
Scope highlights of Annals of Translational Medicine based on a review of the history, definition, and scope of translational medicine
Introduction
Since the launch of volume 1 in April 2013, the journal Annals of Translational Medicine (ATM) has continuously published high-quality scientific articles for over 10 years. During this period, ATM has published more than 9,000 articles in the field of translational medicine, adhering to the founding mission of the journal to ‘expedite the translation of scientific discovery into new or improved standards of management and health outcomes practice’. ATM has been indexed in PubMed since September 5th, 2014, and indexed in Science Citation Index Expanded since March 2018. This remarkable journey is built on the joint efforts of wide-reaching authors, a team of more than 100 outstanding editorial board members from 18 countries, and thousands of dedicated reviewers. On February 16th, 2023, ATM received an email from Web of Science informing that it had decided not to index publications from ATM any longer because of two articles published outside the scope of translational medicine (1). In response, we aim to write this article to re-clarify the scope of ATM by reviewing the definition and intent of translational medicine. We also would like to share with the editorial board, reviewers, authors, and readers the policy alignment of the editorial office.
History of translational medicine
Geraghty first introduced the concept of translational medicine in the Lancet in 1996 (2). However, the origin of this concept dates back to the mid-19th century when the scientific community began to achieve several scientific breakthroughs in the field of basic research, while the corresponding application to clinical practice at bedside was delayed and fraught with inherent barriers.
In 1966, McKinney and Stavely published an article entitled ‘From Bench to Bedside: The Biologist in Drug Development’, emphasizing collaboration and communication among multidisciplinary experts in the pharmaceutical industry to facilitate a meaningful shift in clinical outcomes (3). In 1974, Wolf discussed that the reason for the lag in the translation of bench findings lay in our failure to understand and integrate available data well in an era of knowledge explosion and data explosion (4). At that point, the concept of translational medicine was budding but had not been formally developed. The idea of translation remained in a “one-way” phase, focusing only on the translation of bench results into bedside treatment. Later, in 1992, Choi argued that research should move from bench to bedside, then from bedside to bench, and ultimately for the benefit of all (5). At that point, the idea of translation began to evolve from a one-way to a two-way shift.
In 1996, Geraghty formally introduced the concept of translational medicine, intending to facilitate effective connections between bench researchers and bedside caregivers (2). In July 2003, Marincola published an article further emphasizing that translational research should be a two-way road: bench to bedside and bedside to bench (6). In October of the same year, the National Institutes of Health made public the design of a translational medicine roadmap (7). Further, they established a two-way translational research system between bench and clinical research, aiming to establish new partnerships between clinical and community physicians, patients, and academic researchers. Since then, the concept of translational medicine has formally moved into a two-way translational phase, and the number of studies related to translational medicine has grown exponentially (8).
Definition of translational medicine
When translational medicine was first proposed, it embodied the concept that ‘Translational medicine is the integration of new discoveries in basic science and clinical practice’, albeit lacking clear definition (2). Terms similar to translational medicine have emerged, such as translational research and translational science. Although these terms have been used interchangeably, there has been a paucity of an authoritative and widely accepted definition of translational medicine, translational research, or translational science. For example, the definition of translational research by Broder et al. is limited to the translation of results in the field of cancer (9). Furthermore, some dictionaries either do not have a definition of translational medicine or define it in a narrow unidirectional dimension—‘Translational Medicine: The branch of medicine that deals with using knowledge gained from basic scientific research to develop practical applications, such as new treatments, devices, drugs or policies’ (10).
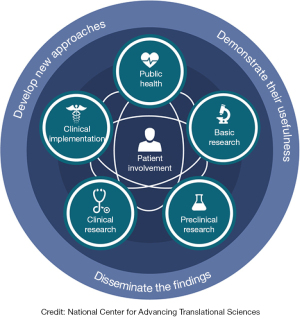
It was not until 2015 that the European Society for Translational Medicine (EUSTM) published a paper entitled ‘Translational Medicine definition by the European Society for Translational Medicine’ (11), which gives a clear and authoritative definition of translational medicine:
‘The EUSTM defines Translational Medicine as an interdisciplinary branch of the biomedical field supported by three main pillars: benchside, bedside and community. The goal of Translational Medicine is to combine disciplines, resources, expertise, and techniques within these pillars to promote enhancements in prevention, diagnosis, and therapies.’
