
Morbidity and mortality in Schaaf-Yang syndrome
Schaaf-Yang syndrome (SYS), first described in 2013, is caused by pathogenic variants in the paternal allele of MAGEL2 (1), one of the protein-coding genes in the Prader-Willi critical region on chromosome 15q11.2. Due to the phenotypical overlap with Prader-Willi syndrome (PWS), the disorder was initially termed “Prader-Willi-like syndrome”. As more patients were discovered, the phenotype caused by truncating pathogenic variants in MAGEL2 became increasingly distinct, and the name was changed to SYS (OMIM 615547). Today, more than 300 individuals with SYS have been identified (personal communication, Dr. Schaaf).
As SYS is a newly discovered neurodevelopmental disorder, the phenotypic spectrum is still being investigated and continues to expand. One of the most prevalent postnatal features of SYS is severe hypotonia, which contributes to respiratory and feeding difficulties (2). Developmental delay (DD) and intellectual disability (ID) become evident during childhood, with varying levels of severity (3). Hyperphagia, often seen in PWS, is also described for SYS, but appears to be less prevalent and has a later onset (4). A typical feature of SYS is autism spectrum disorder (ASD), which has been reported in three quarters of all patients (2).
So far, the literature has focussed on characterizing the clinical phenotype of children and adults with SYS, but little is known about the early symptoms and clinical complications during the neonatal period. Huang et al. now report on a large cohort of neonates, highlighting the specific morbidities and the high mortality of young individuals with SYS (5).
Critically ill infants with SYS
As part of the China Neonatal Genomes Project (CNGP), 42,257 hospitalised neonates were subjected to whole exome sequencing (WES). The goal of the CNPG is to expand the knowledge of single-gene diseases while improving the clinical use of WES.
Huang and colleagues have taken on the task of reviewing the CNGP data for patients with SYS. They identified 12 new individuals with SYS, representing the largest SYS patient cohort to be published since 2018 (2). Should the CNGP cohort be representative, their findings would imply that 1 in 3,500 hospitalized neonates suffers from SYS.
Because symptoms of SYS vary in prevalence and severity, an association between specific genomic variant and clinical phenotype is being discussed (2). Over the last decade, considerable effort has been made to understand this association. Huang and colleagues set out to further improve our understanding of SYS, focussing on neonatal morbidity and mortality both in their own patient cohort and the entire existing literature.
Pathogenic variants in MAGEL2 (NM_019066.5) are not evenly spread throughout the gene (Figure 1). Most of them are found in the middle of the gene, especially in the c.1990 to c.1996 region. This mutational hotspot consists of seven cytosines and 42% of all SYS patients have a variant within this hotspot (5). It is presumed that this sequence presents a challenge for the DNA polymerase, potentially leading to polymerase slippage. Cytosine duplications (c.1996dupC) account for almost 90% of the genetic variation in this region (5). The remaining 10% are caused by deletions (c.1996delC) (5). Huang et al. propose c.1912 as a possible second hotspot with ten reported cases all exhibiting the same pathogenic variant (c.1912C>T), conceivably related to the CpG dinucleotide at that site.
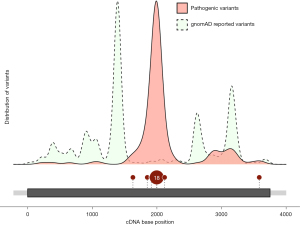
Apart from c.1996 and c.1912 most other variations are considered private mutations. Huang et al. identified six novel pathogenic variants in MAGEL2. As the case numbers for these variants are still very low, it is hard to conclude any genotype-phenotype relationships decisively.
The comprehensive summary by Huang et al. contains 23 cases of premature death within the SYS cohort (mortality 17%). Most of them (80%) died within the first month after birth. They identified c.1996dupC and c.1996delC as the two variants almost exclusively responsible for early death. The mortality is 24% or 100% for individuals carrying a c.1996 duplication or deletion, respectively. While the duplication mainly entails death by respiratory failure, the deletion has a far more severe phenotype, with individuals often not surviving gestational week 24. For deletions, the main cause of death is fetal akinesia. The authors report no differences in phenotype with regards to sex or ethnicity.
Interestingly, although SYS is caused by de novo mutations in approximately 50% of all cases, almost all deceased individuals revealed a paternally inherited genetic variant (3).
Mutations in the hotspot region (c.1990–1996) also result in a more severe postnatal phenotype. For instance, individuals with the c.1996dupC mutation have an average intelligence quotient (IQ) of 14, whereas the average for all other mutations combined was 53 (2). Whole gene deletions, on the other hand, have been reported to manifest a very mild phenotype or no discernible phenotype at all (4). A possible explanation would be that paternal whole gene deletions result in leaky expression from the normally imprinted maternal allele (4). A dominant negative (or neomorphic) effect by the truncated protein is also being discussed. This could explain why shared phenotypic features of SYS and PWS (for example ID and ASD) are more pronounced in SYS.
The most frequent and severe phenotypes are caused by pathogenic variants causing changes to the amino acid sequence in the middle of the protein (Figure 1). Of the 12 infants that were found in Huang’s study, four did not survive. Two had a duplication in the c.1990–1996 region and died within the first month. The third lethal case carried a point mutation at position c.1912. Notably, the fourth lethal variant was located far from the middle of the gene (c.3583delA). The respective individual did not only deviate from the norm in terms of mutation location, but also regarding clinical phenotype, with hemophagocytic syndrome as the reported cause of death. Whether this is causally related to SYS or should rather be considered an independent cause of death, remains subject to future investigation of larger patient cohorts.
