
A treatment algorithm for reconstruction of soft tissue defects in the hand: a narrative review
Introduction
Soft tissue defects of the hand commonly arise from trauma, burns, infections or oncological resections (1). Treatment considerations of hand soft tissue defects have a few distinctive features which are unique. Dorsal skin is loose, whereas palmar glabrous skin is thicker and less mobile as it is anchored by the palmar fascial septations to resist shear stress (2). Critical nerves, vessels and tendons lie just beneath the skin. The hand is richly innervated that allows appropriate response to stimuli and higher order functional tasks. Thus, sensation is crucial for a functional hand and should be considered especially in defects of the thumb and fingers.
Reconstructive planning depends obviously on the extent of the injury, but patient and surgeon factors should not be overlooked (3). A clean wound bed prior to reconstruction is essential. The reconstructive elevator, instead of a ladder, concept is highly applicable to hand soft tissue reconstruction as the simplest option for coverage might not be the best option in terms of form and function (4). Achieving defect coverage is not sufficient in the modern era of reconstructive surgery and the restoration of form and function is critical in a highly visible area of the body, such as the hand, opposed to the lower limb.
In this narrative review, we focus on soft tissue reconstruction in the hand and digits. We review the literature and propose treatment algorithms to ease preoperative planning of hand defects. We also illustrate a general approach to hand soft tissue reconstruction. We present this article in accordance with the Narrative Review reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-23-201/rc).
Methods
The PubMed and Cochrane Databases were used for the literature search. Randomized controlled trials, systematic reviews and review articles were searched specifically from year 1953 up till end of November 2022. Table 1 summarizes the used search strategy. Table 2 confers a detailed tracking sheet. We included articles written in English with free or institutional full-text availability for this narrative review. The final article selection process to screen the relevant articles for this topic was conducted by the authors.
Table 1
| Items | Specification |
|---|---|
| Date of search | 15th of November 2022 |
| Databases and other sources searched | PubMed, Cochrane Database |
| Search terms used | Please see Table 2 |
| Timeframe | From year 1953 to November 2022 |
| Inclusion criteria | Free or institutional full text availability English written |
| Selection process | By unanimous author agreement |
Table 2
| Database | Search terms | Article type | Results by year | No. of hits |
|---|---|---|---|---|
| PubMed | Reconstruction of soft tissue defects in the hand | Systematic Review | 2016–2022 | 6 |
| Reconstruction of soft tissue defects in the hand | Meta-analysis | 2017–2022 | 2 | |
| Reconstruction of soft tissue defects in the hand | Review | 1997–2022 | 55 | |
| Reconstruction of soft tissue defects in the hand | RCT | – | 0 | |
| Reconstruction of soft tissue defects in the hand | All | 1970–2022 | 449 | |
| Thumb soft tissue defect | All | 1976–2022 | 370 | |
| Finger soft tissue defect | All | 1956–2022 | 1,010 | |
| Hand soft tissue defect | All | 1953–2022 | 2,354 | |
| Cochrane Database | Reconstruction of soft tissue defects in the hand | Systematic Review | – | 0 |
| Thumb soft tissue defect | Systematic Review | – | 0 | |
| Finger soft tissue defect | Systematic Review | – | 0 | |
| Hand soft tissue defect | Systematic Review | – | 0 |
RCT, randomized controlled trial.
Discussion
General considerations
The key for successful reconstruction is a clean wound bed, where all non-viable or tumorous tissue has been excised (3,5). Tissue cultures post debridement with directed antimicrobial therapy should be routine and one may consider on infectious disease specialist input in atypical or more complex cases. Preliminary goals should also include stable skeletal fixation and re-establishing patent vascularity prior for more definitive goals of tendon and nerve repair and coverage. Occasionally, soft tissue reconstruction must be staged.
