
Two-stage free latissimus dorsi flap for the management of a voluminous enterocutaneous fistula in a patient with Ehlers-Danlos syndrome: a case report
Highlight box
Key findings
• Abdominal wall reconstruction is challenging and may require two-stage free flaps in large full thickness defects.
What is known and what is new?
• Ehlers-Danlos syndrome is a rare genetic disorder that affects the quality of connective tissues, making reconstructive surgery arduous for affected patients. Additional precautions should be taken for microsurgical transfers.
• The two-stage free flap transfer strategy helps address any flap complication without jeopardizing the abdominal reconstruction, preventing potentially lethal complications due to the exposure of viscera and vital organs in case of flap loss.
What is the implication, and what should change now?
• This approach allowed for optimal tissue coverage and full abdominal restoration while minimizing the risk of complications.
• A two-stage procedure should be considered whenever the reconstruction endangers the functional or vital prognosis of the patient.
Introduction
Background
Reconstruction of the abdominal wall is a challenging task. While most abdominal wall defects can be addressed with locoregional options (1), complex cases with extensive exposure to intraperitoneal organs may require free flaps to achieve optimal tissue coverage and full abdominal restoration (2-4). However, free tissue transfers are not deprived of complications, and even the most experienced microsurgeons face the risk of vascular thrombosis and flap loss. Flap failure has been reported to occur in up to 8.5% of cases in trunk and extremity reconstruction (5). In full-thickness abdominal wall reconstruction cases, free flap failure is not an option as it may lead to vital organ exposure and potentially lethal complications like infection, bleeding, organ failure, and loss of fluids (6).
Rationale and knowledge gap
Ehlers-Danlos syndrome (EDS) is a rare genetic disorder (with a prevalence ranging from 1/50,000 to 1/200,000) that affects the chain of type III procollagen, a major protein in vessel walls and hollow organs (7). It induces an abnormal structure and collagen production that seriously impacts connective tissue quality. The vessels and skin are fragile, and the wound-healing process is altered. Therefore, reconstructive surgery can be arduous in patients with EDS, and additional precautions should be taken for microsurgical transfers.
Objective
This report uses a two-stage free flap transfer strategy to describe the management of a voluminous full-thickness abdominal wall defect in a patient with vascular EDS. We present this article in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-23-826/rc).
Case presentation
This case relates to the reconstruction of an abdominal wall defect in a 27-year-old male patient who is a heavy active smoker (>20 cigarettes/day) and suffers from vascular EDS. During childhood, the patient exhibited a certain degree of cutaneous fragility, with thin, transparent skin and particularly visible veins. He also experienced hematomas after minimal trauma. After several digestive complications, including peritonitis, intestinal perforation, hemoperitoneum, and years of diagnostic uncertainty, the patient was referred to the French reference center for rare vascular diseases. Genetic analysis of the COL3A1 gene confirmed the diagnosis. There were no other affected relatives in the patient’s family.
The patient presented with a significant gastrointestinal history, starting with spontaneous peritonitis 10 years ago, which resulted in a 10 cm small bowel resection. Two years later, the patient suffered from a second peritonitis after a spontaneous sigmoid perforation. The treatment initially consisted of a sigmoid resection with colorectal anastomosis and protective ileostomy. A subtotal colectomy with ileorectal anastomosis was completed 5 months later.
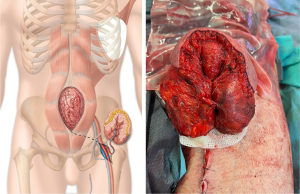
Seven years later, a new spontaneous jejunal perforation was treated by segmental bowel resection with double end stoma. One stoma was placed in the right hypochondrium, and the other in the left flank. The postoperative course of this procedure was marked by an evisceration complicated by a complex enterocutaneous fistula (Figure 1) with multiple open intestinal loops in the middle of the abdominal wall. Evisceration is a frequent and typical complication in patients with EDS. The patient underwent extensive intestinal resection (two segmental resections of the small intestine and colon) and had a significantly reduced intestinal absorption surface, making enteral feeding impossible. The patient relied exclusively on parenteral nutrition. Considering the high surgical risk due to the patient’s condition, the large full thickness abdominal defect, and the lack of locoregional reconstructive options, a two-stage free flap transfer was decided to cover the defect after treatment of the enterocutaneous fistula.

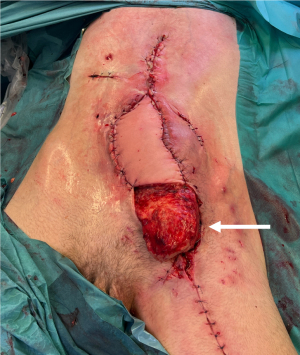
Surgery was performed one year later by the first author (AGL). First, a left latissimus dorsi free flap with a 10 cm × 20 cm skin paddle was harvested (8) and transferred to the left inguinal groin (Figure 2). Arterial and venous anastomoses were performed end-to-side to the femoral artery with interrupted sutures of Ethilon 8/0 (Ethicon Inc, Somerville, NJ, USA) and end-to-end on the greater saphenous vein with a 3-mm Microvascular Anastomotic Coupler Device (Myovatec Surgical Systems, New Delhi, India). The flap was then folded and sutured directly onto itself. Flap survival was monitored with the Licox® PtO2 system (9) and clinically. Low molecular weight heparin was used for postoperative thromboprophylaxis. No major surgical or medical event occurred in between the two stages of reconstruction.
