Sofosbuvir and velpatasvir: a stellar option for patients with decompensated hepatitis C virus (HCV) cirrhosis
The treatment of hepatitis C virus (HCV) in patients with advanced liver disease has consistently been associated with higher failure rates and increase rates of adverse events (1,2). The advent of highly effective direct acting antiviral (DAA) therapy in the management of these difficult-to-treat chronic hepatitis C subpopulation has significantly improved sustained virologic response (SVR, absence of HCV RNA in plasma 12 weeks after cessation of therapy), while maintaining an excellent safety profile (3-5). Achieving SVR has resulted in reversal of the degree of fibrosis, reduction in the risk of hepatocellular carcinoma, lowers the rate of hepatic decompensation, and perhaps curtails the need for liver transplantation (2,6,7). However, lack of availability of a pan-genotypic regimen has limited our ability to expand HCV treatment beyond patients infected with genotype 1 thus far. In particular, there are limited options to treat patients with HCV genotype 3, especially with decompensated liver disease (8).
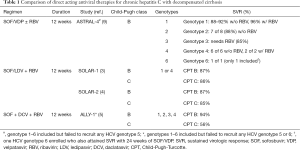
Recently, Dr. Michael P. Curry and the ASTRAL-4 investigators conducted a study using a once daily fixed dose combination regimen containing a widely used nucleotide analogue NS5B polymerase inhibitor, sofosbuvir, and a more recently FDA-approved novel NS5A inhibitor, velpatasvir, with or without the use of ribavirin to treat chronic hepatitis C adult patients with decompensated cirrhosis (9). Both sofosbuvir and velpatasvir are pan-genotypic agents, that when co-administered, have been proven to be highly efficacious in earlier ASTRAL-1, ASTRAL-2, and ASTRAL-3 trials that involved both patients without cirrhosis and those with compensated cirrhosis, with an overall SVR rate of 95–99% (10,11). The ASTRAL-4 study is a phase 3, open label, randomized, multicenter trial that included a total of 267 chronically HCV infected patients with Child-Pugh-Turcotte (CPT) class B. Those with prior use of NS5A or NS5B inhibitors, very low platelets (30,000 per µL or less), significant renal dysfunction (<50 mL/min) and post-transplant status at screening were excluded in this study. It should be noted that at baseline, there were a majority of whites (88–91%) and males (63–76%); and more than three-fourths (76–79%) of the subjects were infected with HCV genotype 1. Only one patient had HCV genotype 6 and none had HCV genotype 5. The overall SVR rate using 12 weeks of sofosbuvir and velpatasvir was at 83%, while treatment with 24 weeks of sofosbuvir-velpatasvir cured 86% of subjects. When weight based ribavirin was added to the 12-week sofosbuvir-velpatasvir regimen, 94% of patients attained SVR. The differences between groups were not statistically significant. The combined overall SVR rate using sofosbuvir-velpatasvir with or without ribavirin was high at 88% (9).
The current AASLD/IDSA guidelines include two 12-week HCV regimens recommended and approved for use in patients with decompensated cirrhosis: sofosbuvir-ledipasvir with ribavirin for HCV genotypes 1 or 4; and sofosbuvir, daclatasvir with ribavirin for HCV genotypes 1, 2, 3, and 4 (12). Both recommended regimens involve a NS5B polymerase inhibitor (sofosbuvir), a NS5A inhibitor (either ledipasvir or the pan-genotypic daclatasvir), and the guanosine analogue ribavirin. The data supporting the sofosbuvir-ledipasvir plus ribavirin recommendation comes from SOLAR-1 study conducted in the U.S., and SOLAR-2 trial conducted in Europe, Australia, New Zealand, and Canada (3,4). SOLAR-1 study, which included a total of 108 HCV genotype 1 or 4 subjects, resulted in a combined SVR in 87% of patients treated for 12 weeks, as compared to 89% when treated for 24 weeks among patients with CPT class B HCV liver cirrhosis, that were treated pre-transplant (3). Similar cure rates were seen when treating subjects with more advanced CPT class C disease—SVR of 86% and 87%, for those treated for 12 and 24 weeks, respectively (3). In the SOLAR-2 trial, among decompensated cirrhotic patients with HCV genotype 1 and CPT class B, 87% achieved SVR with 12 weeks therapy while SVR was 96% if treated for 24 weeks (4). Likewise, a 12-week combination therapy with sofosbuvir, daclatasvir, and ribavirin can be used to treat HCV-infected patients of any genotype with decompensated liver disease, based on ALLY-1 trial results whereby 94% of CPT class B subjects achieved SVR (12). In both currently recommended regimens, addition of ribavirin is recommended as it consistently resulted in numerically superior SVR (3-5,7,13).