The paper at hand suggests that some genotype-phenotype correlation exists for pathogenic variants in MAGEL2 and SYS, including morbidity and mortality. Huang’s study established respiratory failure (in live-born patients) and fetal akinesia (in foetuses) as the main causes of death. In addition, they observed that patients with a c.1996dupC mutation require mechanical ventilation twice as often—a finding that may provide anticipatory guidance to future affected families.
Diagnostic testing, surveillance, and treatment intervention in the genomic era of medicine
Understanding genomic variants is important for predicting disease progression and treatment intervention. WES as a diagnostic tool has been available since 2011 and is widely adopted for clinical use today. Trio exome sequencing further improves the diagnostic yield and simplifies the detection of de novo variants. In 2018, Tong et al. showed that trio exome sequencing can avoid unnecessary examinations, enhance diagnostic efficiency, and provide a more precise diagnosis in paediatric intensive care units (6).
With the help of WES, more pathogenic variants in MAGEL2 are being discovered, leading to the rapid growth of the SYS cohort. This will ultimately help scientists and physicians to develop a personalised treatment intervention plan.
Anticipatory guidance and a structured plan of recommended examinations provide important parts of care for individuals with rare, multi-system disorders. For detailed recommendations regarding SYS, see Schaaf & Marbach (3). Huang et al. also identified mild cardiac malformations as a common finding in their SYS cohort (92%). This may suggest that echocardiography should be considered as a recommended investigation at time of diagnosis for all individuals with SYS.
Recombinant growth hormone (rGH) as a possible therapeutic has been used in the treatment of PWS for several years. First results from SYS cohorts suggest that rGH should also be considered for this clinical entity (7). It has been shown that GH has a positive effect on body height and body composition (7). Nonetheless, more studies are needed to evaluate the benefit and to better anticipate possible adverse effects. Several factors, including respiratory impairment and apnea, have been suggested as contraindications for GH treatment in PWS. Giving GH to critically ill SYS infants struggling with respiratory problems could lead to lymphoid tissue growth [due to increased insulin like growth factor 1 (IGF-1) levels], thereby further impeding their breathing (8).
Treatment of a Magel2 knockout (KO) mouse strain with oxytocin rescued suckling deficits, as well as nearly all the deficits in social behaviour, and could therefore be considered as a potential future pharmaceutic for SYS, even though clinical data is not available yet (9).
Reversal of maternal imprinting of the intact MAGEL2 allele may represent the most elegant therapeutic option. This attempt is currently pursued in research groups for PWS. Considering the analogy in the pathogenesis of SYS and PWS, scientific achievements in Prader-Willi research might also aid SYS patients.
Acknowledgments
We thank Moritz Wimmer, Jannis Bücking, Laura Dötsch and Henning Fröhlich for their support in writing this editorial.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Translational Medicine. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1718/coif). C.P.S. reports that he received funding from the Foundation of Prader-Willi Research, the German Ministry of Health and the German Ministry of Research and Education. He received royalties for a genetics textbook by Springer. Payment for expert testimony (provided to Hayes solicitors) and payment for his work on a ClinGen working group is paid to his institution. He is a member of the Scientific Program Committee of ESHG. His travel has been supported by ESHG and the German Society of Human Genetics. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schaaf CP, Gonzalez-Garay ML, Xia F, et al. Truncating mutations of MAGEL2 cause Prader-Willi phenotypes and autism. Nat Genet 2013;45:1405-8. [Crossref] [PubMed]
- McCarthy J, Lupo PJ, Kovar E, et al. Schaaf-Yang syndrome overview: Report of 78 individuals. Am J Med Genet A 2018;176:2564-74. [Crossref] [PubMed]
- Schaaf CP, Marbach F. Schaaf-Yang Syndrome. 2021 Feb 11. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews®. Seattle (WA): University of Washington, Seattle; 1993-2023.
- Fountain MD, Aten E, Cho MT, et al. The phenotypic spectrum of Schaaf-Yang syndrome: 18 new affected individuals from 14 families. Genet Med 2017;19:45-52. Erratum in: Genet Med 2016;18:1066. [Crossref] [PubMed]
- Huang Z, Lu W, Zhang P, et al. Early onset critically ill infants with Schaaf-Yang syndrome: a retrospective study from the China neonatal genomes project and literature review. Ann Transl Med 2023;11:312. [Crossref] [PubMed]
- Tong W, Wang Y, Lu Y, et al. Whole-exome Sequencing Helps the Diagnosis and Treatment in Children with Neurodevelopmental Delay Accompanied Unexplained Dyspnea. Sci Rep 2018;8:5214. [Crossref] [PubMed]
- Hebach NR, Caro P, Martin-Giacalone BA, et al. A retrospective analysis of growth hormone therapy in children with Schaaf-Yang syndrome. Clin Genet 2021;100:298-307. [Crossref] [PubMed]
- Deal CL, Tony M, Höybye C, et al. GrowthHormone Research Society workshop summary: consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J Clin Endocrinol Metab 2013;98:E1072-87. [Crossref] [PubMed]
- Althammer F, Muscatelli F, Grinevich V, et al. Oxytocin-based therapies for treatment of Prader-Willi and Schaaf-Yang syndromes: evidence, disappointments, and future research strategies. Transl Psychiatry 2022;12:318. [Crossref] [PubMed]