Size, location and depth are key features of the soft tissue defect when the reconstruction is planned (6). The relative defect size depends on its location, a medium or large sized finger defect might be considered as small in the dorsal hand. Thus, some authors base their size definitions on different surfaces that the defect involves (7). Definition by exact measurement is objective. The defect location and size, possible extension to the thumb and/or fingers is important. Injury mechanism and zone of injury extent should not be underestimated, while aiming for “like with like” tissue match (3). In addition to the defect, patient and surgeon related factors determine the plan for reconstruction (8). Patient factors include age, possible comorbidities, smoking status, hand dominance, patient compliance and functional demands. Surgeon related factors are more technical—does the repertoire include all levels of the reconstructive elevator? All surgical incisions and coverage should be planned in a manner to minimize scarring and granulation that limits wrist and/or digit motion. Joint sites, web spaces and preservation or restoration of tendon gliding surfaces is critical. Having a pre-operative template can significantly ease the coverage planning process that often requires three-dimensional portraying of the defect and surrounding vascular supply (8). Also, aesthetic features should be considered, and not only in terms of coverage, but also in donor site appearance (7). Rehabilitation is a multidisciplinary effort and a key determinant of the ultimate functional outcome.
Only minor defects without exposed critical structures can be left to heal by secondary intention. Healing by secondary intention requires prolonged wound care and leads to a higher degree of scar contraction and adhesions to underlying structures (9). Primary closure is rarely achievable in soft tissue defects of the hand, the hand dorsum being a limited exception. A full- or split-thickness skin graft may be considered if primary closure is not achievable, no critical structures are exposed, and the wound bed is vascularized to take a graft (10). Glaborous skin grafts should be favored for palmar defects and full-thickness grafts contract less than split-thickness grafts. In selected situations, dermal substitutes may be used in combination with delayed skin grafting, but there is no clear consensus how advantageous they after all are (11,12).
Negative pressure wound therapy (NPWT) can be a useful supplementary tool to temporize the defect prior to definitive coverage (13). For practical reasons, such as resource arrangements, additional time might be bridged with NPWT, although early coverage has advantages such as shorter hospitalization time, lower cost of care and reduced post-operative infection risk. NPWT might also serve to mature the wound bed in selected cases, if patient related factors exclude more complex reconstruction options, but the treatment is often prolonged and functionally suboptimal if used definitively (11). Furthermore, so called miniaturized negative pressure therapy devices offer a more patient friendly alternative with equal results in many instances (14). As the functionally critical structures of the hand lie just underneath our skin, they become easily exposed and warrant coverage. Local and perforator-based flaps are suitable for small sized defects with a limited zone of injury (7). Axial pattern pedicled flaps are suitable for medium-sized defects with a limited reach. Proximally and longitudinally upper extremity skin has more laxity compared to the distal or transverse axis due to the tapered design of our extremity that limits the use of loco-regional options in the hand (6). Distant and free flaps are not limited by local tissue availability and are thus the only options for coverage of large defects. Pedicled groin and hypogastric flaps can be used for complex hand soft tissue defects. They are flaps that are relatively easy to raise, do not require microsurgical expertise or sacrifice a major upper extremity vessel (6). The major disadvantage is the need for secondary division and stiffness from immobility that impedes early rehabilitation.
Free flaps offer versatile coverage options without local tissue limitations or the disadvantages of a distant flap. Available free flaps can broadly be divided into free fasciocutaneous and muscle flaps with numerous available options, where the final decision is made based on defect size, location, volume replacement needs and necessary pedicle length (15). Ability to contour the flap to match the defect should also be considered especially in digital reconstruction despite that flaps can be thinned with repeated excisions or liposuction if necessary to improve esthetics and function (16). Particularly, first web space reconstruction, extensive dorsal hand defects and multi-digit coverage are clinical situations especially suitable for microsurgical free tissue transfer (17).
Previously published systematic reviews of soft tissue defect reconstruction in the upper extremity focus on perforator-based propeller flaps, venous flaps, radial dysplasia reconstruction, medial plantar flap after sarcoma resection and adverse events after local digital flaps (18-23). There are some reconstruction algorithms published. Most of the algorithms focus on fingertip and palm injuries (24-26). One of the previously published algorithms focuses specifically on pedicled coverage options for traumatic upper extremity soft tissue defects (27). There is also an algorithm for microsurgical coverage options (16). One algorithm collates the soft tissue reconstruction options for non-fingertip injuries of the upper extremity (28). These algorithms reflect the varied nature of hand soft tissue defects—patient specific factors remain paramount; an algorithm may make the pre-operative decision making process more streamline. Thus, we have reconciled the main determinants and the reconstructive elevator to ease this process in a manner led by color theory and named the illustration as the reconstructive color wheel (Figure 1). We subdivided the hand into dorsal/palmar hand, thumb and fingers.

