
The role of reconstructive microsurgeons in liver transplantation—a narrative review
Introduction
Liver transplantation is widely accepted worldwide as a life-saving procedure for suitable patients with end-stage liver failure. The first living donor liver transplantation was reported in 1989, and the procedure has gained traction ever since (1). Due to a limited supply of deceased liver donors and an ever-increasing demand for liver transplant, living donor liver transplantation has become ubiquitous in tertiary institutions (2,3). While living donor liver transplantation accounts for only 5.3% of all liver transplants in the United States (4), living donor liver transplantation programs have reported better patient survival, decreased length of stay, decrease hospital costs, decreased need for post-transplant dialysis, and less intraoperative blood product usage than decreased donor liver transplantation (5-7).
Liver transplantations are also associated with significant complications (8-10). One of the most feared complications in the acute postoperative phase of liver transplantation is hepatic artery thrombosis. This devastating event has a 3.8% to 9.0% incidence rate and results in critical ischemia of the biliary tree (11-14). This in turn may lead to septicaemia and hepatic necrosis (15,16). It is poorly tolerated with high mortality rate of around 50% and re-transplantation rate as high as 75% (15,17,18). As the biliary system is supplied entirely by the hepatic artery, the ischemic compromise of the biliary tree from hepatic artery thrombosis also leads to biliary strictures, biliary leakage or other complications (19,20). These biliary complications result in significant morbidity such as biliary stasis requiring drainage catheters, biliary sepsis and eventually liver failure (21,22).
Until recently, a donor liver graft with hepatic artery of less than 2 mm in diameter was a contraindication for liver transplantation due to high risk of hepatic artery thrombosis (23). Fortunately, the application of expert microsurgical techniques for hepatic arterial reconstruction has greatly reduced the risk of hepatic artery thrombosis and increased the pool of suitable donors, improving the lot of patients on liver transplant lists worldwide (24).
We have performed a narrative review on the current literature of the technical considerations and challenges of microsurgery in hepatic artery anastomosis for liver transplantation. We present this article in accordance with the Narrative Review reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-23-519/rc).
Methods
The search strategy summary is presented in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 3 December 2022 |
| Databases and other sources searched | PubMed, Web of Science, Google |
| Search terms used | “liver transplantation” and “microsurgery”, “living donor liver transplantation” and “microsurgery”, “deceased donor liver transplantation” and “microsurgery”, “hepatic artery” and “microsurgery”, “hepatic artery thrombosis” and “microsurgery”, “liver transplantation” and “microsurgical anastomosis”, “hepatic artery reconstruction” |
| Timeframe | 1985–2022 |
| Inclusion and exclusion criteria | Inclusion criteria: studies written in or translated to English. All study designs were included. Relevant articles pertaining to the challenges and technical considerations of microsurgery in liver transplantation were included. Studies not in English language were excluded |
| Selection process | Title, abstract and full text reviews were performed independently by two authors |
All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this article and accompanying images.
Preparation for microsurgery in liver transplant
Hepatic artery anastomosis demands the highest level of surgical expertise and surgeons who lead this key step of the liver transplantation must be adept at microsurgical techniques, regardless of their original specialty training. Prior to the liver transplantation surgery, we recommend a multi-disciplinary review of the case with the anaesthetists, transplant surgeons, microsurgeons, and radiology to discuss the anticipated technical challenges and optimization of the conditions, as each case is unique. In the following sections, we describe in some detail, the technical tips and tricks for a successful hepatic artery anastomosis.
Positioning
The operating microscope is positioned at the patient’s left, with its base in line with the thorax. The main surgeon stands at the patient’s right axilla, while the first assistant is at the patient’s left axilla. The main surgeon should have a long jeweller’s forceps (Figure 1) in his left hand and use his right hand to handle the curved micro-scissors or needle holder (Figure 2). The first assistant should have two long jewellers at his disposal as well as a straight micro-scissors to cut the micro-sutures. Adequate exposure is essential for success of the anastomosis, and this responsibility lies with the second assistant (23,25). The second assistant also stands at the patient’s left side, and manually retracts any protrusive loops of bowel. Forceful retraction is usually required but too much force will cause undue tension on the vascular anastomosis. The donor liver is positioned depending on the lie of the vessels. Ideally, it is usually easiest to orient the vessels with the cut ends at 45° angle sloping from the main surgeon’s left to right; however, this may not be possible. The main surgeon should be prepared and adept to perform the microsurgical anastomosis in every angle of the vessel cut ends. Back hand microsurgical stitching may be necessary. Furthermore, the first assistant, who may have a better angle or position, may also perform the microsurgical anastomosis as an additional measure.

