation myelopathy was found after 17 months of follow-up (16).
Conclusions
MSCC is an oncological emergency that carries significant morbidity if not treated and diagnosed in a timely fashion. It is estimated to occur in approximately 15% of cancer patients. Lung, breast and prostate cancer are the most common histologies, with the thoracic spine as the most common site of cord compression. MRI evaluating the whole spine is the modality of choice as disease can involve multiple levels. Treatment includes corticosteroids in conjunction with radiotherapy alone, or with surgery followed by adjuvant radiotherapy. The most common maximal dose of corticosteroid utilised is 16 mg of dexamethasone daily, as the therapeutic ratio diminishes beyond this. In terms of radiotherapy vs. surgery + radiotherapy, a phase 3 trial demonstrated better outcomes with a surgical approach. However, the indications for patients’ suitability for the combined approach are very narrow. Consequently, corticosteroids and radiotherapy alone are the cornerstone of treatment for most individuals with MSCC. Various radiotherapy treatment regimens can be utilised and are broadly classified as short course (1×8 or 5×4 Gy) or long course (10×3 or 20×2 Gy). Comparison of these regimens has been the subject of several clinical trials. Longer courses of radiotherapy demonstrate more durable local control and are suited to better prognosis patients. In intermediate prognosis patients 5×4 Gy is similar in outcomes to 10×3 Gy. In poor prognosis patients, 1×8 Gy has been shown to be a non-inferior regimen to 5×4 Gy in terms of functional outcomes. In addition to prognosis, other treatment considerations shaping a decision for radiotherapy include the degree of neurological impairment, radiosensitivity of the tumour and the mechanical stability of the spine. Thus, treatment must be individualised based on thorough clinical assessment (Figure 4).

Future directions
An emerging modality of treatment is the combination of separation surgery and adjuvant SABR. Retrospective data suggests that this approach provides very high rates of 1-year local control and ablative doses of radiotherapy appear to provide local control of radioresistant tumour types. The use of SABR alone in the management MSCC is underexplored, but limited data suggests a role. The use of adjuvant SABR in the management of MSCC requires further prospective evaluation. A purely exploratory field is the potential synergistic antitumour effects of combined SABR and systemic therapies such as immunotherapy and targeted therapies for molecularly appropriate tumours. Although not utilised in MSCC this maybe an avenue of future exploration.
Acknowledgments
The authors thank Dr. Richard Foster (North West Cancer Centre, Burnie, Tasmania, Australia) for thoughtful comments on the manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3076/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3076/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3076/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rajer M, Kovač V. Malignant spinal cord compression. Radiol Oncol 2008;42:23-31.
- Klimo P Jr, Thompson CJ, Kestle JR, et al. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol 2005;7:64-76. [Crossref] [PubMed]
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [Crossref] [PubMed]
- George R, Jeba J, Ramkumar G, et al. Interventions for the treatment of metastatic extradural spinal cord compression in adults. Cochrane Database Syst Rev 2015;2015:CD006716. [Crossref] [PubMed]
- Loblaw DA, Mitera G, Ford M, et al. A 2011 updated systematic review and clinical practice guideline for the management of malignant extradural spinal cord compression. Int J Radiat Oncol Biol Phys 2012;84:312-7. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366:643-8. [Crossref] [PubMed]
- Hoskin PJ, Hopkins K, Misra V, et al. Effect of Single-Fraction vs Multifraction Radiotherapy on Ambulatory Status Among Patients With Spinal Canal Compression From Metastatic Cancer: The SCORAD Randomized Clinical Trial. JAMA 2019;322:2084-94. [Crossref] [PubMed]
- Rades D, Lange M, Veninga T, et al. Final results of a prospective study comparing the local control of short-course and long-course radiotherapy for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys 2011;79:524-30. [Crossref] [PubMed]
- Rades D, Šegedin B, Conde-Moreno AJ, et al. Radiotherapy With 4 Gy × 5 Versus 3 Gy × 10 for Metastatic Epidural Spinal Cord Compression: Final Results of the SCORE-2 Trial (ARO 2009/01). J Clin Oncol 2016;34:597-602. [Crossref] [PubMed]
- Bilsky MH, Angelov L, Rock J, et al. Spinal radiosurgery: a neurosurgical perspective. J Radiosurg SBRT 2011;1:47-54.
