
Practical oncoplastic surgery techniques needed for practice
Highlight box
Surgical highlights
• Oncoplastic breast surgery allows immediate reconstruction combined with traditional breast conversation therapy.
What is conventional and what is novel/modified?
• Both volume displacement and volume replacement techniques can be employed based on patient characteristics, with both techniques described within.
What is the implication, and what should change now?
• Oncoplastic breast surgery should be presented as an option to all women requiring breast surgery.
Introduction
Oncoplastic breast surgery (OPS), a form of breast-conservation surgery (BCS), has been shown to be a safe and effective treatment for breast cancer. OPS comprises both volume displacement and volume replacement techniques. The American Society of Breast Surgeons defines these techniques by the percentage of tissue excised (1). Volume displacement is further subdivided into level I (<20% breast tissue removed) and level II (20–50% breast tissue removed) techniques. Specifically, level II volume displacement techniques utilize mastopexy and reduction mammaplasty designs (e.g. Wise-pattern, circumvertical) with a contralateral symmetry operation (2).
Compared to standard partial mastectomy (PM), OPS is considered a safe oncologic and reconstructive technique that allows for a significant surgical resection followed by local tissue rearrangement. By performing local tissue rearrangement, the surgeon is able to restore symmetry and arrive at an aesthetically pleasing result. Given the volume of resected tissue in the setting of an oncoplastic partial mastectomy, there is a decreased positive margin rate (5–10%) as compared to standard breast conserving therapy with a partial mastectomy alone (20–30%) (3). More importantly, there is no difference in 3-year overall survival and recurrence-free survival when comparing OPS to PM (3).
OPS also improves patient quality of life measured by patient-reported outcome measures (PROMs), such through a BREAST-Q survey (4-6). Measures such as psychosocial well-being have been shown to be higher in patients treated with OPS as compared to BCS. Other measures including physical well-being, satisfaction with breast, and sexual well-being have trended toward favoring OPS, but have not shown statistical significance (4).
With the above-mentioned benefits, this primer intends to describe the work up, indications, preoperative markings, anatomical considerations, surgical technique, and complications of both volume displacement and volume replacement procedures. We present this article in accordance with the SUPER reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1536/rc).
Preoperative preparation
Over time, the indications for OPS have changed substantially. With an appropriately selected patient, most patients are candidates for oncoplastic surgery, with up to 91% of patients able to undergo breast conservation (3). The most important aspect of oncologic breast surgery is removing the entire tumor with adequate margins. OPS typically allows for larger resections, which can decrease the positive margin rate (3). If oncoplastic surgery is desired and breast conservation is contraindicated given the size of the tumor or concern for positive margins, neoadjuvant therapy can be administered to reduce the size of the tumor prior to reconsideration of the surgical approach. In these cases, we recommend a MRI prior to neoadjuvant therapy and after completion of their treatment to evaluate the response. Post neoadjuvant therapy imaging can help determine if these patients have converted into candidates for oncoplastic surgery.
Anatomical considerations in oncoplastic surgery
It is critical to have a thorough understanding of the anatomy in order to maximize reconstructive outcomes. Partial breast reconstruction relies on tissue perfusion to ensure nipple areolar complex (NAC) perfusion, reduce development of fat necrosis, and decrease skin flap necrosis. Additionally, understanding the nerve supply can help surgeons preserve normal sensation to the skin of the breast and nipple, and reduce the risk of long-term pain syndromes associated with breast surgery.
The blood supply should be thought in terms of glandular flaps and pedicles as it is critical to maintain perfusion when performing autoaugmentation during the oncoplastic surgery. The superior and superomedial pedicle of volume displacement techniques relies on the perforators branching off the internal mammary artery (IMA) medially (Figure 1). These perforators are found in the superficial subcutaneous tissue in a radial fashion, approximately 1 cm deep to the skin. Specifically, the superomedial pedicle is supplied by the second and third intercostal branches of the IMA, with additional vascular supply laterally from the lateral thoracic artery and thoracoacromial branches. The main blood supply of the inferior pedicle is from the fourth deep intercostal artery. Venous drainage of both the superomedial and inferior pedicles is provided by the subdermal plexus. The NAC receives innervation primarily from the deep branch of the fourth intercostal nerve in addition to the medial intercostal and supraclavicular nerve branches (7).
The relationship of tumor anatomy interplays with the surgical options available to patients. Body habitus, breast ptosis and breast size in combination with tumor location further narrows the OPS techniques that can be offered (2). As a basic guide, patients with larger breasts and greater ptosis are more ideal candidates for volume displacement reconstruction. Women with smaller breasts or petite body habitus typically can be considered for volume replacement techniques. In such situations, the flap can act as a tissue “fill in” allowing the patient excellent reconstruction while having unilateral surgery.
Aesthetically placed incisions include periareolar incisions, doughnut or crescent mastopexy incisions, inframammary incisions and axillary incisions (2). These techniques usually require tunneling under the skin to the affected segment of breast tissue. Following surgical resection, glandular rearrangement can be performed by dissecting tissue off the pectoralis fascia and into the anterior mammary fascial plane. This allows for mobilization and advancement of the breast parenchyma into the created defect. Care is taken to avoid placing sutures within predominantly fat tissue to avoid shredding of the tissue which will result in bleeding and fat necrosis (8).