Eight days after, the second procedure was performed in a two-team approach. Digestive surgeons (ER, MK) first proceeded to full abdominal viscerolysis, fistula removal, and intestinal continuity restoration with an end-to-end jejunoileal anastomosis. Then, the plastic surgery team unfolded the latissimus dorsi free flap and moved it cephalically to cover the abdominal full-thickness defect. The upper edge was closed in a V-Y fashion, and the muscle surrounding the pedicle was left open to heal in secondary intention to prevent excessive tension on the wound (Figure 3). Resumption of intestinal transit started at postoperative day (POD) 3. At POD 9, leakage of digestive fluid under the flap demonstrated a recurrence of the enterocutaneous fistula. We initiated treatment with somatostatin, antibiotics, negative pressure therapy and nasogastric tube aspiration. The dosage of somatostatin was 6 mg per day. After 1 week of treatment, the output from the enterocutaneous fistula decreased by one-third. Six weeks later, the fistula had completely healed (Figure 4). Follow-up at 6 months showed complete wound healing with no further complication (Figure 5).
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Complex clinical scenarios in reconstructive surgery may require a two-stage strategy, especially when the patient presents severe comorbidities with a high risk of complications. To our knowledge, this case is the first to report the management of a full-thickness defect with voluminous enterocutaneous fistula in a multi-operated patient suffering from EDS. Every surgery performed on Ehlers-Danlos patients is challenging because of the impact of this disease on arterial dissection, wound healing, tissue quality (7), and risk of excessive bleeding (10). In patients with vascular EDS, it is recommended to avoid as much as possible any operative procedure. However, this patient was dependent on parenteral nutrition. The enterocutaneous fistula was highly productive, leading to significant weight loss, as parenteral feeding was insufficient. Restoring gastrointestinal continuity through surgery was therefore essential. It was a life-saving procedure. A one-stage procedure was considered too risky. Ensuring the viability of the flap was a critical requirement for covering the enterocutaneous fistula. Necrosis of the flap after restoring gastrointestinal continuity would have resulted in the patient’s death, as evisceration would occur without the possibility of subsequent coverage.
We believe such patients are good candidates for a staged reconstruction that Servant et al. first described in the chest and abdominal wall reconstruction (4). The main goal of the two-stage strategy is to ensure the reliability of the microsurgical transfer by closely monitoring the flap viability before the visceral surgery and closing of the abdomen. Therefore, any complication on the flap could be addressed without jeopardizing the abdominal reconstruction, thus preventing potentially lethal complications due to the exposure of viscera and vital organs in case of flap loss. In addition, although this method requires two separate surgeries, it reduces the operative time drastically, which is particularly beneficial in fragile patients. In our case, maximizing the chances of success was crucial, considering the impact of EDS on the vessels, skin quality, and wound healing.
Given the abdominal wall’s large central and full-thickness defect in this case, the reconstruction could not be addressed with conventional locoregional options. Component separation was inadequate as it is limited to managing smaller midline defects and requires the integrity of the abdominis rectus muscles (11). Regional pedicled flaps, including the tensor fascia latae, anterolateral of the thigh (ALT), or rectus femoris would not offer sufficient pedicle length to reach the cephalic edge of the defect, and the rectus abdominal flap was unusable. Although the pedicled ALT flap is usually not considered for the reconstruction of the upper umbilical abdominal wall, several authors reported the possibility to reach the epigastrium by tunnelling the flap pedicle beneath the rectus femoris and sartorius muscles (12,13). However, in an anatomical study assessing the arc of rotation of 60 ALT flaps, Tamai et al. showed that less than one-third of the pedicled ALT flaps can reach the umbilicus (14). Suppose the ALT flap in a pedicled fashion is to be considered for lower abdominal wall reconstruction. In that case, it does not offer a reproducible and reliable option for defects extending above the umbilicus. Full-thickness abdominal defects need to reconstruct deep muscular and superficial fasciocutaneous planes to strengthen the abdominal wall and avoid further dehiscence, making the free latissimus dorsi myocutaneous flap the most suitable option in this situation (8,15).
After Servant et al. initial description of the two-stage free flap transfer technique (4), only Fejjal et al. reported a case of abdominal reconstruction with a two-stage latissimus dorsi free flap after resection of a dermatofibrosarcoma, but the peritoneal plane was left intact after oncological resection (16). Other teams developed two-step procedures for abdominal wall reconstruction, including the first step of NPT followed by closure with surrounding skin or distant free flaps (17). However, this strategy has not shown to be effective in managing enterocutaneous fistula, as Fujisawa et al. reported an intestinal perforation during the NPT step that required additional surgery (18). To prevent such complications, we advocate using a free flap coverage of the abdominal defect as a primary reconstructive strategy whenever possible and keeping NPT as a salvage option if an enterocutaneous fistula reoccurs.