Dorsal hand
The dorsal aspect of the hand mainly comprises the extensor tendons as a critical structure for coverage unless the magnitude of the defect extends to bone and joint structures. The ideal coverage for a dorsal defect is a thin and pliable flap that provides a gliding surface for the extensor tendons.
Coverage with direct closure, skin grafts, simple transpositional or rotational flaps, for example the rhomboid flap, pedicled flaps and microvascular free flaps are all options. Skin grafts in general, are suitable only if the wound bed is vascularized including a thin vascular plane covering the extensor tendons (paratenon) and the defect size is not too large [small (<2 cm × 2 cm) – medium ( >2 cm × 2 cm)] (29). For medium sized defects, the dorsal aspect of the hand can be covered by the pedicled posterior interosseus artery flap, the distal ulnar artery perforator flap, or if a major artery can be sacrificed, the reverse radial forearm flap (30-32).
Larger sized defects (≥5 cm × 5 cm) and also some medium sized defects may be more amenable to free flap coverage. The array of options functioning as free flaps in the dorsal hand is vast. However, finding a suitably thin flap is difficult. The majority of thinned microvascular fasciocutaneous flaps still appear bulky in this region. Pure fascial flaps are thin and can cover large areas; however, difficulties with postoperative flap monitoring and occasional problems with skin graft take exist. The choice of the most suitable flap is widely based on surgeon expertise and preference. If a long pedicle is required, a thinned anterolateral thigh (ALT) flap is a good option (33). The medial sural artery perforator (MSAP) flap is another recognized option, providing thin coverage, an average of 3.24 mm thinner than the ALT flap (34). This flap has good skin color match in the dorsum of the hand and has gained increasing popularity in the management of hand defects (35). Finally, certainly worth mentioning is the SCIP (superficial circumflex iliac artery perforator) flap. It has become a popular flap for reconstruction of defects of the extremities. Its shortcomings mainly relate to pedicle length while advantages include a concealed donor site, minimal donor site morbidity and ease of harvest (36).
In situations where good perforators are challenging, such as in diabetics with suspected or confirmed vascular disease, the latissimus dorsi muscle flap is probably the most used one (Figure 2). The contour generally improves after edema settles and the denervated muscle atrophies. Other options include, for example, the serratus and gracilis muscle flaps (37,38). Figure 3 demonstrates the proposed algorithm for hand soft tissue reconstruction.


Palmar hand
The palmar skin is thicker and more resistant to pressure and shear than the dorsal side, which makes the requirements for flap coverage different. The skin on the palmar side is fixed to the palmar fascia making mobilization of skin edges less effective for direct closure of small defects. The general aims for more extensive palm defects are to achieve protective sensation, adequate range of motion, sufficient strength and pain reduction (24). Thickness is usually a problem with most fasciocutaneous flaps. However, flap thinning can be considered as a secondary procedure.
Minor defects can be left to heal by secondary intention. Skin grafts may be used for superficial defects without exposed critical structures. Small (<2 cm × 2 cm) defects may sometimes be closed with local rotational or advancement flaps, if the quality of the skin surrounding the defect is good.
For medium sized (>2 cm × 2 cm) and large (>4 cm × 4 cm) defects, the palm can be covered readily by pedicled flaps or microvascular options. Examples of pedicled flaps include the reverse radial forearm and ulnar artery perforator flaps (30,31). Free style rotational perforator flaps permit reconstruction of even large defects without microsurgical anastomosis.
Free flaps offer an alternative strategy for even small defects in some cases, where the depth of injury does not allow for simple graft coverage. Good alternatives raised as fascial flaps are the ALT (Figure 4), lateral arm and the serratus fascia flaps (39,40). The SCIP flap and MSAP flap are examples of popular fasciocutaneous flaps (34,41). A fasciocutaneous flap may be a better option if staged reconstruction or the need for secondary re-elevation is planned (i.e., later tendon/nerve grafts). Finally, if feasible, with the development of supermicrosurgery, free style free perforator flaps with perforator to perforator anastomosis is also an option (42,43). See Figure 3 for proposed algorithm for hand soft tissue reconstruction.

Thumb
The thumb plays an integral role in hand function and partial loss of a functioning thumb can lead to major disability. Replantation is indicated, when possible, in cases where bone shortening proximal to the lunula would otherwise be required. When planning soft tissue coverage for a defect in the thumb tip, the goal is to provide a sensate cover that is strong and stable enough to withstand rigorous use without slippage, is cosmetically acceptable and won’t impede the range of motion. Dorsally sensation is of less importance but reconstructing the nail complex or the appearance of a nail can be considered for cosmesis. More proximally the flap should provide a gliding surface for tendons, protect volar neurovascular structures and still be aesthetically acceptable.