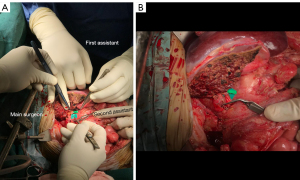
The liver may be retracted superiorly using a Thompson retractor to expose the vessels or a moist penny towel placed in the subdiaphramatic space to bring the liver forward, especially when the vessel stumps are short. A moist sponge may be placed deep to the vessels to elevate the plane of anastomosis and prevent fluid from interfering with field (26). Suction tube hooked to a small (5 French) feeding tube or cannulation tube can be placed beneath gauze or cottonoid to keep the field dry and optimize visualization.
Selection of the donor and recipient vessels
The size and quality of the donor artery will determine the inflow. It is important to preserve all three hepatic arteries (left, middle, and right) during the hepatoduodenal ligament dissection in the recipient (27).
The level of donor and recipient arterial anastomosis ultimately depends on the length and diameter of the vessels. In our experience, we usually anastomose the donor proper hepatic artery for full liver allografts, right hepatic artery or proper hepatic artery for right liver allografts, and left hepatic artery for left liver allografts. The recipient vessel use is usually the proper hepatic artery. The reconstructed hepatic artery should have a tension-free anastomosis with minimal redundancy and be free of kinks when the donor liver is returned to its resting position.
The recipient hepatic artery may be unsuitable for reconstruction in cases of scarring from previous operation, preoperative transarterial chemoembolization, arterial dissection or aneurysm. In such cases, two strategies can be adopted: (I) interposition grafts; (II) extra-anatomical recipient artery. For interposition grafts, various donor vessels have been used, such as the radial artery, iliac artery, inferior mesenteric artery, superior rectal artery, and great saphenous vein (28-31). Some of the extra-anatomical arteries used include left gastric artery, right gastroepiploic artery, right gastric artery, gastroduodenal artery, splenic artery and cystic artery (30,32). These strategies can also be used for re-anastomosis for hepatic artery thrombosis or re-transplantation cases, after all previous anastomoses are excised.
Liver graft with multiple arteries
A “classic” hepatic arterial anatomy is only present in 55–60% of the population with various arterial variations (33,34). Multiple arteries in liver graft are not uncommon, and this is more prevalent in left lobe grafts, where the segment four artery arises from right hepatic artery (35). Other variations include accessory right hepatic artery from superior mesenteric artery or when anterior and posterior divisions of right hepatic artery occur extrahepatically (36).
When managing multiple arteries in grafts for liver transplant, there is controversial evidence regarding the need for reconstruction of all graft arteries. In some studies, there are significantly higher number of biliary complications when one artery was reconstructed compared to multiple arterial reconstructions (37,38) while in other studies, there is no statistical difference in the biliary complications (39,40). Notably, single arterial reconstruction has been advocated to reduce the incidence of arterial complications (41).
Multiple authors have proposed a management algorithm for the need for reconstruction of all graft arteries (40,42). This is based on collateral blood vessels forming a plexus between right and left hepatic arteries, termed as the “hilar plexus”. The authors suggested that multiple arterial reconstructions are unnecessary when (I) there is satisfactory back bleed after division of smaller of arteries prior to graft recovery during the donor surgery, and (II) when there is good pulsatile arterial back bleed from the second artery after the dominant artery reconstruction, with the presence of intrahepatic Doppler flow in the non-arterialized segment of the graft in the recipient. The authors reported no significant incidence in the complications between single and multiple arterial reconstructions.
One way to deal with multiple graft hepatic arteries is the use of unification arterioplasty, which forms a single arterial orifice to anastomose to the recipient artery (32). This can be achieved using side-to-side technique or end-to-side technique (32). When an accessory right hepatic artery from superior mesenteric artery is present, an end-to-end anastomosis of this right branch to the donor stump of the splenic artery can be employed (43,44). Thereafter the donor celiac axis artery can be anastomosed to the recipient artery of choice.
