
Ophthalmic artery reversal predicts contralateral body weakness symptoms better than carotid Doppler velocity—preliminary results
Highlight box
Key findings
• Of 28 patients, 17 (61%) have ophthalmic artery (OA) flow reversal in the presence of carotid stenosis with peak systolic velocity >350 cm/s.
• Of 13 patients presenting with unilateral body or face weakness 10 (77%) have OA flow reversal.
• Measurement of OA flow direction might be a sensitive and specific indicator for middle cerebral artery (MCA) hypotension causing MCA dysfunction.
What is known and what is new?
• Severe carotid stenosis indicates elevated risk of preventable stroke: carotid revascularization reduces the chance of stroke by half; but if untreated only a minority of patients (11% of asymptomatic; 26% of symptomatic) will have stroke.
• This manuscript separates the hazardous pressure reducing carotid stenosis from the benign flow reducing carotid stenosis. OA flow reversal is an indicator of a pressure reducing carotid stenosis.
What is the implication and what should change now?
• Two measurements added to carotid Doppler examination, might identify cases most likely to benefit from treatment.
Introduction
Of the nearly 800,000 strokes in the US each year, about 100,000 are due to severe carotid artery stenosis (sCAS). Anatomic revascularization by carotid endarterectomy (CEA) or inserting a carotid stent (CSt) can prevent many of these strokes. The selection of cases for CEA or CSt is currently based on the anatomic contrast X-ray angiography finding of severe percent diameter reduction (%DR). For asymptomatic patients with CAS >60%DR, the projected risk of death or ipsilateral stroke in 5 years is 11% without CEA/CSt or 5.1% with CEA (1). Although in a patient with sCAS who is asymptomatic, CEA/CSt reduces the chance of stroke by more than half, only 5.9% of these patients are saved from stroke or death by CEA/CSt requiring nearly 17 CEA/CSt procedures to prevent one stroke. In patients with neurological symptoms associated with a sCAS, the risk of stroke is higher and the benefit of CEA/CSt is greater. For symptomatic patients with sCAS >70%DR, the projected risk of ipsilateral stroke in 2 years is 26% without CEA/CSt or 9% with CEA (2). Although in a patient with sCAS who is symptomatic, CEA/CSt reduces the chance of stroke by more than 2/3, only 17% of these patients are saved from stroke by CEA/CSt requiring nearly 6 CEA/CSt procedures to prevent one stroke. The treatment success of CSt is similar to CEA (3).
Because only a minority of patients with sCAS appear to benefit from CEA/CSt by prevention of stroke, three endeavors have been initiated to improve the care of patients with sCAS: (I) identify features that indicate that the carotid atheroma causing the stenosis is vulnerable to rupture causing stroke. Features such as a thin protective cap and hazardous contents such as hemorrhage, lipid or calcium, might indicate that the patient is at higher risk for stroke if the stenosis is not treated. (II) Consider medical treatments for atherosclerotic stenosis, such as the use of statins or bempedoic acid as an alternative to anatomic revascularization. (III) Determine whether sCAS is also a cause of cortical dysfunction such as impaired cognition which can be improved by the treatment of sCAS.
This study explores the hypothesis that pressure reducing sCAS causes middle cerebral artery (MCA) hypotension which is indicated by three independent measurements: (I) elevated peak systolic velocity (PSV) in the sCAS; (II) carotid artery bruit indicating post-stenotic pressure drop; and (III) ophthalmic artery (OA) flow reversal indicating hypotension at the origin of the MCA. This study explores whether this triad of findings indicating sCAS is associated with the stroke symptom of contralateral face or body weakness.