Conclusions
This report suggests the two-stage free flap transfer strategy to manage a voluminous full-thickness abdominal wall defect in a patient with vascular EDS. This approach allowed for optimal tissue coverage and full abdominal restoration while minimizing the risk of complications.
Acknowledgments
The authors thank Charles Boistier (from Coworking space The 4th, Rennes, France) for designing Figures 1,2,5, and Pr. Laurent Lantieri and Dr. Pierre Tawa (both from Department of Plastic and Reconstructive Surgery, European Hospital Georges Pompidou, Public Assistance – Hospitals of Paris, University of Paris, Paris, France) for their valuable advice on this case.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-826/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-826/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-826/coif). AGL serves as an unpaid editorial board member of Annals of Translational Medicine from December 2022 to November 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rohrich RJ, Lowe JB, Hackney FL, et al. An algorithm for abdominal wall reconstruction. Plast Reconstr Surg 2000;105:202-16; quiz 217. [Crossref] [PubMed]
- Wong CH, Lin CH, Fu B, et al. Reconstruction of complex abdominal wall defects with free flaps: indications and clinical outcome. Plast Reconstr Surg 2009;124:500-9. [Crossref] [PubMed]
- Mathes SJ, Steinwald PM, Foster RD, et al. Complex abdominal wall reconstruction: a comparison of flap and mesh closure. Ann Surg 2000;232:586-96. [Crossref] [PubMed]
- Servant JM, Arnault E, Revol M, et al. Reconstruction of large thoracoabdominal defects using two-stage free tissue transfers and prosthetic materials. J Plast Reconstr Aesthet Surg 2006;59:360-5. [Crossref] [PubMed]
- Kwok AC, Agarwal JP. An analysis of free flap failure using the ACS NSQIP database. Does flap site and flap type matter? Microsurgery 2017;37:531-8. [Crossref] [PubMed]
- Guillen B, Cassaro S. Traumatic Open Abdomen. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan.
- Byers PH, Belmont J, Black J, et al. Diagnosis, natural history, and management in vascular Ehlers-Danlos syndrome. Am J Med Genet C Semin Med Genet 2017;175:40-7. [Crossref] [PubMed]
- Vincent A, Hohman MH. Latissimus Dorsi Myocutaneous Flap. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Mar 1.
- Kamolz LP, Giovanoli P, Haslik W, et al. Continuous free-flap monitoring with tissue-oxygen measurements: three-year experience. J Reconstr Microsurg 2002;18:487-91; discussion 492-3. [Crossref] [PubMed]
- Wiesmann T, Castori M, Malfait F, et al. Recommendations for anesthesia and perioperative management in patients with Ehlers-Danlos syndrome(s). Orphanet J Rare Dis 2014;9:109. [Crossref] [PubMed]
- Ramirez OM. Inception and evolution of the components separation technique: personal recollections. Clin Plast Surg 2006;33:241-6. vi. [Crossref] [PubMed]
- Fernandez-Alvarez JA, Barrera-Pulido F, Lagares-Borrego A, et al. Coverage of supraumbilical abdominal wall defects: The tunnelled-pedicled ALT technique. Microsurgery 2017;37:119-27. [Crossref] [PubMed]
- Ting J, Trotter D, Grinsell D. A pedicled anterolateral thigh (ALT) flap for reconstruction of the epigastrium. Case report. J Plast Reconstr Aesthet Surg 2010;63:e65-7. [Crossref] [PubMed]
- Tamai M, Nagasao T, Miki T, et al. Rotation arc of pedicled anterolateral thigh flap for abdominal wall reconstruction: How far can it reach? J Plast Reconstr Aesthet Surg 2015;68:1417-24. [Crossref] [PubMed]
- Bodin F, Dissaux C, Romain B, et al. Complex abdominal wall defect reconstruction using a latissimus dorsi free flap with mesh after malignant tumor resection. Microsurgery 2017;37:38-43. [Crossref] [PubMed]
- Fejjal N, Hafidi J, Belmir R, et al. Two-stage free latissimus dorsi flap: a safe strategy for reconstruction of large defects of the abdominal wall. J Plast Surg Hand Surg 2013;47:232-3. [Crossref] [PubMed]
- Al Zarouni M. Abdominal Wall Reconstruction with the Two-step Technique: Procedure Optimization and Three-year Follow-up in 26 Surgeries. Plast Reconstr Surg Glob Open 2019;7:e2182. [Crossref] [PubMed]
- Fujisawa K, Kitatsuji M, Yamamoto Y. Open Abdomen Negative Pressure Device Applied for Two-stage Closure of Enterocutaneous Fistula. Plast Reconstr Surg Glob Open 2021;9:e3369. [Crossref] [PubMed]