- Laufer I, Iorgulescu JB, Chapman T, et al. Local disease control for spinal metastases following "separation surgery" and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J Neurosurg Spine 2013;18:207-14. [Crossref] [PubMed]
- Redmond KJ, Sciubba D, Khan M, et al. A Phase 2 Study of Post-Operative Stereotactic Body Radiation Therapy (SBRT) for Solid Tumor Spine Metastases. Int J Radiat Oncol Biol Phys 2020;106:261-8. [Crossref] [PubMed]
- Yamada Y, Katsoulakis E, Laufer I, et al. The impact of histology and delivered dose on local control of spinal metastases treated with stereotactic radiosurgery. Neurosurg Focus 2017;42:E6. [Crossref] [PubMed]
- Ryu S, Rock J, Jain R, et al. Radiosurgical decompression of metastatic epidural compression. Cancer 2010;116:2250-7. [Crossref] [PubMed]
- Lee I, Omodon M, Rock J, et al. Stereotactic radiosurgery for high-grade metastatic epidural cord compression. J Radiosurg SBRT 2014;3:51-8.
- Ghia AJ, Guha-Thakurta N, Hess K, et al. Phase 1 Study of Spinal Cord Constraint Relaxation With Single Session Spine Stereotactic Radiosurgery in the Primary Management of Patients With Inoperable, Previously Unirradiated Metastatic Epidural Spinal Cord Compression. Int J Radiat Oncol Biol Phys 2018;102:1481-8. [Crossref] [PubMed]
- Perrin RG, Laxton AW. Metastatic spine disease: epidemiology, pathophysiology, and evaluation of patients. Neurosurg Clin N Am 2004;15:365-73. [Crossref] [PubMed]
- Urch C. The pathophysiology of cancer-induced bone pain: current understanding. Palliat Med 2004;18:267-74. [Crossref] [PubMed]
- He S, Ye C, Gao X, et al. Distribution and predictive value of initial presenting symptoms in spinal metastases from primary cancer patients. Eur Spine J 2020;29:3148-56. [Crossref] [PubMed]
- Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol 1978;3:40-51. [Crossref] [PubMed]
- Griessenauer CJ, Raborn J, Foreman P, et al. Venous drainage of the spine and spinal cord: a comprehensive review of its history, embryology, anatomy, physiology, and pathology. Clin Anat 2015;28:75-87. [Crossref] [PubMed]
- Loblaw DA, Perry J, Chambers A, et al. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: the Cancer Care Ontario Practice Guidelines Initiative's Neuro-Oncology Disease Site Group. J Clin Oncol 2005;23:2028-37. [Crossref] [PubMed]
- Pollono D, Tomarchia S, Drut R, et al. Spinal cord compression: a review of 70 pediatric patients. Pediatr Hematol Oncol 2003;20:457-66.
- Tantawy AA, Ebeid FS, Mahmoud MA, et al. Spinal cord compression in childhood pediatric malignancies: multicenter egyptian study. J Pediatr Hematol Oncol 2013;35:232-6. [Crossref] [PubMed]
- Schiff D, O'Neill BP, Suman VJ. Spinal epidural metastasis as the initial manifestation of malignancy: clinical features and diagnostic approach. Neurology 1997;49:452-6. [Crossref] [PubMed]
- Levack P, Graham J, Collie D, et al. Don't wait for a sensory level--listen to the symptoms: a prospective audit of the delays in diagnosis of malignant cord compression. Clin Oncol (R Coll Radiol) 2002;14:472-80. [Crossref] [PubMed]
- DeAngelis LM, Posner JB. Metastases. In: Kufe DW, Pollock RE, Weichselbaum RR, et al. editors. Holland-Frei Cancer Medicine. 6th edition. Hamilton, ON: BC Decker; 2003.