Each internal carotid artery (ICA) supplies ipsilateral cerebral arteries feeding portions of the cerebral cortex via the circle of Willis (coW). The coW can provide collateral connections to one or both of the alternative supplies: the contralateral ICA or the basilar artery (BA). On autopsy, more than 28% of carotid arteries have no or inadequate collateral connections to either the contralateral ICA or BA (Figure 1) leaving the ICA as the sole supply to the ipsilateral MCA cerebral territory and adjacent connected cerebral artery territories. In such cases, excepting collateral supply from the OA and leptomeningeal collaterals (LAs), the mandatory ICA systolic flow rate might be as low as 3.3 cc/s (supplying MCA only) or as high as 8.3 cc/s (supplying four cerebral arteries) to perfuse the dependent portions of cerebral cortex (Figure 1) (4-6).

The ICA is connected to the MCA in every case. The hypothesis of this study is that a sCAS can be flow reducing if the ICA is connected to either the contralateral ICA or BA via the coW so that the alternate supply can make up the deficit flow required by the cerebral arteries. In that case, the MCA pressure and flow reserve are normal, even with an anatomical sCAS. But if the ICA is isolated, connected to neither the contralateral ICA or BA, the sCAS is pressure reducing because nearly all of the MCA flow must be supplied by the stenotic ICA, supplemented only by the OA and LAs. The resulting MCA hypotension causes impaired MCA cerebral territory function in nearly 30% of cases with sCAS including the anterior cerebral artery (ACA) territories in 21% of all cases with sCAS and the ipsilateral posterior cerebral artery (PCA) territories in 6% of cases.
The purpose of this study is to identify measurements and criteria for assessing pressure reducing sCAS that can be easily incorporated into a conventional carotid duplex ultrasound examination protocol with currently used equipment and little additional time. These measurements, in theory, will more accurately identify patients who are most likely to benefit from treatment of sCAS. We present this article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1681/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of University of Washington, Human Subjects Division in the Office of Research [IRB A (IRB00000241); FWA (FWA00006878); study protocol (STUDY00005630_Moore)]. Individual consent for this retrospective analysis was waived because the anonymized data were acquired as part of a retrospective review of medical records. For the years 2011 to 2019, clinical reports from the vascular laboratory and from the cerebrovascular laboratory at the University of Washington Harborview Medical Center in Seattle were reviewed. Patients were selected if both their carotid arteries were examined (7) at the vascular laboratory and their intracranial arteries were examined (8) at the cerebrovascular laboratory (Figure 2).

Clinical examinations in both laboratories were performed by sonographers holding the registered vascular technologist (RVT) credential issued by the American Registry of Diagnostic Medical Sonographers (ARDMS). Each sonographer reviewed the clinical indications for the examination before performing the examination. The hypothesis tested here was developed after the chart review data were assembled for analysis so the sonographer’s report was not influenced by the hypothesis.
Five measurements were selected for analysis: highest brachial systolic blood pressure (SBP); ICA PSV; the presence of a post-stenotic carotid bruit (Bruit); OA reversal (OAr) vs. OA forward (OAf); and the presenting symptom of face droop or arm weakness contralateral to the sICA (Weak).
Without referring to the clinical presentation during chart review, each OAr measurement was verified by A.E.M. and each PSV measurement was verified by K.W.B. and each ICA spectral waveform was examined for evidence of bruit by K.W.B.
Statistical analysis
Data were presented as cumulative distribution plots, receiver operator curves supplemented with 95% confidence areas (9) and scattergrams.
Results
Records for 28 patients with 55 examined patent ICAs were available. Patient ages spanned between 44 and 87 years with a median of 65 years. Referral symptoms included: 12 cases with unilateral weakness; 1 case with unilateral facial droop; 9 cases with speech difficulties; 3 cases with confusion; 2 cases with amaurosis fugax; 2 cases with blurred vision; 2 cases with syncope; 1 case with numbness; 1 case with bruit; 1 case for preoperative heart surgery workup. The maximum interval between the ICA and the OA examinations was 2 months with 3/4 of the examinations completed within 1 week including 1/3 within 1 day. SBP ranged between 80 and 208 mmHg with the majority having an arm-to-arm difference <11 mmHg, but in three cases, the left SBP was >30 mmHg less than the right SBP. The highest SBP was used for analysis. Of the 19 OAs with OAr, 17 (89%) had PSV >350 cm/s; of the 36 OAs with normal direction, 11 (31%) had PSV >350 cm/s (Figure 3).