Although Moberg, Foucher and Littler flaps and their modifications are considered the classical flaps of the thumb, the treatment decision should be made according to the size and location of the defect (44-46). Small defects (<1.5 cm in diameter) of the thumb tip with no exposed bone can be left to heal by secondary intention with an occlusive dressing without surgical intervention. Small defects (<1.5 cm in diameter) with exposed bone can be covered by V-Y advancement flaps (Figure 5) depending on the defect type: Atasoy or Hueston flaps for transverse; Venkataswami & Subramanian for oblique or unilateral defects (47-49). Medium-sized (>1.5 cm but <3 cm in diameter) volar defects requiring more advancement are readily covered by advancement flaps such as the Moberg flap or, if more advancement is required, with a modification, e.g., O’Brien or Elliot & Wilson (44,50,51). Larger defects (≥3 cm in diameter) will require a heterodigital island flap like the Foucher flap or a heterodigital (neurovascular) island flap from the ring finger and skin graft for the donor site (45). Dorsal and nail complex defects can be covered by the Brunelli dorsoulnar flap (52).
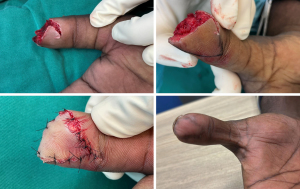
Defects encompassing most of the thumb and spanning to the level of the metacarpal will generally require either a pedicled flap from the forearm (posterior interosseous artery flap, radial forearm flap) or a free flap. A groin/abdomen flap can also be considered, especially as a salvage procedure. There are numerous options for microvascular coverage. For medium-sized defects the MSAP; radial artery superficial palmar branch flap (RASP), which provides a flap with a consistent innervation by the palmar cutaneous branch of the median nerve (PCMN), but has a short pedicle; or a venous flap; and for large defects a fascial flap such as the ALT or lateral arm flap with a skin graft (53). To reconstruct a complete thumb loss, an experienced microsurgeon may do a complete second toe transfer or partial greater toe flap in case of a partial avulsion injury. See Figure 6 for proposed algorithm for thumb soft tissue reconstruction.

Fingers
Most of the basic requirements that apply to the thumb also apply to finger defect coverage. Fingertip amputations have commonly been classified according to Allen’s or Tamai’s classifications (54,55). As fingertip coverage primarily depends on the amount of available volar skin, a useful classification that also guides the treatment is volar favourable, volar neutral and volar unfavourable (56). Small defects (≤1.5 cm in diameter) of the fingertip with no exposed bone can be left to heal by secondary intention. When exposed bone is present, the choice of reconstruction will depend primarily on the amount of volar skin available. Options will include bone shortening and primary closure or V-Y advancement flaps (Atasoy, Venkatswami & Subramanian, Kutler) which are simple and low-risk options that provide good results (57). Nail complex defects can be covered with an adipofascial dorsal flap. Complete pulp and nail complex defects can be reconstructed microsurgically with a partial 2nd toe transfer, but this is not required to achieve a functional tip that is acceptable in appearance. Medium-sized defects (>1.5 cm but <2 cm in diameter) of the tip can be covered with homodigital island flaps, such as an adipofascial dorsal flap or reverse neurovascular island flap, or by free flaps such as a partial toe flap, venous flap, MSAP or RASP). Cross-finger flaps, thenar flaps and heterodigital neurovascular island flaps have been broadly used, but with the development and popularization of homodigital flaps and thinner free flaps, their use should be carefully judged due to their greater donor site morbidity and risk for donor finger stiffness.
In dorsal defects with the paratenon still present and in volar defects with adequate neurovascular, tendon and bone soft tissue coverage, a skin graft (STSG or FTSG) is still viable, but we should avoid using them in pulp defects because they decrease discriminative sensitivity (58). Finger defects excluding the fingertip can be covered by local adipofascial, rotator or transposition flaps, or especially dorsal defects by local propeller flaps such as the Quaba flap (Figure 7) (59,60).