- Rades D, Dunst J, Schild SE. The first score predicting overall survival in patients with metastatic spinal cord compression. Cancer 2008;112:157-61. [Crossref] [PubMed]
- Spinazzé S, Caraceni A, Schrijvers D. Epidural spinal cord compression. Crit Rev Oncol Hematol 2005;56:397-406. [Crossref] [PubMed]
- Bach F, Larsen BH, Rohde K, et al. Metastatic spinal cord compression. Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir (Wien) 1990;107:37-43. [Crossref] [PubMed]
- Harel R, Angelov L. Spine metastases: current treatments and future directions. Eur J Cancer 2010;46:2696-707. [Crossref] [PubMed]
- Roodman GD. Mechanisms of bone metastasis. N Engl J Med 2004;350:1655-64. [Crossref] [PubMed]
- Sciubba DM, Petteys RJ, Dekutoski MB, et al. Diagnosis and management of metastatic spine disease. A review. J Neurosurg Spine 2010;13:94-108. [Crossref] [PubMed]
- Helweg-Larsen S, Sørensen PS. Symptoms and signs in metastatic spinal cord compression: a study of progression from first symptom until diagnosis in 153 patients. Eur J Cancer 1994;30A:396-8. [Crossref] [PubMed]
- Abdi S, Adams CI, Foweraker KL, et al. Metastatic spinal cord syndromes: imaging appearances and treatment planning. Clin Radiol 2005;60:637-47. [Crossref] [PubMed]
- Schiff D, O'Neill BP, Wang CH, et al. Neuroimaging and treatment implications of patients with multiple epidural spinal metastases. Cancer 1998;83:1593-601.
- van der Sande JJ, Kröger R, Boogerd W. Multiple spinal epidural metastases; an unexpectedly frequent finding. J Neurol Neurosurg Psychiatry 1990;53:1001-3. [Crossref] [PubMed]
- Posner JB. Spinal metastases. In: Posner JB. Neurologic complications of cancer. Philadelphia, PA, USA: FA Davis Company; 1995:111-42.
- Maranzano E, Bellavita R, Rossi R, et al. Short-course versus split-course radiotherapy in metastatic spinal cord compression: results of a phase III, randomized, multicenter trial. J Clin Oncol 2005;23:3358-65. [Crossref] [PubMed]
- Maranzano E, Trippa F, Casale M, et al. 8Gy single-dose radiotherapy is effective in metastatic spinal cord compression: results of a phase III randomized multicentre Italian trial. Radiother Oncol 2009;93:174-9. [Crossref] [PubMed]
- Rades D, Douglas S, Veninga T, et al. Validation and simplification of a score predicting survival in patients irradiated for metastatic spinal cord compression. Cancer 2010;116:3670-3. [Crossref] [PubMed]
- Graham PH, Capp A, Delaney G, et al. A pilot randomised comparison of dexamethasone 96 mg vs 16 mg per day for malignant spinal-cord compression treated by radiotherapy: TROG 01.05 Superdex study. Clin Oncol (R Coll Radiol) 2006;18:70-6. [Crossref] [PubMed]
- Heimdal K, Hirschberg H, Slettebø H, et al. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol 1992;12:141-4. [Crossref] [PubMed]
- Vecht CJ, Haaxma-Reiche H, van Putten WL, et al. Initial bolus of conventional versus high-dose dexamethasone in metastatic spinal cord compression. Neurology 1989;39:1255-7. [Crossref] [PubMed]
- Sørensen S, Helweg-Larsen S, Mouridsen H, et al. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: a randomised trial. Eur J Cancer 1994;30A:22-7. [Crossref] [PubMed]
- Molina C, Goodwin CR, Abu-Bonsrah N, et al. Posterior approaches for symptomatic metastatic spinal cord compression. Neurosurg Focus 2016;41:E11. [Crossref] [PubMed]
- Rades D, Huttenlocher S, Dunst J, et al. Matched pair analysis comparing surgery followed by radiotherapy and radiotherapy alone for metastatic spinal cord compression. J Clin Oncol 2010;28:3597-604. [Crossref] [PubMed]
- Young RF, Post EM, King GA. Treatment of spinal epidural metastases. Randomized prospective comparison of laminectomy and radiotherapy. J Neurosurg 1980;53:741-8. [Crossref] [PubMed]
- Cole JS, Patchell RA. Metastatic epidural spinal cord compression. Lancet Neurol 2008;7:459-66. [Crossref] [PubMed]
- Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976) 2010;35:E1221-9. [Crossref] [PubMed]
- Sahgal A, Myrehaug SD, Siva S, et al. Stereotactic body radiotherapy versus conventional external beam radiotherapy in patients with painful spinal metastases: an open-label, multicentre, randomised, controlled, phase 2/3 trial. Lancet Oncol 2021;22:1023-33. [Crossref] [PubMed]