Level I volume displacement defects can typically be filled by advancement of adjacent tissue. When performing level II volume displacement excisions, the tumor location becomes relevant to the available surgical techniques. Clough et al. devised “an Atlas” for each tumor location. To briefly review, we must break the breast into quadrants, including the lower pole, lower inner pole, lower outer pole, upper inner pole, and upper outer pole. The first quadrant to be recognized as a high-risk location for breast deformity (9-11) was the inner pole which can be approached with a superomedial pedicle, superior pedicle with inverted T incision, or anterior intercostal perforator-based flap (2,8). The lower inner quadrant can be addressed by an inferior pedicle. The upper inner quadrant has historically been a limitation of BCS, Anderson et al. proposed a “batwing” excision pattern utilizing the standard keyhole incision with triangular incisions extended laterally on each side to approach a central and upper inner quadrant tumor (8,12). However, an inferior based-pedicle design has been used by these authors with success in resecting tumors of this location, as this approach is generally adapted for upper pole tumors as well (2,8). The upper outer breast quadrant is classically more forgiving in that standard BCS resections does not lead to significant breast deformities (8). In practice, these authors typically approach upper outer quadrant based tumor with either an inferior or a superomedial pedicle design using volume displacement and a lateral intercostal artery perforator (LICAP) flap for a volume replacement approach. Lower outer quadrant tumors can be approached in a similar fashion to lower-pole tumors with a superior or superomedial pedicle based design.
Centrally located tumors can be a surgical challenge. Oncoplastic surgery allows for central resection with or without resection of the NAC. Options for central tumor resection can be approached utilizing both volume replacement and volume displacement techniques. The Grisotti technique can be used to resect the tumor and achieve a satisfactory reconstructive outcome by creating a pedicled flap, typically an inferior pedicle based upon the intercostal artery perforators and leaving a skin island on the de-epithelized inferior pedicle (13). This skin island is measured to be similar as the contralateral NAC. The flap is advanced into the defect and the skin island is sutured into the superior keyhole. Another technique the authors perform is volume replacement techniques using perforator-based flaps. Following surgical resection of the centralized tumor, local tissue rearrangement is tunneled into the central defect to autoaugment the resection. A skin island is created at the appropriate location of the flap, potentially at the previous NAC site. Details on pedicle-based flap reconstruction will be discussed in detail below. When the nipple cannot be spared based on tumor location, reconstruction of the NAC can be performed in the future, using three-dimensional nipple tattooing, which has emerged as a popular option for nipple reconstruction following surgical healing.
Volume displacement
Volume displacement techniques are typically performed most often through a Wise-pattern skin incision, most commonly when 20–50% of the breast volume is resected. While this procedure can be performed for unilateral cases, in our experience, we often perform contralateral surgery frequently to achieve symmetry. Although beyond the scope of OPS, patients who are otherwise not candidates for a nipple sparing mastectomy, given their large breast size or degree of ptosis, can be offered level II oncoplastic breast reduction using contralateral mammoplasty or mastopexy procedures to enable these patients to be candidates for a nipple sparing mastectomy in a staged fashion (14). Our practice is to perform the second stage nipple sparing mastectomy around 6 weeks after index procedure, allowing for revascularization of the skin flaps and NAC. Waiting 6 weeks allows staying within the recommended timeframe of initiating radiation therapy in the breast conservation setting.
Tissue rearrangement using vascularized pedicles is performed most often using full thickness dermo-glandular flaps which are advanced into the defect. Types of pedicle designs include superior, superomedial, inferior, lateral, and central mound techniques. However, for the purposes of this article, the authors have chosen to elaborate on the inferior-based and superomedial-based pedicle designs, which, in our opinion, offer the greatest utility in the oncoplastic surgical management for most breast cancers. It is important to avoid excessive undermining of the glandular flaps and to assess perfusion of the flap by ensuring the distal tip of the flaps exhibit arterial bleeding and/or using a tissue perfusion scan (15).
Indications
Volume displacement OPS is ideally suited for patients with moderate- to large-sized breasts and grade II or grade III ptosis (2). The Wise-pattern design is ideal for ptotic breasts as it facilitates a reduction in both overall breast volume as well as the skin envelope. Moreover, women with symptomatic macromastia who are assigned the diagnosis of breast cancer may benefit most from OPS as they receive symptomatic relief in addition to its oncologic advantage over PM (2).
There are relatively few absolute contraindications to OPS and they are inherently similar to those associated with PM, namely a history of chest wall radiation and inflammatory breast cancer (2).