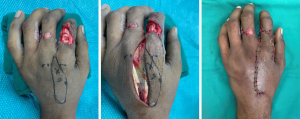
Larger defects and multi-digit defects will generally require a free flap or groin/abdomen flap. In complex hand traumas the use of available “spare parts” is always recommended, and in these cases fillet flaps (either as local, pedicled or free flaps) should be utilized when available. To cover multiple digits simultaneously, it is sometimes required to use multilobed abdominal flaps. See Figure 8 for proposed algorithm for finger soft tissue reconstruction.

Conclusions
The literature on soft tissue reconstruction of the hand is sparse. The development of advanced microsurgical techniques has brought about a vast number of reconstructive options. Although many algorithms may be composed incorporating advanced techniques, the ultimate decision on how to reconstruct depends mainly on the patient, defect and surgeons experience with available resources. The modern reconstructive surgeon should always aim to use the most suitable and appropriate reconstruction option, which might not be the simplest available option.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Johnny Ionut Efanov) for the series “The Modern Plastic and Reconstructive Surgeon—Collaborator, Innovator, Leader” published in Annals of Translational Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-201/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-201/coif). The series “The Modern Plastic and Reconstructive Surgeon—Collaborator, Innovator, Leader” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Luginbuhl J, Solarz MK. Complications of Hand Infections. Hand Clin 2020;36:361-7. [Crossref] [PubMed]
- Barron JN. The structure and function of the skin of the hand. Hand 1970;2:93-6. [Crossref] [PubMed]
- Miller EA, Friedrich J. Soft tissue coverage of the hand and upper extremity: the reconstructive elevator. J Hand Surg Am 2016;41:782-92. [Crossref] [PubMed]
- Gottlieb LJ, Krieger LM. From the reconstructive ladder to the reconstructive elevator. Plast Reconstr Surg 1994;93:1503-4. [Crossref] [PubMed]
- Talbot SG, Athanasian EA, Cordeiro PG, et al. Soft tissue reconstruction following tumor resection in the hand. Hand Clin 2004;20:181-202. [Crossref] [PubMed]
- Das De S, Sebastin SJ. Considerations in Flap Selection for Soft Tissue Defects of the Hand. Clin Plast Surg 2019;46:393-406. [Crossref] [PubMed]
- Ono S, Sebastin SJ, Ohi H, et al. Microsurgical Flaps in Repair and Reconstruction of the Hand. Hand Clin 2017;33:425-41. [Crossref] [PubMed]
- Yannascoli SM, Thibaudeau S, Levin LS. Management of soft tissue defects of the hand. J Hand Surg Am 2015;40:1237-44; quiz 1245. [Crossref] [PubMed]
- Demmer W, Sorg H, Steiert A, et al. Wound Healing and Therapy in Soft Tissue Defects of the Hand and Foot from a Surgical Point of View. Med Sci (Basel) 2021;9:71. [Crossref] [PubMed]
- Simman R. Wound closure and the reconstructive ladder in plastic surgery. J Am Col Certif Wound Spec 2009;1:6-11. [Crossref] [PubMed]
- Watt AJ, Friedrich JB, Huang JI. Advances in treating skin defects of the hand: skin substitutes and negative-pressure wound therapy. Hand Clin 2012;28:519-28. [Crossref] [PubMed]
- Choughri H, Weigert R, Heron A, et al. Indications and functional outcome of the use of integra® dermal regeneration template for the management of traumatic soft tissue defects on dorsal hand, fingers and thumb. Arch Orthop Trauma Surg 2020;140):2115-27.
- Buchanan PJ, Kung TA, Cederna PS. Evidence-Based Medicine: Wound Closure. Plast Reconstr Surg 2016;138:257S-70S. [Crossref] [PubMed]
- Mitchell SL, Ray E, Cordeiro PG. Minituarized negative pressure wound therapy for split-thickness skin graft donor sites. J Reconst Microsurg Open 2018;3:e46-49.