ATM recognizes and takes EUSTM’s definition of translational medicine as an interdisciplinary branch of biomedicine, which is the interdisciplinary translation of the three main pillars of benchside, bedside, and community.
The scope of translational medicine
The perception of the scope of translational medicine has gone through different stages, with several models proposed as below.
The 2T model scope (Figure 1A)
In 2002, the Institute of Medicine (US) Clinical Research Roundtable proposed that there are barriers to translation in different research stages, i.e., ‘translation block’. The Institute of Medicine (US) Clinical Research Roundtable further proposed the original 2T model to represent the coverage of translational research. That is, the first translational block (T1) is translational research between basic biomedical research and clinical science and knowledge, i.e., translation from basic science to human studies; the second translational block (T2) is translational research between clinical science and knowledge and improved health, i.e., the translation of new knowledge into clinical practice and health decision-making (12).
The 3T model scope (Figure 1B)
In 2007, Westfall et al. pointed out that although many interventions are effective in clinical research, few actually work in clinical practice or have difficulty in achieving the therapeutic effects of clinical research in the real world (13). Westfall et al. proposed to add practice-based research to the 2T model to shift the 2T model into a 3T model, i.e., the T1 stage is the translation between basic science research and human clinical research; the T2 stage is the translation between human clinical research and practice-based research, in the form of clinical guidelines, systematic reviews and meta-analyses; T3 is the translation between practice-based research and clinical practice, with dissemination and implementation research as the primary focus (13). This 3T model closely links the bench, bedside, and daily clinical practice. Later in 2008, Dougherty and Conway published an article in JAMA that further clarified the 3T model and what it entails (Figure 1B) (14).
The 4T model scope (Figure 1C)
Khoury et al. proposed the 4T model (15) using genomic studies as an example. While the first three phases are similar to that proposed by Dougherty and Conway (14), the T4 phase translates from the clinical practice implementation process to assess the impact of the application of these evidence-based treatments on population health outcomes. The ‘Biomedical Research Translation Continuum’ framework constructed by Drolet and Lorenzi also describes the progression from basic science to public health improvement (16).
The 4T with a T0 model scope
The 4T with a T0 model scope in addition to T1 to T4, there is also the T0 stage of proposal—‘scientific discovery’ (Figure 1D). Broadly speaking, the T0 stage is not only limited to basic laboratory discoveries (mechanisms of action, target biomarkers, animal and preclinical studies, etc.), but also includes interdisciplinary science such as epidemiological and genome-wide association studies, which is increasingly recognized as the key to new discoveries (17-19). The University of Arkansas for Medical Sciences Translational Research Institute explicitly explains each translation phase from T0 to T4 (Figure 2) (20).
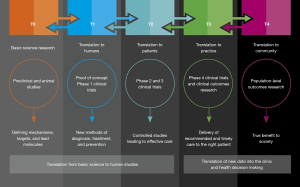
The full spectrum scope
The National Center for Advancing Translational Sciences (NCATS) divided translational research into different stage segments according to different phases (21). In this full spectrum scope last updated on November 10th 2021 (Figure 3), translational research includes (I) basic research, those studies that explore the basic mechanisms of biology, disease, and behavior; (II) preclinical research, those studies that link basic research to human medicine, where scientists test scientific hypotheses through cellular models, animal models, and samples of human or animal tissues; (III) clinical research, including clinical trials on the safety and efficacy of interventions in humans, and behavioral or observational studies; (IV) clinical implementation research, which includes the adoption and application of interventions in routine care; and (V) public health research, which includes the study of the broad impact of disease at the population level and the health outcomes of prevention, diagnosis, and treatment of disease. Of note, the coronavirus disease 2019 pandemic has taught us that few global public health systems were prepared for the devastating impact of the rapidly spreading severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. In the immediate future, public health research and its clinical application are expected to play an increasingly crucial role in preventing future epidemics that overwhelm normal patient planning and care. Highlighting public health in the full spectrum scope of translational research will contribute to better preparing for the future.