Preoperative markings
For both the superomedial and inferior pedicle Wise-pattern techniques, initial markings are similar. Patients should be standing during the marking. These markings should be made to mark the IMFs, sternal midline, and midclavicular lines, splitting the breast in half. The new nipple position should meet 3 requirements: (I) 19–23 cm from sternal notch to nipple and equidistant from the sternal midline; (II) at the midpoint of the humerus when arms relaxed at sides; and (III) at the projection of the original IMF. Beginning at the point of the new nipple position, an equilateral triangle is created with 7 cm limbs for smaller patients to 8–9 cm limbs in larger patients. A superior keyhole designed for a 42-mm NAC is marked at the apex of the triangle. Next, a gentle sloping curve is marked starting at either side of the base of the triangles and extending the medial and lateral IMF (Figure 2). The base and ultimate size of the superomedial or inferior pedicle varies based upon the tumor location and size of lumpectomy required, but a 10-cm base for the inferior pedicle is standard to begin with. With regard to the superomedial pedicle, the design is based from the 10 or 2 o’clock marking of the superior keyhole and carried around the NAC to the corner of the triangle (16).
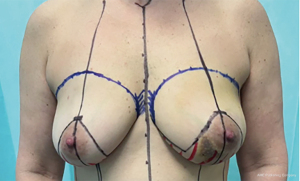
Surgical technique
De-epithelialization
Using an assistant to place the NAC in full stretch, a 38–42 mm cookie cutter and marking pen are used to mark the size of the new NAC centered over the nipple. The marked pedicle, whether inferior or superomedial, is de-epithelialized the assistant provides countertension (16). The de-epithelialized skin is discarded.
Resection of the tumor
The marked lines are next incised partial thickness. Full-thickness incisions are eventually made through all marked lines, with the exception of the base of the pedicle. It is essential to not cut through the base of the pedicle as this is where its blood supply arises. The tumor is resected with wide margins in the standard fashion with wire-localization by taking advantage of the wide exposure that the Wise-pattern allows. If there is concern for close margins, it is reasonable to take additional shave margins as well.
Marking the tumor bed
Once the tumor has been resected and properly marked for pathology, the tumor bed is marked with surgical clips in all 6 borders (superior, inferior, medial, lateral, anterior, and posterior). It is critical that the borders of the tumor are well-marked as this allows for one to re-access the tumor cavity in the case of positive margins, as well as for radiation oncologists to identify the tumor bed postoperatively for boost radiation.
Accessing the sentinel lymph node
Given the wide exposure of the Wise-pattern, the sentinel lymph node is easily accessed by dissecting laterally into the axilla or through a separate axillary incision if preferred. The sentinel lymph node biopsy is performed in the standard fashion with dual tracer.
Superomedial pedicle
The superomedial pedicle is performed following standard Wise-pattern skin incisions (Figure 3). The superomedial pedicle is declared on its medial and lateral aspects with electrocautery and a “finger-spreading” retraction technique to the chest wall, which takes care not to distort the surrounding tissues. The pedicle is then grasped as a “bucket-handle”, and the pedicle is elevated off the chest wall (17,18). The elevation of the pedicle allows for rotation of the pedicle and NAC into the superior keyhole. The pedicle length is designed based upon the tumor cavity size and location. The pedicle is tailor tacked into place and the NAC is inset into the superior keyhole prior to closing the pillars. The skin edges are closed in several layers. The critical suture is the three-point stitch at the intersection of the breast meridian at the inframammary crease and the inferior aspect of the medial and lateral pillars. Drains are left as needed.
Inferior pedicle
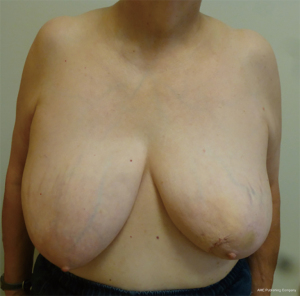
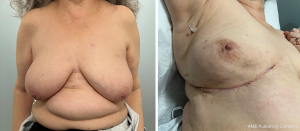
The inferior pedicle is performed following standard Wise-pattern skin incisions. We introduce a representative case of a woman with left-sided breast cancer with plan for level II volume displacement (Figure 4) (Case 1). The inferior pedicle, medial and lateral wings are de-epithelized in the standard fashion (Figure 3). We de-epithelialize the medial and lateral wings as this tissue is sometimes used to fill in the tumor resection cavity as a bi-pedicled flap. The inferior pedicle is declared using a base diameter typically measuring 10–12 cm. The pedicle is declared down to the chest wall using a “finger spreading” technique. Care is taken to preserve the vessels, which are based upon the intercostal artery perforators. Over dissection of these vessels can lead to nipple and fat necrosis. Once the pedicle is elevated off the chest wall, it is advanced superiorly into the superior keyhole. The medial and lateral wings are excised based upon the tumor location and resection. For tumors located in the medial breast, the medial wing is advanced into the tumor resection cavity. The inferior pedicle is also advanced into the cavity and superiorly into the superior keyhole to help reduce breast asymmetry. The pedicle is tailor tacked into place and the NAC is inset into the superior keyhole prior to closing the pillars. The skin edges are closed in several layers. The critical suture is the three-point stitch at the intersection of the breast meridian at the inframammary crease and the inferior aspect of the medial and lateral pillars. Again, drains are left as needed. Postoperative outcome one month following left-sided partial mastectomy and oncoplastic reduction with right-sided symmetry reduction in Case 1 is demonstrated in Figure 5.