Similarly, in ASTRAL-4, there is a numerical higher SVR with addition of ribavirin to the sofosbuvir-velpatasvir regimen (overall SVR of 94% as opposed to 83–86%). Although, this was not statistically significant as this study was not powered to detect significant differences between the three treatment arms (9). This higher rate of cure with addition of ribavirin is statistically significant among patients with HCV genotype 3. In this subpopulation, HCV genotype 3 subjects achieved 85% SVR with sofosbuvir-velpatasvir and ribavirin, compared to 50% in the non-ribavirin groups (9). This needs to be further studied and validated in prospective clinical trials. On the other hand, all 6 HCV genotype 4 and 7 of 8 HCV genotype 2 subjects, as well as a high proportion of HCV genotype 1 (88–92%) attained SVR even in the absence of ribavirin (9). Larger trials will be needed to demonstrate if a ribavirin-free regimen will be adequate to achieve a comparably high SVR. In the meantime, sofosbuvir-ledipasvir with ribavirin is recommended (12) to maximize the chance to attain cure, given that decompensated HCV cirrhotic patients have a high risk of dying from complications of chronic HCV infection.
Sofosbuvir, velpatasvir with or without ribavirin regimens appear to be well tolerated by HCV patients despite decompensated cirrhosis. Only 9 of the total 267 subjects discontinued the assigned regimen due to adverse reactions (9). The three most common side effects are fatigue (23–39%), nausea (20–25%), and headaches (19–26%) which is no different from other regimens used to treat decompensated cirrhotic subjects (3-5,9). As expected, there were significantly more cases of anemia (23% vs. 8–9% if without ribavirin) and hyperbilirubinemia (as a function of hemolysis) in the ribavirin-containing treatment arm (9). Though there were a total of nine deaths during the course of the study, three from each group, all deaths occurred after discontinuation of therapy, and none of which were considered to be related to HCV therapy by the investigators (9). Majority of deaths were attributed to complications of end-stage liver disease (such as liver failure and sepsis) (9).
Successful treatment with the use of combination DAAs for chronic HCV infection with advanced liver disease has consistently shown early improvements in hepatic function, as evidenced by improvement in both CPT and Model for End Stage Liver Disease (MELD) scores (3-5,9). With the use of sofosbuvir-ledipasvir and ribavirin, there was a 67–72% improvement of MELD score with those decompensated HCV cirrhotic patients who attained SVR (3,4). A 47% better MELD score found in those cured with sofosbuvir, daclatasvir, and ribavirin (5). These early improvement in hepatic function was also seen in the ASTRAL-4 trial. Forty seven (47%) percent of those who achieved SVR with sofosbuvir-velpatasvir with or without ribavirin had improvement in their CPT scores—mostly as a function of decrease in bilirubin and increase in albumin levels, but 11% had worse scores (9). While 51% of those with lower baseline MELD scores showed improvement in liver function, a larger proportion (81%) of patients with more severe (MELD greater than or equal to 15) hepatic dysfunction at baseline improved (9). Nevertheless, the impact of lowering MELD scores on wait time for organ availability of patients with decompensated cirrhosis remains unclear.