- Dibbs R, Grome L, Pederson WC. Free tissue transfer for upper extremity reconstruction. Semin Plast Surg 2019;33:17-23. [Crossref] [PubMed]
- Reuben CM, Bastidas N, Sharma S. Power-assisted suction lipectomy of fasciocutaneous flaps in the extremities. Ann Plast Surg 2010;65:60-5. [Crossref] [PubMed]
- Horta R, Silva P, Costa-Ferreira A, et al. Microsurgical soft-tissue hand reconstruction: an algorithm for selection of the best procedure. J Hand Microsurg 2011;3:73-7. [Crossref] [PubMed]
- Vitse J, Bekara F, Bertheuil N, et al. Perforator-based propeller flaps reliability in upper extremity soft tissue reconstruction: a systematic review. J Hand Surg Eur Vol 2017;42:157-64. [Crossref] [PubMed]
- Wharton R, Creasy H, Bain C, et al. Venous flaps for coverage of traumatic soft tissue defects of the hand: a systematic review. J Hand Surg Eur Vol 2017;42:817-22. [Crossref] [PubMed]
- Roberts JM, Carr LW, Haley CT, et al. Venous flaps for revascularization and soft-tissue coverage in traumatic hand injuries: a systematic review of the literature. J Reconstr Microsurg 2020;36:104-9. [Crossref] [PubMed]
- Murphy GRF, Logan MPO, Smith G, et al. Correction of "Wrist" Deformity in Radial Dysplasia: A Systematic Review and Meta-Analysis. J Bone Joint Surg Am 2017;99:2120-6. [Crossref] [PubMed]
- Troisi L, Berner JE, West EV, et al. Medial Plantar Flap for Hand Reconstruction: A Systematic Literature Review and Its Application for Post-Sarcoma Excision. Ann Plast Surg 2019;82:337-43. [Crossref] [PubMed]
- Karjalainen T, Jokihaara J. A review and meta-analysis of adverse events related to local flap reconstruction for digital soft tissue defects. Hand Clin 2020;36:107-21. [Crossref] [PubMed]
- Lemmon JA, Janis JE, Rohrich RJ. Soft-tissue injuries of the fingertip: methods of evaluation and treatment. An algorithmic approach. Plast Reconstr Surg 2008;122:105e-17e. [Crossref] [PubMed]
- Engelhardt TO, Rieger UM, Schwabegger AH, et al. Functional resurfacing of the palm: flap selection based on defect analysis. Microsurgery 2012;32:158-66. [Crossref] [PubMed]
- Abi-Chahla ML, Alet JM, Fabre T, et al. Treatment of defects in the tip and palmar surface of the fingers. Hand Surg Rehabil 2018;37:4-11. [Crossref] [PubMed]
- Naalla R, Chauhan S, Dave A, et al. Reconstruction of post-traumatic upper extremity soft tissue defects with pedicled flaps: An algorithmic approach to clinical decision making. Chin J Traumatol 2018;21:338-51. [Crossref] [PubMed]
- Bas S, Sizmaz M, Aydin A, et al. An algorithm for the reconstruction of nonfingertip upper extremity soft tissue defects. Turk J Plast Surg 2021;29:43-50.
- Ho W, Jones CD. Geometry and design of a rhomboid flap. J Plast Surg Hand Surg 2021;55:249-54. [Crossref] [PubMed]
- Kelada MN, Salem RR, Eltohfa YA, et al. Posterior interosseus artery flap for hand reconstruction: anatomical basis and clinical application. BMC Musculoskelet Disord 2022;23:662. [Crossref] [PubMed]
- Becker C, Gilbert A. The ulnar flap. Handchir Mikrochir Plast Chir 1988;20:180-3.
- Hovius SE, Sluimers JE, Van Adrichem LN, et al. The radial forearm flap. Neth J Surg 1988;40:69-75.