In summary, the scope of translational research has expanded from the initial ‘Bench to Bedside’ to ‘Bench to Community’, which is consistent with the 2015 EUSTM definition of translational medicine. Specifically, the scope of translational research is comprehensive and includes all phases of T0, T1, T2, T3, and T4 research, as well as basic research, preclinical research, clinical research, clinical implementation research, and public health research.
The scope of ATM
We recognize that the broad scope of translational medicine could create potential confusion for authors. As Kane et al. wrote in the chapter ‘Evaluating Translational Research’ in the book ‘Translational Medicine-What, Why and How: An International Perspective’ (22), researchers often describe their research findings as novel, promising, unique, or potentially useable, and applicational. Thus, there are few studies that do not have ‘translational’ characteristics in some form. Therefore, to provide more explicit guidance to authors and readers, we are herein obliged to further clarify the priority areas that ATM will cover in the future.
ATM is a multidisciplinary and comprehensive journal focusing on translational medicine. The T0 to T4 model of translational research emphasizes the continuity and interaction of the various stages involved, which is consistent with the vision of ATM. Therefore, ATM’s scope will cover translational research phases from T0 to T4, including basic research, preclinical research, clinical research, clinical practice research, and health policy research. Of note, although ATM covers a wide range of research, those studies that are translational but have a focus other than the human body, medicine, or disease are not included in the scope of ATM.
While ATM focuses on many areas of translational medicine, there are several defining specialized sections that differentiate the aims and mission the journal:
- Global Burden of Diseases—including those with high morbidity and mortality such as cancers, cardiovascular and neurological diseases, metabolic diseases, critical and emergent diseases.
- Omics Sciences—including genomics and genetics, epigenomics, transcriptomics, proteomics, metabolomics, medical bioinformatics, and radiomics.
- Data Sciences—including big biomedical data mining, machine learning, artificial intelligence, and translational imaging.
- Basic and Mechanism Sciences—including physiology, unknown etiology and pathology, mechanisms of disease and behavior, immunology such as immune-oncology and vaccine development.
- Biomarkers Sciences—including disease biomarkers mining, molecular diagnosis and therapies.
- Drug and Biomaterials Screening and Development—including disease modeling and simulation platforms, biomaterials and pharmaceutical formulation, clinical pharmacology such as pharmacokinetics and pharmacodynamics.
- Emerging Therapeutics—such as personalized therapy, precision therapy, cell, tissue and gene therapy, immunotherapy, immune checkpoint therapy, antibody therapy, and cancer transformation therapy.
- Emerging Medical Technology Innovation and Translation—such as gene editing technology, tissue engineering and regenerative medicine, RNA interference technology, medical device and diagnostic tool development, medical app, 3D printing, and mobile medicine.
- Clinical Studies—including design, implementation, data management and analysis of case reports, observational studies, and phases 1–4 clinical trials.
- Data-Driven Clinical Practice and Policy Making—including health economics, clinical guidelines, health policy-making, and barriers to clinical practice and population adoption.
Summary and next steps
Translational medicine has advanced from the initial narrow concept of ‘benchside to bedside’ to a comprehensive concept encompassing bench, bedside, and community-focused research. Furthermore, the scope of translational medicine encompasses all research from T0 to T4, including basic research, preclinical research, clinical research, clinical implementation research, and public health research. Specifically, the scope of ATM shall include all translational research on the human body, medicine, or disease, with ten specialized sections. In hindsight, while ATM has published a few articles that extend beyond translational medicine, this reflects the opportunity to better align editorial workflow and the content focus of the journal. In the past, the editorial office has expressed differing opinions regarding the overarching reach of the journal. Moving forward, ATM will strengthen editorial training and add a mandatory review of submitted articles to assess their scope and relevance. Lastly, we would like to take this opportunity to thank editorial board members, reviewers, authors, and readers for their attentiveness and support for ATM, and we hope that ATM’s future alignment and editorial policy adjustment will contribute to the continuing improvement of the journal.
Acknowledgments
We would like to thank Drs. Xiaosong Gu, Jianxing He and Susanna S. Park for their advice on this paper.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Translational Medicine. The article has undergone external peer review.