In June 28, 2016, the fixed dose combination pill containing sofosbuvir and velpatasvir (Epclusa®, Gilead, USA) was approved by FDA for the treatment of chronic hepatitis C (14). It has since been added to the AASLD/IDSA guidelines as a treatment option for those with decompensated HCV cirrhosis (12).
In conclusion, ASTRAL-4 study was able to demonstrate that use of sofosbuvir and velpatasvir with or without ribavirin is comparably safe and effective in HCV adult patients with advanced liver disease, and thus adds an excellent option in the treatment of these more challenging to treat chronic hepatitis C subgroup (see Table 1 for tabulated comparison with other approved HCV regimens). Except with HCV genotype 3, combination therapy with sofosbuvir and velpatasvir retains an acceptably high efficacy rate. However, several questions remain unanswered. As ASTRAL-4 enrolled only adult HCV patients with moderate liver decompensation (CPT B), future studies are need to see if the same benefit afforded to CPT B patients can be generalized to more severely liver impaired HCV population (CPT C). Lastly, though hepatic improvement was rapidly seen with achieving SVR, long-term follow-up of these patients is crucial to be able to appreciate if successful treatment of patients with decompensated HCV cirrhosis with sofosbuvir-velpatasvir with or without ribavirin would translate into long-term survival benefit and improvement in quality of life.
Full table
Acknowledgements
None.
Footnote
Provenance: This is a Guest Editorial commissioned by Managing Editor Bing Gu, MD (Department of Laboratory Medicine, the Affiliated Hospital of Xuzhou Medical University, Xuzhou, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lens S, Mariño Z, Forns X. Efficacy of new direct acting antivirals in transplant recipients and patients with advanced disease. Dig Liver Dis 2014;46 Suppl 5:S197-205. [Crossref] [PubMed]
- Bunchorntavakul C, Reddy KR. Treat chronic hepatitis C virus infection in decompensated cirrhosis - pre- or post-liver transplantation? the ironic conundrum in the era of effective and well-tolerated therapy. J Viral Hepat 2016;23:408-18. [Crossref] [PubMed]
- Charlton M, Everson GT, Flamm SL, et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology 2015;149:649-59. [Crossref] [PubMed]
- Manns M, Samuel D, Gane EJ, et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis 2016;16:685-97. [Crossref] [PubMed]
- Poordad F, Schiff ER, Vierling JM, et al. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology 2016;63:1493-505. [Crossref] [PubMed]
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol 2014;61:S58-68. [Crossref] [PubMed]
- Foster GR, Irving WL, Cheung MC, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol 2016;64:1224-31. [Crossref] [PubMed]
- Kattakuzhy S, Levy R, Rosenthal E, et al. Hepatitis C genotype 3 disease. Hepatol Int 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Curry MP, O'Leary JG, Bzowej N, et al. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. N Engl J Med 2015;373:2618-28. [Crossref] [PubMed]
- Feld JJ, Jacobson IM, Hézode C, et al. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N Engl J Med 2015;373:2599-607. [Crossref] [PubMed]
- Foster GR, Afdhal N, Roberts SK, et al. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N Engl J Med 2015;373:2608-17. [Crossref] [PubMed]
- AASLD. HCV Guidance: recommendations for testing, managing, and treating hepatitis C. Unique patient populations: patients with decompensated cirrhosis. Updated July 6, 2016. Cited July 20, 2016. Available online: http://hcvguidelines.org/full-report/unique-patient-populations-patients-decompensated-cirrhosis
- Gane EJ, Hyland RH, An D, et al. Sofosbuvir/ledipasvir fixed dose combination is safe and effective in difficult-to-treat populations including genotype-3 patients, decompensated genotype-1 patients, and genotype-1 patients with prior sofosbuvir treatment experience. J Hepatol 2014;60:S3-4. [Crossref]
- FDA News Release. FDA approves Epclusa for treatment of chronic Hepatitis C virus infection. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm508915.htm