Volume replacement
Volume replacement oncoplastic surgery is a powerful breast conservation tool when performing a large breast cancer resection and reconstruction. The reconstruction element involves the use of regional flaps adjacent to the breast area footprint. The two adjacent regions to the breast that naturally have redundant tissue and healthy perforator blood supply are immediately lateral and inferior to the breast. As such, these donor sites for pedicled, perforator-based flaps can often be used without donor site morbidity since these flaps are typically fasciocutaneous and transposing them into the breast defect is naturally accompanied with the collapse of the donor site itself. Schaverien et al. described the most common perforator vessels available for volume displacement oncoplastic surgery (19). Surgical options for volume replacement include chest wall perforator-based flaps, such as lateral thoracic artery perforator (LTAP) flap, anterior intercostal artery perforator (AICAP) flap and LICAP flap; as well as larger tissue based flaps such as, thoracodorsal artery perforator (TDAP) flap and latissimus dorsi myocutaneous flap (LD flap). In the following sections we specifically elaborate on the more recently described techniques of AICAP and LICAP flaps due to their versatility and decreased donor site morbidity in comparison to TDAP and LD flaps (20).
Indications
There are three common indications for volume replacement oncoplastic surgery. First, the American Society of Breast Surgeons classified volume replacement oncoplastic surgery as a breast conservation modality that one would use when greater than 50% of the breast was removed as part of the oncologic resection with reconstruction (1). While this is certainly an indication, there are additional indications for when volume replacement oncoplastic surgery is reasonable. A second indication would be for patients who have smaller breasts with minimal ptosis and smaller breasts but who have cancers in the lower pole where a resection would compromise the pillar foundations of the breast itself, regardless of the resected breast percentage. In patients with higher adipose to glandular tissue, performing a level 1 volume displacement doughnut mastopexy may lead to higher fat necrosis than an AICAP flap (8). Without an AICAP flap in this situation, a patient’s breast would likely experience a bird’s beak deformity with the collapse of the breast foundation (8,9). One would see this more often after resorption of the seroma and the additive radiation contracture. A third indication for volume replacement would involve breast cancer patients who have any grade of ptosis with moderate to large breasts whom prefer unilateral surgery. These are patients who are satisfied with their breast form, have no macromastia symptoms but do not want the aesthetic defect that a partial mastectomy alone may create (12) or want the benefit of a lower positive margin rate that oncoplastic surgery provides over partial mastectomy alone (17,21,22). As such, a LICAP or AICAP regional flap affords the patient an ipsilateral oncoplastic reconstruction without operating on the contralateral breast that a level II volume displacement reduction design would require, especially in patients with larger/ptotic breasts.
Inferiorly based flap: AICAP flap
The inferior defects are approached predominantly dependent upon the AICAPs (Figure 6). The redundant supply of blood vessels originating from inferior edges of the ribs is reliable and perfuses the lower region of the superior abdominal wall just inferior to the inframammary crease. As such, an AICAP flap can fill in volume from the inferior pole preventing bird beak deformities (8). AICAP flaps can be used in a turnover fashion or rotational fashion depending upon where the lower pole breast defect occurs. There are several other names in the literature describing these flaps such as “angel wings”, “crescent flaps”, etc., but the authors submit that an anatomical description based on blood supply is the most easily understood description for this set of flaps.
Technique
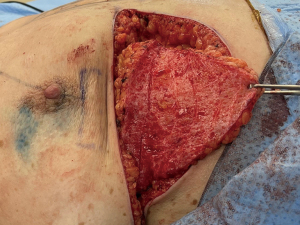
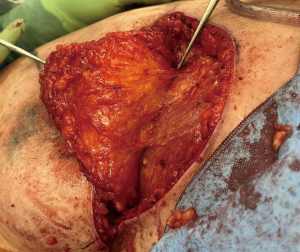
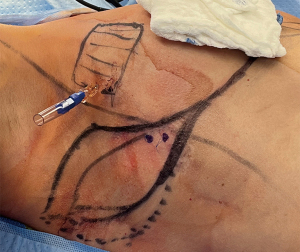
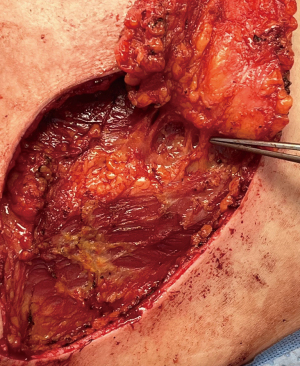
As with most flaps, markings are done with the patient standing and lying in the preoperative setting (Figure 7) (Case 2). The dissection of this flap begins with a pinch test to ensure that the surgeon can close the donor site when transposing the flap to the recipient site, followed by de-epithelialization of the flap. Then, a full thickness incision is made at the inframammary crease allowing the breast surgeon excellent access to the cancer region which can be aggressively resected. The AICAP flap is elevated (Figure 8) from an inferior to superior dissection taking the underlying abdominal wall muscular fascia. As the surgeon approaches closer to the inframammary crease the dissection slows down to ensure the intercostal artery perforators are identified and protected (Figure 9). These arteries typically arise from the inferior edges of the ribs close to the inframammary crease. The flap is then manipulated into the resection defect, typically necessitating rotation of the flap itself (Figure 10). Flap turnover can be done without visualization of the perforators, then there is no need to unnecessarily skeletonize the vessels if one can verify their normality with a Doppler device. Any additional length for the turnover flap may necessitate the need to skeletonize the vessels or in the most extreme case sacrifice a vessel if several perforators have been identified. The viability of the flap can be ensured with distal dermal tip punctate bleeding or with intraoperative indocyanine green (ICG) technology (15). In Case 2, we demonstrate a woman with right-sided breast cancer of the lower inner pole, Figure 11 represents postoperative outcome 1 month following right sided partial mastectomy with oncoplastic reconstruction using an AICAP flap.