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1798/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1798/coif). KZ, YC, YP and FJ report that they are the full-time staffs of AME Publishing Company (the publisher of Annals of Translational Medicine). CSHN serves as the Editor-in-Chief of Annals of Translational Medicine. RR serves as the Honorary Editor-in-Chief of Annals of Translational Medicine from June 2019 to May 2024. JTE serves as an unpaid editorial board member of Annals of Translational Medicine from October 2022 to September 2024. AL serves as an unpaid editorial board member of Annals of Translational Medicine from September 2022 to August 2024. FN serves as an unpaid editorial board member of Annals of Translational Medicine from March 2023 to February 2025. RW serves as an unpaid editorial board member of Annals of Translational Medicine from August 2022 to July 2024. SU serves as an unpaid editorial board member of Annals of Translational Medicine from November 2021 to October 2023. MTC serves as an unpaid editorial board member of Annals of Translational Medicine from November 2022 to October 2024. RD serves as an unpaid editorial board member of Annals of Translational Medicine from November 2022 to October 2024. CZ has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Editorial Office. Updated on Web of Science inclusion of ATM articles. In: Annals of Translational Medicine. 2023. Available online: https://atm.amegroups.com/announcement/view/%203875
- Geraghty J. Adenomatous polyposis coli and translational medicine. Lancet 1996;348:422. [Crossref] [PubMed]
- McKinney GR, Stavely HE. From bench to bedside: the biologist in drug development. Bioscience 1966;16:683-7.
- Wolf S. Editorial: The real gap between bench and bedside. N Engl J Med 1974;290:802-3. [Crossref] [PubMed]
- Choi DW. Bench to bedside: the glutamate connection. Science 1992;258:241-3. [Crossref] [PubMed]
- Marincola FM. Translational Medicine: A two-way road. J Transl Med 2003;1:1. [Crossref] [PubMed]
- Zerhouni E. The NIH roadmap. Science 2003;302:63-72. [Crossref] [PubMed]
- van der Laan AL, Boenink M. Beyond bench and bedside: disentangling the concept of translational research. Health Care Anal 2015;23:32-49. [Crossref] [PubMed]
- Broder S, Cushing M. Trends in program project grant funding at the National Cancer Institute. Cancer Res 1993;53:477-84.
-
The Free Dictionary - Cohrs RJ, Martin T, Ghahramani P, et al. Translational Medicine definition by the European Society for Translational Medicine. New Horiz Transl Med 2015;2:86-8.
- Institute of Medicine (US) Clinical Research Roundtable. In: The Role of Purchasers and Payers in the Clinical Research Enterprise: Workshop Summary. Tunis S, Korn A, Ommaya A, editors. Washington (DC): National Academies Press (US); 2002.
- Westfall JM, Mold J, Fagnan L. Practice-based research--"Blue Highways" on the NIH roadmap. JAMA 2007;297:403-6. [Crossref] [PubMed]
- Dougherty D, Conway PH. The "3T's" road map to transform US health care: the "how" of high-quality care. JAMA 2008;299:2319-21. [Crossref] [PubMed]
- Khoury MJ, Gwinn M, Yoon PW, et al. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genet Med 2007;9:665-74. [Crossref] [PubMed]
- Drolet BC, Lorenzi NM. Translational research: understanding the continuum from bench to bedside. Transl Res 2011;157:1-5. [Crossref] [PubMed]
- Thornicroft G, Lempp H, Tansella M. The place of implementation science in the translational medicine continuum. Psychol Med 2011;41:2015-21. [Crossref] [PubMed]
- Khoury MJ, Gwinn M, Ioannidis JP. The emergence of translational epidemiology: from scientific discovery to population health impact. Am J Epidemiol 2010;172:517-24. [Crossref] [PubMed]
- Fort DG, Herr TM, Shaw PL, et al. Mapping the evolving definitions of translational research. J Clin Transl Sci 2017;1:60-6. [Crossref] [PubMed]
- University of Arkansas for Medical Sciences Translational Research Institute. What is Translational Research? Available online: https://tri.uams.edu/about-tri/what-is-translational-research/
- National Center for Advancing Translational Sciences. Translational Science Spectrum. Available online: https://ncats.nih.gov/translation/spectrum
- Kane C, Rubio D, Trochim W. Evaluating Translational Research. In: Translational Medicine-What, Why and How: An International Perspective. Alving B, Dai K, Chan SHH, editors. Basel: Karger; 2012.