According to the laws of Torricelli, Bernoulli and Venturi, the pressure drop across a stenosis in a blood vessel is ∆P (mmHg) = 4 × [PSV (cm/s/100)]2. Thus, a stenosis with PSV =350 cm/s and a post-stenotic bruit would cause a systolic pressure drop of 49 mmHg; a PSV =500 cm/s would cause a pressure drop of 100 mmHg. A patient with SBP =120 mmHg and a PSV =400 cm/s with post-stenotic bruit would have an MCA systolic pressure of 120−64=56 mmHg. A patient with SBP =144 mmHg could not have a PSV >600 cm/s (A transected artery with SBP of 144 mmHg has a bleeding jet velocity of 600 cm/s during systole according to Torricelli; according to Venturi, a patient with a brachial blood pressure 120/80 and carotid velocities of 400 cm/s systolic: 200 cm/s diastolic will have a transmural blood pressure at the stenosis of 56/64, paradoxically higher during diastole than during systole) (10). The relationship between PSV and SBP in these patients is shown in Figure 4.

Figure 4 shows the relationship between the SBP and PSV for each ICA. Seven ICAs have PSV greater than the Bernoulli limit; this is not possible. PSV values are computed from the Doppler “angle correction” formula based on Doppler examination angle (Ɵ): PSV = (f × λ)/2cos(Ɵ), where: 2 accounts for the round trip of the ultrasound from the transducer to the blood sample volume and back; λ is the wavelength of ultrasound in blood; f is the Doppler frequency shift; and Ɵ is the angle between the ultrasound beam path and the assumed direction of flow of the blood. In all of these cases, the Doppler angle (Ɵ) was set to 60 degrees, doubling the PSV value toward the transducer to correct for the angle. Unfortunately, because of turbulence and uncertainty of the measured angle, some components of the blood velocity vector could have been aligned with the ultrasound beam (Ɵ=0) doubling a portion of the PSV value in error. Above the red line are cases exceeding the PSV limit for a post-stenotic ICA & MCA pressure of 60 mmHg for brain perfusion pressure; 60 mmHg is accepted as the minimum allowable cerebral artery systolic pressure. Of the 13 ICAs with bruit, 11 are above the “Bernoulli 60 mmHg” line. Of the 22 points above the “Bernoulli 60 mmHg” line, half have bruit.
The merit of a diagnostic test is often represented by sensitivity and specificity displayed as a receiver operator characteristic (ROC) curve for various criterion threshold values. Figure 5 (9,11-13) compares the ROC for PSV with the values for OAr and bruit using the symptom of contralateral facial droop or body weakness indicating transient ischemic attack (TIA) or stroke as the reference standard rather than a %DR angiographic stenosis.
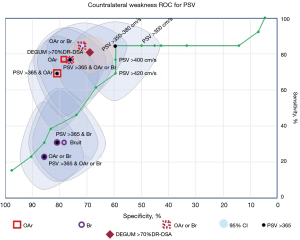
Of the three measurements (PSV >350 cm/s, OAr, and bruit), the combination of OAr or bruit has a superior combination of sensitivity and specificity. The sensitivity and specificity for OAr or bruit are 84.6%±5.4%, specificity 71.4%±2.1%; the sensitivity and specificity for PSV >350 cm/s are 84.6%±5.4%, 59.5%±2.3%. The sensitivity and specificity for OAr or bruit for contralateral weakness is superior to the German Society of Ultrasound in Medicine (DEGUM) sensitivity and specificity method for classifying >70%DR ICA stenosis. This is a comparison of a method using a physiology/functional reference to a method using an anatomic (%DR) reference.