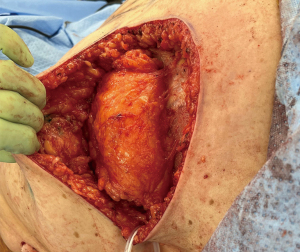
Laterally based flap: LICAP flap
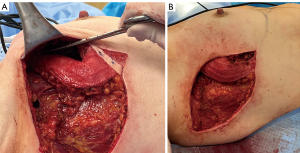
The laterally based flaps have several perforator options that often overlap in design but collectively are very effective in perfusing tissue from the axillary region for transposition into the upper outer quadrants and central regions of the breast. The advantage of this a LICAP flap compared to more laterally based perforator flaps, such as the TDAP flap or LD flap, is that utilizing the LICAP does not sacrifice the thoracodorsal pedicle, which can be particularly useful in cases where oncologic margins are uncertain and a staged procedure may be necessary (23). Also, in the authors’ opinion, a further advantage of the LICAP is that is can be performed with a small axillary roll underneath the patient without having the reposition the patient during the case.Similar to an AICAP, a LICAP flap arises from underneath the rib into the superficial tissue and is easily identifiable with a Doppler or as one slowly dissects to its location. One of the reliable ways to find the LICAP, resides in the lower outer quadrant edge of the breast region where the lateral point of the wing in a Wise-pattern incision would end (Figure 12).
Technique
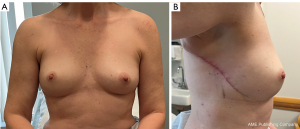
Preoperative markings for the LICAP flap are done with the patient standing with shoulders in the resting position (Figure 13), and also in 90-degree abducted position (Case 3). The dissection of the flap begins with a pinch test to ensure the donor site can be closed. Attempt to Doppler the LICAPs in the region where the tip or apex point of the lateral wing in a Wise-pattern skin incision would lie (Figure 14). De-epithelialize the planned area of the flap, which can include most of the axilla approaching the inferior axillary hairline. Of note, this flap offers an excellent opportunity for donor site reduction in appropriate patients with excessive axillary tissue. Dissection should occur superiorly and laterally towards the final location of dissection terminating in the inferior, medial region where the perforator vessels reside (Figure 15). These flaps are principally rotationally based on the pivot point at the lateral intercostal region (Figure 16). The underlying muscular fascia can be included as part of this dissection as necessary, if this is the case, skeletonizing the perforator is unnecessary as long as a Doppler can confirm existence of the perforator. The viability of the flap should be addressed by observing bleeding at the dermal edges of the distal region or by using ICG perfusion technology (15). Figure 17 demonstrates one-month postoperative outcomes of a woman following right sided partial mastectomy with oncoplastic reconstruction using a LICAP flap.
Postoperative considerations
Postoperative monitoring requires stay in a designated post-anesthesia care unit (PACU) only, or one night inpatient observation if no immediate complications are encountered. Unlike autologous free-flap, perforator-based flaps do not require inpatient monitoring for viability confirmation (i.e., scheduled Doppler checks). All patients undergoing OPS will follow up with their surgeon within a week if drains are placed to monitor output and determine removal. The majority of patients undergo postoperative breast radiation—as with any patient undergoing BCS alone. Not only is marking the cavity used for future radiation planning and future breast imaging, it can also help identify accurate resection sites if the pathology yields compromised margins. There are several commercially available devices to help mark the partial mastectomy cavity, for example radiopaque sutures. However, at this time titanium clips provide a relatively cheap and reliable way to mark the cavity. It is important to communicate with the Radiology and Radiation Oncology teams on the placement of surgical bed markings.
Regardless if a dual or single-surgeon model is utilized, the surgeon specifically trained in breast oncology will continue to follow patients for years to monitor for recurrence. Ongoing scheduled postoperative clinic visits at the surgeon(s)’ discretion is necessary to monitor healing, for example if epidermolysis or minor wound dehiscence is identified early, local wound care can attempt to prevent progression.
