
Autogenous breast reconstruction for total mastectomies: a narrative review
Introduction
A partial or total mastectomy profoundly affects a woman’s body image, leading to a significant socio-psychological impact and motivating patients to undergo breast reconstruction. Breast reconstruction following mastectomy is associated with higher patient satisfaction and quality of life (1). According to the American Society of Plastic Surgeons (2), less than half of all women who require a mastectomy are doing breast reconstruction surgery, and fewer than a quarter of them understand the wide range of breast reconstruction options available. Moreover, less than 20% are elected to undergo immediate reconstruction (2).
Czerny realized one of the first breast reconstruction attempts in 1895 using a lipoma for a lumpectomy. The first breast reconstruction with implant occurred in 1971, in a subcutaneous plan. Then it was combined with a latissimus dorsi (LD) flap in the late 1970s. Since then, implant-based breast reconstruction has gained popularity and acceptance among the public (3,4). Silicone breast implants have been the subject of many successive controversies questioning the use of these implants: capsular retraction, device failure, anaplastic large cell lymphoma (ALCL) and more recently, breast implant illness (BII) (5). Acellular dermis matrices have improved significantly the results of implant-based breast reconstruction (6) but remain a supplementary foreign body with its own share of problems (7,8).
The use of autologous tissue for breast reconstruction exploded following Taylor’s research on angiosomes, microsurgery development, and, more recently, the standardization of fat grafting (9,10). There are numerous advantages to using autogenous solutions over implants: more natural feeling and texture, no implant surveillance, a better patient satisfaction rate and better resistance to radiation therapy (11). In addition, the tissue modeling can be specific for each patient’s anatomic features, such as the thorax width, the contralateral breast volume, or the desired volume in case of bilateral mastectomies and the overall appearance. However, donor site outcomes, surgery duration and safety concerns have emerged regarding the risk of cancer recurrences due to growth factors within the fat graft and the postoperative calcifications (12).
This narrative review aims to provide the readers a comprehensive, evidence-based overview of state-of-the-art autologous breast reconstruction after total mastectomy, including the selection of the flaps, the outcomes, and the recent advancements. We present this article in accordance with the Narrative Review reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1471/rc).
Methods
We conducted a narrative review of the literature searching for papers published between January 2010 and December 2022 about autogenous breast reconstruction. We used the MeSH terms with different combinations to identify articles for inclusion in this review, including: “breast reconstruction”, “deep inferior epigastric perforator flap”, “DIEP”, “transverse upper gracilis”, “TUG”, “Transverse Myocutaneous Gracilis Flaps”, “TMG”, “superior gluteal artery perforator”, “SGAP”, “inferior gluteal artery perforator”, “IGAP”, “free fasciocutaneous infragluteal”, “FCI flap”, “latissimus dorsi flap”, “latissimus dorsi”, “LD”, “autologous fat graft”, “lipofilling”, “lipomodeling”, “Breast Q”, “complications” and “nipple-areola complex reconstruction” (Table 1). Backward chaining of reference lists from retrieved papers was also used to expand the search. Articles were limited to those published in English and from peer-reviewed journals with an impact factor of over 0.5. Retrospective and prospective primary clinical studies were included. Conference abstracts, case reports, small case series (≤10 cases), letters to the editor, brief communications, editorial comments, and animal studies were excluded.