In this group of patients, 20 CEAs were performed and one CSt was placed. All 21 revascularizations had PSV >350 cm/s, 7 patients with PSV >350 cm/s did not receive revascularization. Of the 21 revascularized arteries, 8 had bruit, 13 had OAr (15 had either bruit or OAr or both).
Discussion
The purpose of the carotid stenosis examination is to identify patients who will benefit from therapy and to provide guidance for the type of therapy best suited to the case. Treatment of a sCAS might prevent stroke or reduce the risk of stroke; alternatively, treatment might improve brain function such as cognition. Stroke will occur in a minority of patients with sCAS. Efforts to identify the atheroma vulnerable to rupture require prolonged randomized multicenter clinical trials to validate. Demonstrating a significant improvement of cerebral function after sCAS treatment might be conducted with fewer patients, each over a short interval. Some tests of cognition involve ACA territories which might be supplied by either ICA. Motor and sensory tasks of the hand and arm involve only the contralateral MCA territory always supplied by the corresponding ICA. Therefore, a quantitative test of MCA cerebral territory function might be useful to demonstrate that treatment of sCAS is beneficial to the patient.
The brain can be divided into “cerebrosomes” (cerebral regions supplied by a single branch of a cerebral artery). Resting brain tissue requires about 1.3 Watts/100 cc brain. The resting blood flow rate of 1 mL/s/100 cc brain results in an oxygen extraction of about 3 µM of O2 (20% O2 extraction) and production of 3 µM of CO2 per second. During activation, the local power density demand increases to about 5 Watts/100ccBrain quadrupling the CO2 production resulting in local vasodilation. The increase in local CO2 reduces local arteriolar/capillary resistance which doubles local perfusion rate accompanied by doubling the oxygen extraction fraction to supply the required increase in power demand. The resultant oxygen saturation decrease is most easily measured with transcranial functional near-infrared spectroscopy (fNIRS, pulse-oximetry of the brain) (14) or fMRI blood oxygenation level dependent (BOLD) methods (15). To deliver the required increase in power density demand, the arteries supplying each cerebrosome must be adequate caliber and be unobstructed including the aorta, carotids and cerebral arteries.
At rest, regional brain blood flow rates can be measured with MRI methods. Zarrinkoob et al. (16) measured flow rates in the extracranial arteries (ICAs and BA) and the flow rates in the cerebral arteries (ACA, MCA and PCA) at rest. Two groups of patients were compared, patients with mild CAS and patients with sCAS (Figure 6).

The distribution of flow rates in the cerebral arteries was similar for the moderate and severe CAS patients, but in the cases of sCAS, the flow rate in the ipsilateral ICA, averaged across the 25 cases, was lower than the MCA flow rate, requiring collateral supply via the coW from either the contralateral ICA or the BA. In these cases, on average, 1% of the cerebral flow was provided by flow reversal through the ipsilateral OA. Of course, in the Zarrinkoob study, and in the present analysis, patients have been pre-selected by survivorship bias; patients without adequate coW collateral die from severe carotid stenosis.
The cause of stroke in sCAS patients is usually attributed to the release of emboli from the atheroma causing the stenosis. In September 1980, in a Seattle talk sponsored by Merrill Spencer, John Johnson (17,18) explained the formation of an ulceration on a carotid atheroma: “and then the top blows off, just like Mt. St. Helens”. That is possible if the pressure in the ICA lumen is depressed by Venturi (10) and Coanda effects below the pressure of the intra-atheroma neovascularization. Inflated neovascularization in the atheroma is sometimes called intraplaque hemorrhage (IPH) (19). IPH is most likely with PSV >400 cm/s (20). Depressed intrastenotic pressure in systole has been documented by observing paradoxical arterial pulsation at the stenosis using ultrasound tissue Doppler (10,21,22). Although paradoxical pulsation of arterial diameter can be measured with any conventional ultrasound duplex scanner, a specialized engineering software signal processing modification is required. Prior to atheroma eruption (in asymptomatic patients), intra-atheroma neovascularization is most likely to arise from the adventitial vasa vasorum, after eruption (in symptomatic patients), the neovessels more often arise from the arterial lumen (23).