Outcomes of microsurgical hepatic arterial reconstruction
Multiple studies have compared hepatic artery reconstruction with surgical loupes versus operating microscope. While it has been reported that microsurgical anastomosis using operating microscope significantly reduced the rate of hepatic artery thrombosis in some studies (45-48), others found little notable differences compared to using surgical loupes (49,50). Table 2 summarizes these studies. It is interesting to note that in the studies that show no significant difference, surgical loupes of at least 5.0 times magnification was used, while the studies that show significant difference did not specify the magnification of the surgical loupes.
Table 2
| Studies | Number of patients | Risk of hepatic artery complications | LDLT or DDLT | P value | |||
|---|---|---|---|---|---|---|---|
| Without microsurgical reconstruction | With microsurgical reconstruction | Without microsurgery | With microsurgery | ||||
| Studies that show significant difference in the hepatic artery complications | |||||||
| Tan et al. [2021] (45) | 80 | 48 | 6.2% (early hepatic artery thrombosis) | 2.1% (early hepatic artery thrombosis) | LDLT | 0.280 | |
| 35% (hepatic artery complications) | 5.3% (hepatic artery complications) | 0.022 | |||||
| Yoon et al. [2021] (46) | 342 | 128 | 7.6% (hepatic artery complications) | 4.7% (hepatic artery complications) | DDLT | 0.264 | |
| Nickel et al. [2021] (47) | 180 | 51 | 8.3% (hepatic artery thrombosis) | 2% (hepatic artery thrombosis) | LDLT | 0.114 | |
| Dziodzio et al. [2021] (48) | 58 | 21 | 24.1% (hepatic artery thrombosis) | 0% (hepatic artery thrombosis) | LDLT and DDLT | 0.013 | |
| 17.2% (retransplantation) | 0% (retransplantation) | 0.042 | |||||
| Studies that did not show significant difference in the hepatic artery complications | |||||||
| Jwa et al. [2019] (49) | 101 | 136 | 2% (hepatic artery thrombosis) | 1.5% (hepatic artery thrombosis) | LDLT | 0.763 | |
| Seo et al. [2021] (50) | 150 | 150 | 1.3% (hepatic artery complications) | 1.3% (hepatic artery complications) | LDLT | >0.99 | |
LDLT, liver donor liver transplant; DDLT, deceased donor liver transplant.
In the authors’ experience for hepatic arteries 4 mm in diameter or less, using the operating microscope with all the tools and techniques in microsurgery for hepatic artery anastomosis allows for surveillance of dissociation between the intimal and medial vessel layers of the artery, thrombus formation within the artery, and discrepancies in the arterial wall thickness, which are all potential risk factors associated with hepatic artery thrombosis. For hepatic arteries that have a larger diameter, the advantages of using surgical loupes which afford wider operative field of view and more flexibility in suturing technique need to be weighed against the lower surgical efficiency that comes with the use of an operating microscope, as well as a less ergonomic position for the main surgeon and the assistant.
Microsurgical techniques in liver transplant
Preparation of donor and recipient vessels
The dissection of the recipient artery is carried as proximal as necessary to obtain vessel of good calibre and optimal blood inflow, and this is usually 1 to 2 cm proximal to the take-off of the gastroduodenal branch (44). The ideal recipient artery should have adequate length, minimal fibrosis and adequate flow (51).
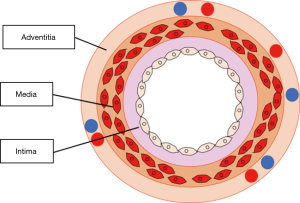
The cross-sectional anatomy of an artery is made up of the intima, media and adventitia (Figure 3). The adventitia is the layer with the highest tensile strength (52,53) and hence should not trimmed excessively to minimise the risk of suture cut out. Before the anastomosis, it is important to check for risk factors that can cause hepatic artery thrombosis. These include soft thrombus in the artery (these must be removed), intimal or medial wall dissection (vessel should be cut back to an area that is healthy), or intimal flaps protruding into the lumen (26). Intimal flaps may cause turbulence and should be trimmed off circumferentially.