Table 1
| Items | Specification |
|---|---|
| Date of search | December 1st, 2022 |
| Databases and other sources searched | PubMed |
| Search terms used (including MeSH and free text search terms and filters) | “Breast reconstruction”, “deep inferior epigastric perforator flap”, “DIEP”, “Tram flap”, “Transverse rectus abdominis muscle flap”, “SIEA flap”, “transverse upper gracilis flap”, “TUG”, “Transverse Myocutaneous Gracilis Flaps”, “TMG”, “profunda artery perforator flap”, “PAP flap”, “superior gluteal artery perforator flap”, “SGAP”, “inferior gluteal artery perforator”, “IGAP”, “free fasciocutaneous infragluteal”, “FCI flap”, “latissimus dorsi flap”, “LD flap”, “extended LD flap”, “lipofilling”, “autologous fat graft”, “Lipomodeling”, “Breast Q”, “complications”, “nipple areola complex reconstruction” |
| Timeframe | From January 2010 to December 2022 |
| Inclusion and exclusion criteria | Inclusion criteria: |
| ❖ English language articles | |
| ❖ Peer-reviewed journal | |
| ⬥ Impact factor ≥0.5 | |
| ⬥ Meta-analyses | |
| ⬥ Systematic review | |
| ⬥ Retrospective and prospective primary clinical studies | |
| Exclusion criteria: | |
| ❖ Implant-based breast reconstructions | |
| ❖ Conference abstracts | |
| ❖ Case reports | |
| ❖ Small case series (≤10 cases) | |
| ❖ Letters to the editor | |
| ❖ Brief communications | |
| ❖ Editorial comments | |
| ❖ Animal studies | |
| Selection process | Three authors independently screened the initial retrieved of articles |
DIEP, deep inferior epigastric perforator; SIEA, superficial inferior epigastric artery; TUG, transverse upper gracilis; TMG, transverse myocutaneous gracilis; PAP, profunda artery perforator; SGAP, superior gluteal artery perforator; IGAP, inferior gluteal artery perforator; FCI, fasciocutaneous infragluteal; LD, latissimus dorsi.
An initial retrieval of 2,823 articles were identified using these parameters. After screening article titles and abstracts independently by three authors, 66 papers and their reference lists were included for full-text review. The articles have been classified according to the objectives displayed and the used techniques. The extracted data included the year of publication, the study design, the total number of patients and number of procedures, the type of technique, surgical outcomes, complications, and patients’ satisfaction. Then, we selected the relevant papers for our narrative review. We included only techniques presented in a minimum of ten articles over five different years as representing methods having found a permanent audience in the surgical community.
Results and considerations
The search for autogenous solutions has remained constant over the year, the main concerns being having a sufficient donor site, lowering the donor site morbidity, and looking for a natural appearance and texture. There are two main categories of autogenous breast reconstruction: the flaps (pedicled or free flaps) and the autologous fat transfer.
We divided the different autologous breast reconstruction options according to the donor sites: the abdomen, the back, the inner thigh, and the gluteal area. We added a paragraph on the autologous fat graft.
Flaps using the abdominal tissues
The use of the abdomen for breast reconstruction is compelling for two reasons: the considerable volume of tissue that may be available, and the fact that abdominal scars can be cleverly hidden with the underwear. Furthermore, it allows an improvement of the abdominal contours.
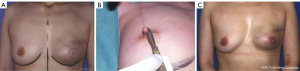
Flap including rectus abdominis muscles: transverse rectus abdominis myocutaneous (TRAM) flap (Figure 1)
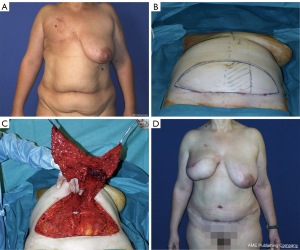
The pedicled rectus abdominis muscle flap use started in the 1970s. First associated with a vertical incision, Hartrampf transferred to a transversely oriented abdominal musculocutaneous island (TRAM) flap (13). The technique consists of using the ipsilateral TRAM flap with 180° rotation, pedicled on the superior epigastric vessels. It had been a popular surgery for 15 years known for a significant number of complications, either on the reconstructed breast with distal necrosis, or in the donor site with significant alteration of the abdominal wall. Scheflan and Dinner confirmed that the lower abdominal tissue is vascularized mainly by the deep epigastric inferior vessels (14). Thanks to these breakthroughs, after different attempts to secure the TRAM vascularization using the delay phenomenon (15), a free TRAM flap based on the deep inferior epigastric vessels connected with microsurgery became the gold standard, especially after the implementation of the internal mammary recipient vessels in replacement of the circumflex scapular vessels from the ipsilateral axilla. On one hand, it improves the results of the breast because of a more reliable blood supply and on the other hand, it improves the outcomes of the abdomen by avoiding the “pedicle bulge” (14) and using a lower abdominal skin flap. However, the TRAM flap was gradually set aside because it alters the integrity of the abdominal wall with a high rate of hernia and abdominal bulge.