In 1977, Kartchner and McRae followed the clinical course of 1,287 patients referred to their laboratory for noninvasive evaluation of cerebrovascular (carotid artery) obstruction (24). The cases were followed for an average of 24 months (max 70 months) after testing. They tested for the presence eyeball pulse delay (indicating low OA pressure) and systolic carotid bruit; these two measures are independent indicators of pressure reducing carotid stenosis. The follow-up included tabulations of death, CEA and stroke (Table 1).
Table 1
| OPG | Death/all† | CEA/all | Stroke & noCEA/noCEA† | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No CPA bruit | CPA bruit | No CPA bruit | CPA bruit | No CPA bruit | CPA bruit | P | |||
| Normal | 63/877 (7.2%) | 12/107 (11.2%) | 15/877 (1.7%) | 13/107 (12.1%) | 3/862 (0.3%) | 1/94 (1.1%) | 0.00001 | ||
| Delay | 10/156 (6.4%) | 17/147 (11.6%) | 29/156 (18.6%) | 78/147 (53.1%) | 2/127 (1.6%) | 11/69 (15.9%) | |||
†, stroke vs. OPG delay + CPA bruit. OPG, oculoplethysmography; CPA, carotid phonoangiography; CEA, carotid endarterectomy.
The percentage of deaths was higher in patients with carotid bruit. Patients with carotid bruit or with ocular pulse delay were likely to be selected for CEA; more than half of the patients with both carotid bruit and ocular pulse delay were selected for endarterectomy. In those 1,152 patients that did not have CEA, only 17 of 1,152 (1.5%) had a stroke during follow-up. However, 11 of 17 (65%) of the strokes were in patients who had both carotid bruit AND ocular pulse delay. The stroke rate among those with bruit and ocular pulse delay was 15.9% (11 of 69). For those patients, only six CEAs were required to prevent one stroke. However, because the examination method was cumbersome and required specialized equipment, and had a poor sensitivity for X-ray angiography anatomic carotid stenosis, the method was abandoned.
Nearly a decade later, Bill Gee reported on the pressure in the OA in cases of sCAS (25,26). The OA pressure was derived from a method of compressing the eyeball to suppress pulsation. In normal patients OA pressure measurements between 100 and 140 mmHg were recorded: OA pressures were near SBP if OA pressure was 100 mmHg but if OA pressures were near 140 mmHg, some patients had SBP of 200 mmHg. Patients with sCAS were found to have an ipsilateral OA pressure as low as 60 mmHg.
The conventional classification of carotid stenosis primarily utilizes %DR of the ICA lumen as the standard supplemented with atheroma content and post-stenotic turbulence (Bruit). The most common method of ICA examination is ultrasonic duplex Doppler measurement of PSV converted to %DR (27) categories by either of two widely accepted methods (Figure 7): the “consensus” method (28,29) widely used in the US and the DEGUM method (11-13) used in the EU.

The PSV thresholds differ between the two methods and the DEGUM method specifically includes bruit (confetti sign) and the presence of collateral circulation. Using the PSV and %DR data from published articles, the overlap in classification can be appreciated. The data can be rendered as ROC curves (Figure 8) to show that the best combination of sensitivity and specificity occur at PSV values much lower than the 350 cm/s value that best correlated with MCA territory weakness.

The standard carotid Doppler examination does not currently include measurement of the direction of flow in the OA or supraorbital artery. Measurement of OA flow direction is often done as part of a transcranial Doppler (TCD) examination, but this exam is usually performed in a separate laboratory with dedicated TCD equipment. In the US, according to the Intersocietal Accreditation Commission, there are 1,280 laboratories accredited in carotid testing and 66 facilities accredited in TCD testing (30). OA direction measurement is an option for the cerebrovascular Doppler examination (31,32). Measurement of OA Doppler waveform is now advocated as part of a standard obstetrical examination during the third trimester using a conventional duplex scanner for prediction of pre-eclampsia (33). The OA examination technique, performed at the end of a regular ultrasound exam, can be learned with “minimal training” (34). The examination method has been used for a quarter of a century (35,36).