Stitching techniques
A double clamp, such as an Ikuta or Acland is placed and the vessels are approximated until the vessel ends are close to each other. Clamps should be released before suturing to check for adequacy of the arterial flow at the recipient vessel, which should be pulsatile and strong. The release of clamps also confirm that arterial flow comes from the lumen of the vessel, rather than from between the intima and media layers of the vessel, in the case of arterial dissection. The lumen of the donor and recipient vessels should be flushed with heparinized saline (25).
One method is to use stay sutures with 8/0 or 9/0 sutures are placed at 0° and 180° and cut long to be used for retraction of the vessel edges. The rest of the sutures are distributed evenly along the edges (26) and the knots are not tied immediately after sutures are placed. The lumen is also irrigated with heparinized saline whenever required to give a clear view so as to avoid suturing the back wall. After confirming the suture orientation and placement, as well as free back wall, the sutures are then tied with at least three throws. Care is taken not to create too much tension at the anastomosis when tying the sutures as this creates turbulence of the blood flow that can result in thrombosis (25). Another method is the back wall up technique that the authors’ prefer for its simplicity and ability to handle vessel size mismatch better in the hands of the authors.
Simple interrupted or continuous techniques have been used for microsurgical anastomosis of the vessels. Proponents of continuous technique argue for its speed and ease of suturing, while proponents of simple interrupted technique report lower hepatic artery complications (54). Tzeng et al. found no difference in the rates of hepatic artery complications between simple interrupted or continuous techniques (55). Suffice to say, operator competence is the main determinant of a successful anastomosis.
Staggered stitching technique
A staggering stitching technique (Figure 4) was described especially for friable vessels, especially those that have undergone chemoembolization (51,56). For this technique, interrupted stitches were performed at different distances from the vessel edge, so as to prevent a linear line of suture holes in the vessel wall that has a tendency to fissure.

Techniques to handle the adventitia
One technique is to anastomose the intimal and medial layer with 8/0 or 9/0 sutures and then reinforce the anastomosis with adventitial stitches using 9/0 or 10/0 sutures and subsequently with fibrin sealant (51). These adventitial stitches can seal the suture hole gaps which become obvious only when the vessel clamps are released. One theoretical risk is triggering the coagulation cascade from an inadvertent exposure of the blood stream within the vessel to the adventitia. As such, the authors’ preferred technique is to take full-thickness bites of the vessels during microvascular anastomosis that include all three layers of the vessel.
Techniques to handle vessel delamination
For vessels with intimal dissection or tendency for endothelial delamination and trimming of the vessel is not feasible, stitching can be done intraluminally to extraluminally (Figure 5) past the point of the dissection or delamination (51,56). If there is dissection or delamination in both donor and recipient vessels, a single strand of double arm suture can be used so that the suture can be introduced from intraluminally on both sides. This prevents further lifting off of the endothelium or the intimal layer.

The problem of sewing in a deep abdominal cavity with limited space
The site of hepatic artery anastomosis is usually deep within the abdominal cavity, which results in limited surgical motion and field of vision (57,58). This limited space may also cause kinking of the vessels post anastomosis and manipulation of the bile ducts may push the vessels into an undesirable orientation (26). To overcome this challenge, as mentioned previously, the role of the second assistant to manually retract the surrounding bowel loops and donor liver cannot be understated. Longer instruments of 18–21 cm, especially in the left hand of the main surgeon, are highly recommended (27). Another strategy the authors use when the limited space makes passing the needle difficult at certain sections of the anastomosis is to have the first assistant pass the needle instead of the main surgeon. This is often the case in situations where the main surgeon has to pass the suture via a backhand or any other awkward angle.
Movement during microsurgical anastomosis
Interference from cardiac, aortic, and respiratory movements affects the stability of the small operative field and poses a significant challenge (23,57). Teamwork between the surgical and anaesthesiology teams is essential to maximize the stability. Before the suturing begins, the anaesthesiology team is informed to control the extent of respiratory movements by decreasing the respiratory rate and tidal volume to limit the superior-inferior movement of the surgical field (26,27). In some instances, the main surgeon may even ask that the anaesthesiologist stops respiration completely for a short duration while the needle is passed. This is important especially when placing critical sutures, such as those for haemostasis or at difficult angles and when fatigue is beginning to set in during times where multiple revisions of the anastomosis were deemed to be necessary.