Flaps preserving the rectus abdominis muscle
Deep inferior epigastric perforator (DIEP) flap (Figure 2)
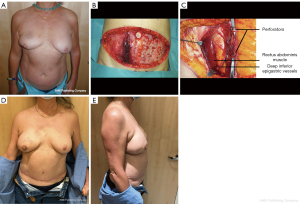
The DIEP flap was first described in 1989 by Koshima and Soeda (16) to reduce the abdominal wall weakening. First applied in breast reconstruction in 1994 by Robert Allen, the DIEP flap remains a gold standard for autologous breast reconstruction (17). Indeed, a prolific number of publications attests to it. The DIEP flap has the considerable advantage of respecting the rectus abdominis muscle and its nerves, preserving as much as possible the abdominal strength. It was achieved at the cost of a more time-consuming and meticulous dissection. Previous abdominal surgeries and incisions (low transverse incision, laparoscopy port and midline incisions) remain frequent. Those scars do not alter the result of breast reconstruction in terms of flap loss. However, abdominal donor-site complications are significantly higher in obese patients (18-22). Regarding the pregnancy concern, there is no evidence that the DIEP flap harvest could affect the pregnancy process or contraindicate vaginal delivery (23).
For cases of bilateral mastectomies (bilateral cancers or a predisposing mutation), bilateral DIEP flaps have become a daily practice. It can only be considered in patients with sufficient abdominal tissue. Beyond the longer operative time, double DIEP procedures are associated with an increased donor site complication rate compared to unilateral DIEP. In a comparative study, a significant difference in the overall complications rate in the donor site was reported as twice as high in bilateral DIEP (24).
In our experience, we tend to oversize the volume of the flap by 30% in case of adjuvant radiation therapy to anticipate the subsequent volume decrease; even though no increase in complication rates of fat necrosis, surgery for removal of fat necrosis was reported in the literature (25). In the case of previous radiation therapy, there was no statistical evidence of an increased flap loss rate (26), but rather a higher risk of developing wound healing disturbances at the recipient site (27).
Endo-robotic approach for DIEP flap harvest
The robotic approach comes from the desire to optimize the DIEP harvesting, especially when it comes to the pedicle length and the incision of the aponeurosis. First reported by Gundlapalli et al. in 2018, the usage of the Da Vinci® robot system to harvest deep inferior epigastric vessels is becoming more and more popular for the many benefits it can provide (28). The minimally invasive technique allows, as early experience suggests, smaller recovery time and less overall postoperative pain (29). In a retrospective study which included 207 patients in total (21 in the robotic DIEP group versus 186 in the regular DIEP group), Lee et al. found a significant reduction in postoperative pain (P=0.001) and reduction of mean hospital stay length (P=0.002) (30). This technique allows the surgeon to harvest the longest possible pedicle length (10 to 15 cm) while performing a very small incision on the abdominal fascia, ranging from 1.5 to 3 cm (29). However, the financial cost is substantially higher, and surgeons will need additional training to master this new skill (29,30). Thus, the robotic DIEP flap cannot be universally used and the patient selection is a crucial part. Ideally, perforators must be of short intramuscular course and of a small number (ideally 1–2), identified on the preoperative computed tomography (CT) scan (29). Otherwise, the required dissection will be greater, and the benefits from using afterwards a minimally invasive approach are reduced (29,31). The surgeon must dissect around the perforators till the posterior sheath. Then, they inflate the abdominal cavity (29). After, the surgeon installs three ports through the fascia on the contralateral side of the flap and located on a line connecting the anterior superior iliac spine and the anterior axillary line. Then, the robotic dissection of the pedicle begins till they reach the perforator. The pedicle can then be dissected till the external iliac vessels and be clamped. It is recommended to close the posterior sheath with a barbed suture (29).