The standard carotid Doppler examination does not currently include measurement of bruit frequency. Lees and Dewey (37,38) found that the angiographic minimum lumen diameter of an ICA carotid stenosis could be accurately estimated from the audio frequency of a carotid bruit: D (mm) = 500 (mm/Hz)/F (Hz), where F is the audible frequency of the bruit. This frequency can be measured from the Doppler spectral waveform by turning down both the wall filter and the velocity range scale (frequency range scale). The presence of a carotid bruit carries a 2.49 (95% CI: 1.77 to 3.52) risk ratio for stroke (39).
The two additional measurements during a conventional ultrasound scan can be accomplished using any conventional duplex Doppler scanner with spectral waveform display and the data can be acquired with a small amount of additional effort. The information might provide the key parameters for differentiating the benign carotid stenosis from the hazardous carotid stenosis (Figure 9).
With modern anti-atherosclerotic medical therapy, there is controversy whether a severe carotid stenosis should be treated with revascularization or medical therapy. In an asymptomatic patient with sCAS and either bruit with measured bruit frequency or OA flow reversal, a trial of medical therapy to determine whether the bruit frequency declines indicating a decrease in stenosis severity and/or a normalization of OA flow direction provides a conspicuous indication that in that patient, medical therapy might provide protection against stroke and perhaps improved cerebral function including cognition. Medical therapy treats all atheromas in the arterial system including coronary and intracranial as well as carotid; endarterectomy or stent treats only the target stenosis.
Other methods have been promoted to identify MCA hypotension such as computational hemodynamics using simplifying assumptions (40). MRI methods have been used to detect OAr (41), but these add cost to the diagnostic procedure. The examination modification proposed here might offer important key information at little additional cost.
Conclusions
To demonstrate the value of the addition of OA flow direction and bruit measurement to the conventional carotid examination, prospective clinical testing is needed. For such testing or clinical trials, ICA PSV values should be preserved rather than converted to the less certain %DR classifications often used. For the outcome variable, specific functional testing of each MCA cerebral territory might provide the most specific and useful outcome variables.
Acknowledgments
The data acquisition and validation were part of the standard quality assurance policies of the University of Washington Harborview Medical Center in Seattle.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1681/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1681/dss
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1681/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1681/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995;273:1421-8.
- North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445-53. [Crossref] [PubMed]
- Brott TG, Howard G, Roubin GS, et al. Long-Term Results of Stenting versus Endarterectomy for Carotid-Artery Stenosis. N Engl J Med 2016;374:1021-31. [Crossref] [PubMed]
- De Silva KR, Silva R, Amaratunga D, et al. Types of the cerebral arterial circle (circle of Willis) in a Sri Lankan population. BMC Neurol 2011;11:5. [Crossref] [PubMed]
- Waaijer A, van Leeuwen MS, van der Worp HB, et al. Anatomic variations in the circle of Willis in patients with symptomatic carotid artery stenosis assessed with multidetector row CT angiography. Cerebrovasc Dis 2007;23:267-74. [Crossref] [PubMed]