Vessel size mismatch
Vessel size mismatch is expected because patients with liver cirrhosis suffer from chronic portal hypertension that cause hypertrophy of the coeliac axis (51). In experimental models, size discrepancy directly results in lower patency (59). The mismatch between donor and recipient arteries may be in the internal diameter as well as intimal thickness. The left or right hepatic artery is the usual choices for recipient artery. For larger calibre donor vessels, the recipient artery can be the bifurcation of the left and right hepatic arteries at the proper hepatic artery or the splenic artery (51).
Ways to overcome marked size discrepancy is to do a slanted cut or “fish mouth” cut with the smaller artery or use differential stitching in the back wall up technique. Some authors also suggest an end-to-side anastomosis if the donor artery is particularly small (60).
Poor quality vessels
The recipient arteries are usually of poor quality with fibrosis from previous peritonitis, atherosclerosis associated with fatty liver, or fragile after rounds of transarterial chemoembolization (56). Tan et al. described an angled cutting platform and an 11 blade on an angled blade holder to trim the diseased vessel to obtain a linear arteriotomy (51). The vessel edges are trimmed until it is healthy (i.e., the intima should be adherent to the media of the vessel wall) but not skeletonised. Other options of recipient artery include gastroduodenal artery, which is usually spared during transarterial chemoembolization, and the common hepatic artery. Excessive stripping of the adventitia should be minimized during the preparation of the vessels.
Short vessel stump
When the donor vessel length is short, arterial or venous interpositional grafts may be used. Venous graft (e.g., from the saphenous or common iliac vein) usually has thinner vessel wall and not be as suitable for transmission of high arterial pressures. Thrombosis rates of venous grafts have been reported to be as high as 23.8% (15). A radial artery interpositional graft is commonly used in the setting of inadequate vessel length (61). Preoperatively, it is important to assess for any percutaneous vascular interventions such as intra-arterial line insertion, coronary angioplasty that may injure the radial artery (62). Allen’s test should be performed. The descending branch of lateral circumflex femoral artery, or a segmental part of recipient hepatic artery, is an alternative if Allen’s test is abnormal. The back-wall first technique will also be necessary as rotation of the donor artery will not be possible.
Conclusions
The goal of succeeding in this life-preserving procedure brings many high-performing individuals together including the hepatobiliary surgeons, microsurgeons and anaesthesiologists. While expecting each of them to give their best is a given, paying attention to transforming them into accomplished individuals to a high-performing team is arguably the most important. Using an article on high functioning teams as a guide (44), we need our team members to communicate often and well (both at work and outside of it) and this is brought into sharp focus during the decision-making on which of the surgeons should perform the anastomosis. Clear criteria should be agreed on beforehand and applied with joint decision-making. Joint decision-making may involve having the microsurgical team present at the hepatic artery anastomosis regardless of which team the main surgeon hails from. This fosters ownership and provides instant support should the anastomosis team run into difficulty.
With the combination of individual professionalism and high-performance team management, microsurgeons will continue to be a priceless resource that all liver transplant teams should have to produce the best outcomes for their patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Johnny Ionut Efanov) for the series “The Modern Plastic and Reconstructive Surgeon – Collaborator, Innovator, Leader” published in Annals of Translational Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-519/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-519/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-519/coif). The series “The Modern Plastic and Reconstructive Surgeon – Collaborator, Innovator, Leader” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this article and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Raia S, Nery JR, Mies S. Liver transplantation from live donors. Lancet 1989;2:497. [Crossref] [PubMed]