Oncoplastic outcomes
OPS, as mentioned, is a safe option for women in comparison to oncologic breast surgery without reconstruction. A systematic review on all OPS outcomes from 2020 documented crude overall survival rates of 95% and disease-free survival rates of 90% (24), which is comparable to rates seen in women undergoing BCS and mastectomies based on a review of SEER database outcomes (25).
Surgical complications
OPS is not unique from traditional breast surgery regarding the possible surgical complications that can arise. Commonly encountered complications include seroma, hematoma, wound infection, dehiscence, fat, skin, and NAC necrosis. OPS surgical complications rates documented included hematoma 2.5%, seroma 1%, wound infections 2%, nipple necrosis 0.4%, fat necrosis 3.3%, skin necrosis 2.2% (24). These rates closely reflect prevalence rates seen in women undergoing BCS without reconstruction (26,27). In a review of NSQIP data of volume-replacement OPS from 2005–2014 the authors found no difference in wound, infectious, or bleeding complications in comparison to BCS (28). OPS can still be offered in more aggressive variants of cancer as well, as postoperative complications in OPS in women undergoing neoadjuvant chemotherapy did not differ from rates of women who did not require preoperative chemotherapy (29). Even in comparison to non-oncologic cases, a recent retrospective review over 6 years found there was no difference between women undergoing standard reduction mammoplasty and oncoplastic resection with symmetry reduction (30). As with any surgical procedure, patient selection is first and foremost. Inappropriately chosen patients together with poorly managed expectations inherently leads to worse surgical outcomes.
Complications between displacement and replacement are similar with paucity of research comparing these surgical complication rates head-to-head. Previous literature has demonstrated complete flap loss of 2.5% in a cohort of 40 patients (20). In addition, volume replacement, does introduce a donor site location. Chest wall perforator flaps do create a large scar, as shown in Figure 17, however this is typically designed to be hidden within the bra line. Although in comparison to TDAP and LD flaps there has been minimal donor site morbidity with LICAP and AICAP flaps with enhanced patient satisfaction (31-33).
Positive margins
As discussed above, OPS is an effective oncologic surgical technique that has a lower positive margin rate compared to standard BCS owing largely to the generous partial mastectomy it allows. Nevertheless, when positive margins do occur, it is important to proceed with reoperation to decrease local recurrence and achieve disease control. Known risk factors for positive margins include higher-grade tumors, invasive lobular carcinoma, larger tumor size, and tumor stage (34).
Multiple involved margins may indicate that the disease burden is too great for breast conservation and the patient should instead be counseled to proceed to mastectomy with reconstruction for safe oncologic treatment. In fact, literature has shown that when positive margins are observed after BCS, completion mastectomy is more common (6.5%) in OPS than with PM (3.8%) (34). This may be secondary to the large initial resection resulting in small breasts that are unable to be tolerate further volume loss without sacrificing aesthetics.
However, if disease is seen at a single margin, it is feasible to perform a re-excision as the resection margins that should be easily identified by the surgical clips placed at the index operation. The authors recommend early return to the OR to allow for easier identification of the involved margin. If a mastectomy is required, the authors recommend waiting a minimum of 3 weeks to allow for perfusion of the NAC (2).
Tips and pearls
- Always assess pedicle perfusion for distal tip bright red bleeding. If any questions, consider ICG angiography for perfusion assessment.
- Bra or Ace wrap for compression post-operatively is advised.
- Drains typically are not needed for volume displacement oncoplastic surgery but may be considered for volume replacement oncoplastic surgery especially with flaps taken from the back that are rotated into the breast.
- In the setting of positive margins after oncoplastic surgery, discussion should be held in a tumor board to assess how many margins are positive. More than one positive margin may necessitate the need for a mastectomy as tumor burden may be too extensive for breast conservation.
Discussion
The main strengths of the aforementioned OPS techniques are that BCS remains possible with larger tumors in patients who were not otherwise candidates for breast conservation, and provides wider resection margins, all the while enhancing aesthetics (3,4). While refinement of surgical techniques is always a future direction, currently the most urgent improvement necessary is enhanced multidisciplinary interaction with earlier collaboration. Once a patient has been diagnosed with breast cancer or atypical pathology requiring resection, a plastic surgeon can be involved informally through tumor board discussions. If all necessary surgeons are present for tumor board discussions, it would empower plastic and reconstructive surgeons to suggest earlier referral when necessary, such as offering volume replacement techniques for majority of postoperative defects without staged surgery.
The advent of the oncoplastic breast surgeon has created controversy of who should be performing these surgeries. The difference lies between two surgical models, one being a single surgeon trained in both plastic surgery and breast oncology, versus a dual-surgeon standard of a breast oncology surgeon performing the resection independently with freedom to involve plastic and reconstructive surgeon when deemed necessary. Karamchandani et al. demonstrated no difference in positive margin rate or surgical complication rate when comparing single versus dual-surgeon model (22). Therefore, current literature does not demonstrate clinically significant differences between the two models, which is encouraging for ongoing collaboration between breast oncology experts and reconstructive experts. Regardless of surgeon model employed in the care of a breast cancer surgical treatment plan, widespread awareness of the variety of OPS techniques are vital to both specialties. Whomever makes the commitment to provide OPS techniques, previous literature is promising in that there is a quick learning curve. A retrospective review of over 200 patients over six years demonstrated competency in performing OPS after 24 procedures and mastery after 74 procedures (35), speaking to the necessity of training both breast and plastic surgeons in the surgical techniques that offer the best oncologic and aesthetic outcomes for eligible patients.