- Zarrinkoob L, Ambarki K, Wåhlin A, et al. Blood flow distribution in cerebral arteries. J Cereb Blood Flow Metab 2015;35:648-54. [Crossref] [PubMed]
- Beach KW, Bergelin RO, Leotta DF, et al. Standardized ultrasound evaluation of carotid stenosis for clinical trials: University of Washington Ultrasound Reading Center. Cardiovasc Ultrasound 2010;8:39. [Crossref] [PubMed]
- Danyel LA, Brachaczek IA, Röhl JE, et al. Validation of an Oscillation Test for the Sonographic Assessment of Fetal-Type Posterior Cerebral Artery Variants in Migraine Patients with Visual Aura. Ultrasound Med Biol 2022;48:512-9. [Crossref] [PubMed]
- Kohn MA, Senyak J. Sample Size Calculators. UCSF CTSI. 20 December 2021. [Accessed 03 November 2023]. Available online: https://www.sample-size.net/
- Ramnarine KV, Hartshorne T, Sensier Y, et al. Tissue Doppler imaging of carotid plaque wall motion: a pilot study. Cardiovasc Ultrasound 2003;1:17. [Crossref] [PubMed]
- Arning C, Widder B, von Reutern GM, et al. Revision of DEGUM ultrasound criteria for grading internal carotid artery stenoses and transfer to NASCET measurement. Ultraschall Med 2010;31:251-7. [Crossref] [PubMed]
- Barlinn K, Rickmann H, Kitzler H, et al. Validation of Multiparametric Ultrasonography Criteria with Digital Subtraction Angiography in Carotid Artery Disease: A Prospective Multicenter Study. Ultraschall Med 2018;39:535-43. [Crossref] [PubMed]
- Winzer S, Rickmann H, Kitzler H, et al. Ultrasonography Grading of Internal Carotid Artery Disease: Multiparametric German Society of Ultrasound in Medicine (DEGUM) versus Society of Radiologists in Ultrasound (SRU) Consensus Criteria. Ultraschall Med 2022;43:608-13. [Crossref] [PubMed]
- Lacerenza M, Frabasile L, Buttafava M, et al. Motor cortex hemodynamic response to goal-oriented and non-goal-oriented tasks in healthy subjects. Front Neurosci 2023;17:1202705. [Crossref] [PubMed]
- Rangaprakash D, Barry RL, Deshpande G. The confound of hemodynamic response function variability in human resting-state functional MRI studies. Front Neurosci 2023;17:934138. [Crossref] [PubMed]
- Zarrinkoob L, Wåhlin A, Ambarki K, et al. Blood Flow Lateralization and Collateral Compensatory Mechanisms in Patients With Carotid Artery Stenosis. Stroke 2019;50:1081-8. [Crossref] [PubMed]
- Johnson JM, Ansel AL, Morgan S, et al. Ultrasonographic screening for evaluation and follow-up of carotid artery ulceration. A new basis for assessing risk. Am J Surg 1982;144:614-8. [Crossref] [PubMed]
- Johnson JM, Kennelly MM, Decesare D, et al. Natural history of asymptomatic carotid plaque. Arch Surg 1985;120:1010-2. [Crossref] [PubMed]
- Saam T, Underhill HR, Chu B, et al. Prevalence of American Heart Association type VI carotid atherosclerotic lesions identified by magnetic resonance imaging for different levels of stenosis as measured by duplex ultrasound. J Am Coll Cardiol 2008;51:1014-21. [Crossref] [PubMed]
- Beach KW, Hatsukami T, Detmer PR, et al. Carotid artery intraplaque hemorrhage and stenotic velocity. Stroke 1993;24:314-9. [Crossref] [PubMed]
- Xu C, Yuan C, Stutzman E, et al. Quest for the Vulnerable Atheroma: Carotid Stenosis and Diametric Strain--A Feasibility Study. Ultrasound Med Biol 2016;42:699-716. [Crossref] [PubMed]
- Bonnefous O, Luizy F, Kownator S. Arterial Wall Motion imaging: A new ultrasound approach to vascular characterization. Medica Mundi 2000;44:37-43.
- Uchihara Y, Saito K, Motoyama R, et al. Neovascularization From the Carotid Artery Lumen Into the Carotid Plaque Confirmed by Contrast-Enhanced Ultrasound and Histology. Ultrasound Med Biol 2023;49:1798-803. [Crossref] [PubMed]
- Kartchner MM, McRae LP. Noninvasive evaluation and management of the "asymptomatic" carotid bruit. Surgery 1977;82:840-7.