- Fayek SA, Quintini C, Chavin KD, et al. The Current State of Liver Transplantation in the United States: Perspective From American Society of Transplant Surgeons (ASTS) Scientific Studies Committee and Endorsed by ASTS Council. Am J Transplant 2016;16:3093-104. [Crossref] [PubMed]
- Sugawara Y, Makuuchi M. Technical advances in living-related liver transplantation. J Hepatobiliary Pancreat Surg 1999;6:245-53. [Crossref] [PubMed]
- Kwong AJ, Kim WR, Lake JR, et al. OPTN/SRTR 2019 Annual Data Report: Liver. Am J Transplant 2021;21:208-315. [Crossref] [PubMed]
- Humar A, Ganesh S, Jorgensen D, et al. Adult Living Donor Versus Deceased Donor Liver Transplant (LDLT Versus DDLT) at a Single Center: Time to Change Our Paradigm for Liver Transplant. Ann Surg 2019;270:444-51. [Crossref] [PubMed]
- Barbetta A, Aljehani M, Kim M, et al. Meta-analysis and meta-regression of outcomes for adult living donor liver transplantation versus deceased donor liver transplantation. Am J Transplant 2021;21:2399-412. [Crossref] [PubMed]
- Black M, Gupta A, Asrani SK, et al. Living donor liver transplantation versus donation after brain death and donation after circulatory death liver transplantation in the US. Proc (Bayl Univ Med Cent) 2022;35:273-7. [Crossref] [PubMed]
- Berg CL, Merion RM, Shearon TH, et al. Liver transplant recipient survival benefit with living donation in the model for endstage liver disease allocation era. Hepatology 2011;54:1313-21. [Crossref] [PubMed]
- Freise CE, Gillespie BW, Koffron AJ, et al. Recipient morbidity after living and deceased donor liver transplantation: findings from the A2ALL Retrospective Cohort Study. Am J Transplant 2008;8:2569-79. [Crossref] [PubMed]
- Olthoff KM, Merion RM, Ghobrial RM, et al. Outcomes of 385 adult-to-adult living donor liver transplant recipients: a report from the A2ALL Consortium. Ann Surg 2005;242:314-23, discussion 323-5. [Crossref] [PubMed]
- Iida T, Kaido T, Yagi S, et al. Hepatic arterial complications in adult living donor liver transplant recipients: a single-center experience of 673 cases. Clin Transplant 2014;28:1025-30. [Crossref] [PubMed]
- Proposito D, Loinaz Segurola C, Garcia Garcìa I, et al. Assessment of risk factors in the incidence of hepatic artery thrombosis in a consecutive series of 687 liver transplantations. Ann Ital Chir 2001;72:187-205.
- Abou Ella KA, Al Sebayel MI, Ramirez CB, et al. Hepatic artery thrombosis after orthotopic liver transplantation. Saudi Med J 2001;22:211-4.
- Song S, Kwon CH, Moon HH, et al. Single-Center Experience of Consecutive 522 Cases of Hepatic Artery Anastomosis in Living-Donor Liver Transplantation. Transplant Proc 2015;47:1905-11. [Crossref] [PubMed]
- Tzakis AG, Gordon RD, Shaw BW Jr, et al. Clinical presentation of hepatic artery thrombosis after liver transplantation in the cyclosporine era. Transplantation 1985;40:667-71. [Crossref] [PubMed]
- Rela M, Muiesan P, Bhatnagar V, et al. Hepatic artery thrombosis after liver transplantation in children under 5 years of age. Transplantation 1996;61:1355-7. [Crossref] [PubMed]
- Stange BJ, Glanemann M, Nuessler NC, et al. Hepatic artery thrombosis after adult liver transplantation. Liver Transpl 2003;9:612-20. [Crossref] [PubMed]
- Bekker J, Ploem S, de Jong KP. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant 2009;9:746-57. [Crossref] [PubMed]
- Northover J, Terblanche J. Bile duct blood supply. Its importance in human liver transplantation. Transplantation 1978;26:67-9.
- Takasaki S, Hano H. Three-dimensional observations of the human hepatic artery (Arterial system in the liver). J Hepatol 2001;34:455-66. [Crossref] [PubMed]
- Lattanzi B, Ott P, Rasmussen A, et al. Ischemic Damage Represents the Main Risk Factor for Biliary Stricture After Liver Transplantation: A Follow-Up Study in a Danish Population. In Vivo 2018;32:1623-8. [Crossref] [PubMed]
- Testa G, Malagó M, Valentín-Gamazo C, et al. Biliary anastomosis in living related liver transplantation using the right liver lobe: techniques and complications. Liver Transpl 2000;6:710-4. [Crossref] [PubMed]
- Mori K, Nagata I, Yamagata S, et al. The introduction of microvascular surgery to hepatic artery reconstruction in living-donor liver transplantation--its surgical advantages compared with conventional procedures. Transplantation 1992;54:263-8. [Crossref] [PubMed]
- Starzl TE, Porter KA, Putnam CW, et al. Orthotopic liver transplantation in ninety-three patients. Surg Gynecol Obstet 1976;142:487-505.