- Wong CH, Wei FC. Anterolateral thigh flap. Head Neck 2010;32:529-40. [Crossref] [PubMed]
- Al-Himdani S, Din A, Wright TC, et al. The medial sural artery perforator (MSAP) flap: A versatile flap for lower extremity reconstruction. Injury 2020;51:1077-85. [Crossref] [PubMed]
- Xie RG, Gu JH, Gong YP, et al. Medial sural artery perforator flap for repair of the hand. J Hand Surg Eur Vol 2007;32:512-7. [Crossref] [PubMed]
- Koshima I, Nanba Y, Tsutsui T, et al. Superficial circumflex iliac artery perforator flap for reconstruction of limb defects. Plast Reconstr Surg 2004;113:233-40. [Crossref] [PubMed]
- Covello GS, Martins DVR, Padilha GC, et al. Serratus anterior muscle flap for reconstruction of extremity injuries. Acta Ortop Bras 2022;30:e250673. [Crossref] [PubMed]
- Pedreira R, Calotta NA, Deune EG. Free Gracilis Muscle Flap for Sarcoma Reconstruction: 19 Years of Clinical Experience. Sarcoma 2019;2019:3975020. [Crossref] [PubMed]
- Kokkalis ZT, Papanikos E, Mazis GA, et al. Lateral arm flap: indications and techniques. Eur J Orthop Surg Traumatol 2019;29:279-84. [Crossref] [PubMed]
- Ulrich D, Fuchs P, Bozkurt A, et al. Free serratus anterior fascia flap for reconstruction of hand and finger defects. Arch Orthop Trauma Surg 2010;130:217-22. [Crossref] [PubMed]
- Altiparmak M, Cha HG, Hong JP, et al. Superficial Circumflex Iliac Artery Perforator Flap as a Workhorse Flap: Systematic Review and Meta-analysis. J Reconstr Microsurg 2020;36:600-5. [Crossref] [PubMed]
- Bravo FG, Schwarze HP. Free-style local perforator flaps: concept and classification system. J Plast Reconstr Aesthet Surg 2009;62:602-8; discussion 609. [Crossref] [PubMed]
- Chang CC, Wong CH, Wei FC. Free-style free flap. Injury 2008;39:S57-61. [Crossref] [PubMed]
- Moberg E. Aspects of sensation in reconstructive surgery of the upper extremity. J Bone Joint Surg Am 1964;46:817-25.
- Foucher G, Braun JB. A new island flap transfer from the dorsum of the index to the thumb. Plast Reconstr Surg 1979;63:344-9. [Crossref] [PubMed]
- Wang H, Yang X, Chen C, et al. Modified Littler flap for sensory reconstruction of large thumb pulp defects. J Hand Surg Eur Vol 2018;43:546-53. [Crossref] [PubMed]
- Atasoy E, Ioakimidis E, Kasdan ML, et al. Reconstruction of the amputated finger tip with a triangular volar flap. A new surgical procedure. J Bone Joint Surg Am 1970;52:921-6.
- Hueston J. Local flap repair of fingertip injuries. Plast Reconstr Surg 1966;37:349-50. [Crossref] [PubMed]
- Venkataswami R, Subramanian N. Oblique triangular flap: a new method of repair for oblique amputations of the fingertip and thumb. Plast Reconstr Surg 1980;66:296-300.
- O'Brien B. Neurovascular island pedicle flaps for terminal amputations and digital scars. Br J Plast Surg 1968;21:258-61. [Crossref] [PubMed]
- Elliot D, Wilson Y. V-Y advancement of the entire volar soft tissue of the thumb in distal reconstruction. J Hand Surg Br 1993;18:399-402. [Crossref] [PubMed]
- Brunelli F, Vigasio A, Valenti P, et al. Arterial anatomy and clinical application of the dorsoulnar flap of the thumb. J Hand Surg Am 1999;24:803-11. [Crossref] [PubMed]
- Yang JW, Kim JS, Lee DC, et al. The radial artery superficial palmar branch flap: a modified free thenar flap with constant innervation. J Reconstr Microsurg 2010;26:529-38. [Crossref] [PubMed]
- Allen MJ. Conservative management of finger tip injuries in adults. Hand 1980;12:257-65. [Crossref] [PubMed]
- Tamai S. Twenty years' experience of limb replantation--review of 293 upper extremity replants. J Hand Surg Am 1982;7:549-56. [Crossref] [PubMed]
- Yeo CJ, Sebastin SJ, Chong AK. Fingertip injuries. Singapore Med J 2010;51:78-86; quiz 87.
- Karjalainen T, Sebastin SJ, Chee KG, et al. Flap Related Complications Requiring Secondary Surgery in a Series of 851 Local Flaps Used for Fingertip Reconstruction. J Hand Surg Asian Pac Vol 2019;24:24-9. [Crossref] [PubMed]
- Adani R, Tang JB, Elliot D. Soft and tissue repair of the hand and digital reconstruction. J Hand Surg Eur Vol 2022;47:89-97. [Crossref] [PubMed]
- Venkatramani H, Varadharajan V. Adipofascial, Transposition, and Rotation Flaps. Hand Clin 2020;36:9-18. [Crossref] [PubMed]
- Tos P, Crosio A, Pugliese P, et al. Propeller Flaps for Hand and Digit Reconstruction. Semin Plast Surg 2020;34:192-9. [Crossref] [PubMed]