- Gee W. Carotid physiology with ocular pneumoplethysmography. Stroke 1982;13:666-73. [Crossref] [PubMed]
- Gee W. Ocular pneumoplethysmography. Surv Ophthalmol 1985;29:276-92.
- Beach KW, Leotta DF, Zierler RE. Carotid Doppler velocity measurements and anatomic stenosis: correlation is futile. Vasc Endovascular Surg 2012;46:466-74. [Crossref] [PubMed]
- Grant EG, Benson CB, Moneta GL, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis--Society of Radiologists in Ultrasound Consensus Conference. Radiology 2003;229:340-6. [Crossref] [PubMed]
- Grant EG, Benson CB, Moneta GL, et al. Carotid artery stenosis: grayscale and Doppler ultrasound diagnosis--Society of Radiologists in Ultrasound consensus conference. Ultrasound Q 2003;19:190-8. [Crossref] [PubMed]
- Marge Hutchisson. Intersocietal Accreditation Commission <https://intersocietal.org/>, private email. 06 December 2022.
- Mohler ER, Gornik HL, Gerhard-Herman M, et al. ACCF/ACR/AIUM/ASE/ASN/ICAVL/SCAI/SCCT/SIR/SVM/SVS 2012 Appropriate Use Criteria for Peripheral Vascular Ultrasound and Physiological Testing Part I: Arterial Ultrasound and Physiological Testing: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American College of Radiology, American Institute of Ultrasound in Medicine, American Society of Echocardiography, American Society of Nephrology, Intersocietal Commission for the Accreditation of Vascular Laboratories, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Interventional Radiology, Society for Vascular Medicine, and Society for Vascular Surgery. J Am Coll Cardiol 2012;60:242-76. [Crossref] [PubMed]
- AIUM Practice Parameter for the Performance of Transcranial Doppler Ultrasound. J Ultrasound Med 2023;42:E36-44. [Crossref] [PubMed]
- Lau KGY, Wright A, Kountouris E, et al. Ophthalmic artery peak systolic velocity ratio distinguishes pre-eclampsia from chronic and gestational hypertension: A prospective cohort study. BJOG 2022;129:1386-93. [Crossref] [PubMed]
- Sarno M, Wright A, Vieira N, et al. Ophthalmic artery Doppler in prediction of pre-eclampsia at 35-37 weeks' gestation. Ultrasound Obstet Gynecol 2020;56:717-24. [Crossref] [PubMed]
- Tranquart F, Bergès O, Koskas P, et al. Color Doppler imaging of orbital vessels: personal experience and literature review. J Clin Ultrasound 2003;31:258-73. [Crossref] [PubMed]
- Matthiessen ET, Zeitz O, Richard G, et al. Reproducibility of blood flow velocity measurements using colour decoded Doppler imaging. Eye (Lond) 2004;18:400-5. [Crossref] [PubMed]
- Duncan GW, Gruber JO, Dewey CF Jr, et al. Evaluation of carotid stenosis by phonoangiography. N Engl J Med 1975;293:1124-8. [Crossref] [PubMed]
- Lees RS, Kistler JP, Sanders D. Duplex Doppler scanning and spectral bruit analysis for diagnosing carotid stenosis. Circulation 1982;66:I102-5.
- Pickett CA, Jackson JL, Hemann BA, et al. Carotid bruits and cerebrovascular disease risk: a meta-analysis. Stroke 2010;41:2295-302. [Crossref] [PubMed]
- Holmgren M, Støverud KH, Zarrinkoob L, et al. Middle cerebral artery pressure laterality in patients with symptomatic ICA stenosis. PLoS One 2021;16:e0245337. [Crossref] [PubMed]
- Miralles M, Dolz JL, Cotillas J, et al. The role of the circle of Willis in carotid occlusion: assessment with phase contrast MR angiography and transcranial duplex. Eur J Vasc Endovasc Surg 1995;10:424-30. [Crossref] [PubMed]