- Wei WI, Lam LK, Ng RW, et al. Microvascular reconstruction of the hepatic artery in live donor liver transplantation: experience across a decade. Arch Surg 2004;139:304-7. [Crossref] [PubMed]
- Lee CF, Lu JC, Zidan A, et al. Microscope-assisted hepatic artery reconstruction in adult living donor liver transplantation-A review of 325 consecutive cases in a single center. Clin Transplant 2017;
- Balci D, Ahn CS. Hepatic artery reconstruction in living donor liver transplantation. Curr Opin Organ Transplant 2019;24:631-6. [Crossref] [PubMed]
- Fong HC, Tan EK, Chew KY, et al. Hepatic Artery Reconstruction in Living Donor Liver Transplantation With the Radial Artery Interpositional Graft. Transplant Proc 2021;53:1659-64. [Crossref] [PubMed]
- Li PC, Thorat A, Jeng LB, et al. Successful application of supraceliac aortohepatic conduit using saphenous venous graft in right Lobe living donor liver transplantation. Liver Transpl 2017;23:976-80. [Crossref] [PubMed]
- Uchiyama H, Shirabe K, Taketomi A, et al. Extra-anatomical hepatic artery reconstruction in living donor liver transplantation: can this procedure save hepatic grafts? Liver Transpl 2010;16:1054-61. [Crossref] [PubMed]
- Lau NS, Liu K, Almoflihi A, et al. Liberal Use of Interposition Grafts for Arterial Reconstruction Is Safe and Effective in Adult Split Liver Transplantation. Transplant Direct 2021;7:e735. [Crossref] [PubMed]
- Li PC, Thorat A, Jeng LB, et al. Hepatic artery reconstruction in living donor liver transplantation using surgical loupes: Achieving low rate of hepatic arterial thrombosis in 741 consecutive recipients-tips and tricks to overcome the poor hepatic arterial flow. Liver Transpl 2017;23:887-98. [Crossref] [PubMed]
- Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 1966;112:337-47. [Crossref] [PubMed]
- Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg 1994;220:50-2. [Crossref] [PubMed]
- Lee KW, Lee S, Oh DK, et al. Outcome of partial reconstruction of multiple hepatic arteries in pediatric living donor liver transplantation using left liver grafts. Transpl Int 2016;29:890-6. [Crossref] [PubMed]
- Lee KW, Lee S, Huh J, et al. Outcome of living donor liver transplantation using right liver allografts with multiple arterial supply. Liver Transpl 2016;22:1649-55. [Crossref] [PubMed]
- Suehiro T, Ninomiya M, Shiotani S, et al. Hepatic artery reconstruction and biliary stricture formation after living donor adult liver transplantation using the left lobe. Liver Transpl 2002;8:495-9. [Crossref] [PubMed]
- Uchiyama H, Harada N, Sanefuji K, et al. Dual hepatic artery reconstruction in living donor liver transplantation using a left hepatic graft with 2 hepatic arterial stumps. Surgery 2010;147:878-86. [Crossref] [PubMed]
- Mehta NN, Mangla V, Varma V, et al. Minimizing Hepatic Artery Thrombosis and Establishing Safety of Grafts With Dual Arteries in Living Donor Liver Transplantation. Transplant Proc 2018;50:1378-85. [Crossref] [PubMed]
- Puri Y, Palaniappan K, Rammohan A, et al. Anatomical Basis for Selective Multiple Arterial Reconstructions in Living Donor Liver Transplantation. Langenbecks Arch Surg 2021;406:1943-9. [Crossref] [PubMed]
- Zhang R, Zhang HZ, Han T, et al. Effect of accessory hepatic artery reconstruction on prognosis in orthotopic liver transplantation: a single center experience. BMC Surg 2023;23:138. [Crossref] [PubMed]
- Ikegami T, Kawasaki S, Matsunami H, et al. Should all hepatic arterial branches be reconstructed in living-related liver transplantation? Surgery 1996;119:431-6. [Crossref] [PubMed]
- Shaw BW Jr, Iwatsuki S, Starzl TE. Alternative methods of arterialization of the hepatic graft. Surg Gynecol Obstet 1984;159:490-3.
- Makowka L, Stieber AC, Sher L, et al. Surgical technique of orthotopic liver transplantation. Gastroenterol Clin North Am 1988;17:33-51.