Conclusions
Innovations in breast reconstruction offer women treatment options that are both oncologically safe and aesthetically preferred. The rise in reconstructive procedures is changing how patients make decisions based on their diagnosis. With both volume displacement and volume replacement techniques, women of all breast sizes can achieve an aesthetic outcome without sacrificing the oncologic resection. The ultimate breast cancer surgical decision should be determined by the patient’s anatomy, patient’s personal preferences, tumor characteristics and presentation in a shared decision-making fashion with a multidisciplinary team. However, OPS should be emphasized to all qualifying patients and thus further education and adaptation of the techniques described in this review are necessary to provide comprehensive breast cancer care to all patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Oscar J. Manrique) for the series “Breast Reconstruction” published in Annals of Translational Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1536/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1536/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1536/coif). The series “Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. SMN received payments from Dilon Technologies, honoraria and support from American Society of Breast Surgeons, American College of Osteopathic Surgeons, and owns stock of Cassava Sciences. Abhishek Chatterjee received payments from 3M, Dilon Technologies, Molynlcke, DeRoyal, Hologic, support from American Society of Breast Surgeons and American College of Osteopathic Surgeons, and holds stock of Dilon. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board through Tufts Medical Center (No. STUDY00003501). Written informed consent was obtained from the patient for publication of this study and all accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chatterjee A, Gass J, Patel K, et al. A Consensus Definition and Classification System of Oncoplastic Surgery Developed by the American Society of Breast Surgeons. Ann Surg Oncol 2019;26:3436-44. [Crossref] [PubMed]
- Patel K, Bloom J, Nardello S, et al. An Oncoplastic Surgery Primer: Common Indications, Techniques, and Complications in Level 1 and 2 Volume Displacement Oncoplastic Surgery. Ann Surg Oncol 2019;26:3063-70. [Crossref] [PubMed]
- Campbell EJ, Romics L. Oncological safety and cosmetic outcomes in oncoplastic breast conservation surgery, a review of the best level of evidence literature. Breast Cancer (Dove Med Press) 2017;9:521-30. [Crossref] [PubMed]
- Rose M, Svensson H, Handler J, et al. Patient-reported outcome after oncoplastic breast surgery compared with conventional breast-conserving surgery in breast cancer. Breast Cancer Res Treat 2020;180:247-56. [Crossref] [PubMed]
- Char S, Bloom JA, Erlichman Z, et al. A comprehensive literature review of patient-reported outcome measures (PROMs) among common breast reconstruction options: What types of breast reconstruction score well? Breast J 2021;27:322-9. [Crossref] [PubMed]
- Char S, Bloom JA, Erlichman Z, et al. How Does Oncoplastic Surgery Compare with Standard Partial Mastectomy? A Systematic Review of Patient-Reported Outcomes. Plast Reconstr Surg 2022;150:950e-8e. [Crossref] [PubMed]
- Hall-Findlay EJ, Shestak KC. Breast Reduction. Plast Reconstr Surg 2015;136:531e-44e. [Crossref] [PubMed]
- Clough KB, Kaufman GJ, Nos C, et al. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol 2010;17:1375-91. [Crossref] [PubMed]
- Clough KB, Soussaline M, Campana F, et al. Mammoplasty combined with irradiation: conservative treatment of breast cancer localized in the lower quadrant. Ann Chir Plast Esthet 1990;35:117-22.
- Clough KB, Nos C, Salmon RJ, et al. Conservative treatment of breast cancers by mammaplasty and irradiation: a new approach to lower quadrant tumors. Plast Reconstr Surg 1995;96:363-70. [Crossref] [PubMed]
- Nos C, Fitoussi A, Bourgeois D, et al. Conservative treatment of lower pole breast cancers by bilateral mammoplasty and radiotherapy. Eur J Surg Oncol 1998;24:508-14. [Crossref] [PubMed]
- Anderson BO, Masetti R, Silverstein MJ. Oncoplastic approaches to partial mastectomy: an overview of volume-displacement techniques. Lancet Oncol 2005;6:145-57. [Crossref] [PubMed]
- Grisotti A. Immediate reconstruction after partial mastectomy. Oper Tech Plast Reconstr Surg 1994;1:1-12.
- Spear SL, Rottman SJ, Seiboth LA, et al. Breast reconstruction using a staged nipple-sparing mastectomy following mastopexy or reduction. Plast Reconstr Surg 2012;129:572-81. [Crossref] [PubMed]
- Lauritzen E, Bredgaard R, Bonde C, et al. Indocyanine green angiography in breast reconstruction: a narrative review. Ann Breast Surg 2022;6:6.