- Tan EK, Tan BK, Fong HC, et al. Impact of Microsurgical Anastomosis of Hepatic Artery on Arterial Complications and Survival Outcomes After Liver Transplantation. Transplant Proc 2021;53:65-72. [Crossref] [PubMed]
- Yoon YI, Lee SG, Moon DB, et al. Microsurgical Hepatic Artery Reconstruction in Deceased Donor Liver Transplantation for Reduced Arterial Complications. Transplant Proc 2021;53:1645-52. [Crossref] [PubMed]
- Nickel KJ, Staples J, Meeberg G, et al. The Transition to Microsurgical Technique for Hepatic Artery Reconstruction in Pediatric Liver Transplantation. Plast Reconstr Surg 2021;148:248e-57e. [Crossref] [PubMed]
- Dziodzio T, Martin F, Gül-Klein S, et al. Hepatic artery reconstruction using an operating microscope in pediatric liver transplantation-Is it worth the effort? Pediatr Transplant 2022;26:e14188. [Crossref] [PubMed]
- Jwa EK, Kim JD, Choi DL. Comparison of hepatic artery reconstruction using surgical loupe and operating microscope during living donor liver transplantation focusing on the beginner's point. Ann Hepatobiliary Pancreat Surg 2019;23:122-7. [Crossref] [PubMed]
- Seo CH, Ahn J, You YK, et al. Single-Center Experience with Hepatic Artery Reconstruction During Living Donor Liver Transplantation: Microscope Versus Surgical Loupe. Ann Transplant 2021;26:e933371. [Crossref] [PubMed]
- Tan BK, Fong HC, Tan EK, et al. Strategies for a successful hepatic artery anastomosis in liver transplantation: A review of 51 cases. Ann Acad Med Singap 2021;50:679-85. [Crossref] [PubMed]
- Holzapfel GA, Sommer G, Regitnig P. Anisotropic mechanical properties of tissue components in human atherosclerotic plaques. J Biomech Eng 2004;126:657-65. [Crossref] [PubMed]
- Teng Z, Tang D, Zheng J, et al. An experimental study on the ultimate strength of the adventitia and media of human atherosclerotic carotid arteries in circumferential and axial directions. J Biomech 2009;42:2535-9. [Crossref] [PubMed]
- Coelho GR, Leitao AS Jr, Cavalcante FP, et al. Continuous versus interrupted suture for hepatic artery anastomosis in liver transplantation: differences in the incidence of hepatic artery thrombosis. Transplant Proc 2008;40:3545-7. [Crossref] [PubMed]
- Tzeng YS, Hsieh CB, Chen SG. Continuous versus interrupted suture for hepatic artery reconstruction using a loupe in living-donor liver transplantation. Ann Transplant 2011;16:12-5. [Crossref] [PubMed]
- Ng SW. Hepatic artery anastomosis in liver transplantation. Ann Acad Med Singap 2021;50:666-8. [Crossref] [PubMed]
- Hernandez JA, Mullens CL, Aoyama JT, et al. Analysis of Outcomes in Living Donor Liver Transplants Involving Reconstructive Microsurgeons. J Reconstr Microsurg 2020;36:223-7. [Crossref] [PubMed]
- Kim BW, Won JH, Lee BM, et al. Intraarterial thrombolytic treatment for hepatic artery thrombosis immediately after living donor liver transplantation. Transplant Proc 2006;38:3128-31. [Crossref] [PubMed]
- Rickard RF, Wilson J, Hudson DA. Characterization of a rodent model for the study of arterial microanastomoses with size discrepancy (small-to-large). Lab Anim 2009;43:350-6. [Crossref] [PubMed]
- Tan BK, Wong CH, Chew W, et al. Use of the slit arteriotomy for end-to-side arterial anastomosis in free-tissue transfers to the extremities. J Plast Reconstr Aesthet Surg 2009;62:1519-23. [Crossref] [PubMed]
- Mizuno S, Yokoi H, Isaji S, et al. Using a radial artery as an interpositional vascular graft in a living-donor liver transplantation for hepatocellular carcinoma. Transpl Int 2005;18:408-11. [Crossref] [PubMed]
- Dietl CA, Benoit CH. Radial artery graft for coronary revascularization: technical considerations. Ann Thorac Surg 1995;60:102-9; discussion 109-10.