- Chatterjee A, Dayicioglu D, Khakpour N, et al. Oncoplastic Surgery: Keeping It Simple With 5 Essential Volume Displacement Techniques for Breast Conservation in a Patient With Moderate- to Large-Sized Breasts. Cancer Control 2017;24:1073274817729043. [Crossref] [PubMed]
- Chatterjee A, Yao M, Sekigami Y, et al. Practical Perspectives Regarding Patient Selection and Technical Considerations in Oncoplastic Surgery. Curr Breast Cancer Rep 2019;11:35-42.
- Clough KB, Ihrai T, Oden S, et al. Oncoplastic surgery for breast cancer based on tumour location and a quadrant-per-quadrant atlas. Br J Surg 2012;99:1389-95. [Crossref] [PubMed]
- Schaverien MV, Kuerer HM, Caudle AS, et al. Outcomes of Volume Replacement Oncoplastic Breast-Conserving Surgery Using Chest Wall Perforator Flaps: Comparison with Volume Displacement Oncoplastic Surgery and Total Breast Reconstruction. Plast Reconstr Surg 2020;146:14-27. [Crossref] [PubMed]
- Agrawal SK, Shakya SR, Nigam S, et al. Chest wall perforator flaps in partial breast reconstruction after breast conservation surgery: an additional oncoplastic surgical option. Ecancermedicalscience 2020;14:1073. [Crossref] [PubMed]
- Margenthaler JA, Dietz JR, Chatterjee A. The Landmark Series: Breast Conservation Trials (including oncoplastic breast surgery). Ann Surg Oncol 2021;28:2120-7. [Crossref] [PubMed]
- Karamchandani MM, De La Cruz Ku G, Gaffney KA, et al. Single Versus Dual Surgeon Approaches to Oncoplastic Surgery: A Comparison of Outcomes. J Surg Res 2023;283:1064-72. [Crossref] [PubMed]
- Mangialardi ML, Baldelli I, Salgarello M, et al. Breast Reconstruction Using the Lateral Thoracic, Thoracodorsal, and Intercostal Arteries Perforator Flaps. Plast Reconstr Surg Glob Open 2021;9:e3334. [Crossref] [PubMed]
- De La Cruz L, Blankenship SA, Chatterjee A, et al. Outcomes After Oncoplastic Breast-Conserving Surgery in Breast Cancer Patients: A Systematic Literature Review. Ann Surg Oncol 2016;23:3247-58. [Crossref] [PubMed]
- Yu P, Tang H, Zou Y, et al. Breast-Conserving Therapy Versus Mastectomy in Young Breast Cancer Patients Concerning Molecular Subtypes: A SEER Population-Based Study. Cancer Control 2020;27:1073274820976667. [Crossref] [PubMed]
- Tenofsky PL, Dowell P, Topalovski T, et al. Surgical, oncologic, and cosmetic differences between oncoplastic and nononcoplastic breast conserving surgery in breast cancer patients. Am J Surg 2014;207:398-402; discussion 402. [Crossref] [PubMed]
- Losken A, Dugal CS, Styblo TM, et al. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg 2014;72:145-9. [Crossref] [PubMed]
- Cil TD, Cordeiro E. Complications of Oncoplastic Breast Surgery Involving Soft Tissue Transfer Versus Breast-Conserving Surgery: An Analysis of the NSQIP Database. Ann Surg Oncol 2016;23:3266-71. [Crossref] [PubMed]
- Adamson K, Chavez-MacGregor M, Caudle A, et al. Neoadjuvant Chemotherapy does not Increase Complications in Oncoplastic Breast-Conserving Surgery. Ann Surg Oncol 2019;26:2730-7. [Crossref] [PubMed]
- Pawlak N, Karamchandani M, Wareham C, et al. Comparing oncoplastic breast reduction with immediate symmetry surgery to standard breast reduction surgery: Are postoperative complications worse? J Surg Oncol 2022;126:956-61. [Crossref] [PubMed]
- Hamdi M, Van Landuyt K, Monstrey S, et al. Pedicled perforator flaps in breast reconstruction: a new concept. Br J Plast Surg 2004;57:531-9. [Crossref] [PubMed]
- Hamdi M, De Frene B. Pedicled Perforator Flaps in Breast Reconstruction. Semin Plast Surg 2006;20:73-8.
- McCulley SJ, Schaverien MV, Tan VK, et al. Lateral thoracic artery perforator (LTAP) flap in partial breast reconstruction. J Plast Reconstr Aesthet Surg 2015;68:686-91. [Crossref] [PubMed]
- Clough KB, Gouveia PF, Benyahi D, et al. Positive Margins After Oncoplastic Surgery for Breast Cancer. Ann Surg Oncol 2015;22:4247-53. [Crossref] [PubMed]
- Karamchandani MM, Jonczyk MM, De La Cruz Ku G, et al. The adoption of oncoplastic surgery: Is there a learning curve? J Surg Oncol 2023;128:189-95. [Crossref] [PubMed]