Superficial inferior epigastric artery (SIEA) flap (32,33) (Figure 3)
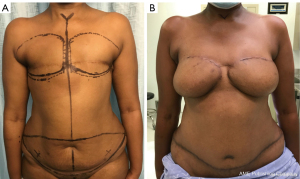
The SIEA flap is a lower hemi-abdominal flap which involves a less extensive dissection compared to DIEP flap since it does not require the opening of the rectus sheath (34). Holmström described in 1979 the first use in superficial inferior epigastric vessels (SIEA flap) in breast reconstruction with a free TRAM flap in which he included the contralateral superficial inferior epigastric vein (SIEV) (35). After Taylor conducted anatomical studies, Arnez et al. published the SIEA flap applied to the breast reconstruction in 1999 (36). The SIEA vascularizes the SIEA flap. This pedicle runs in the lower abdominal fat tissue under the Scarpa or sometimes above the Scarpa, depending on the level of incision. It has the advantage of avoiding intramuscular dissection. However, that pedicle’s vascularization territory is inconsistent from one patient to another. Indeed, the dominance between the DIEP and the SIEA is not predictable preoperatively. For that reason, it cannot be performed in more than 15% of our cases. It is important to ensure the good vascularization of the arterial and venous side of the flap before selecting that pedicle for the abdominal flap. Moreover, a supplementary venous anastomosis with the superficial vein is often necessary because of venous congestion. In our practice, two main problems exist with this flap: the extreme spasticity of the SIEA and the peripheric position of the pedicle entrance in the flap.
Muscle sparing-TRAM (MS-TRAM) flap
MS-TRAM is another technique using the abdomen which consists in harvesting a partial part of the rectus abdominis muscle including a full raw of perforators and the DIEP vessels. We only use that technique when a single or two perforators are not enough to vascularize the flap, which remains rare. No significant differences in the overall incidence of complications between MS-TRAM and DIEP are found in the literature (37).
Flaps from the back (Figure 4)
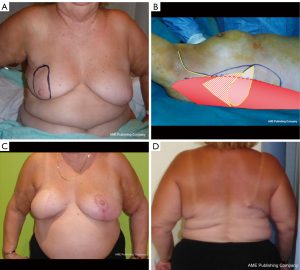
Because of its reliability and proximity to the breast, the LD flap is commonly used in breast reconstruction. After its first description in 1906 by Tansini, it was only in 1976 that Olivari rediscovered it (4). Then, Mühlbauer and Olbrisch in 1977 developed its use to provide muscle coverage of the silicone implant and breast skin replacement (38,39). The LD has maintained popularity in breast reconstruction since the 1990s because of its fundamental qualities: its reliability and relative harvest ease.
Its various indications and the patient’s morphology guide the strategy for harvesting and positioning the flap. The layout of the cutaneous paddle was the subject of many variations in order to optimize its positioning at the thoracic level and reduce the dorsal scar ransom. Mathes and Nahai classified the LD muscle as type V (40); its dominant pedicle (thoracodorsal artery), with a large diameter and minimal anatomic variation, provides a highly reliable blood supply. The vessel enters the underside of the latissimus in the posterior axilla, giving off a branch to the serratus muscle, continues into the muscle and bifurcates into a large lateral descending branch and a small transverse branch (41). In addition, numerous musculocutaneous perforators allow for skin island design anywhere on the muscle if the skin over the proximal third of the muscle is included. If most authors require a position change, Binder et al. and Boa et al. standardized a supine position harvest technique with an oblique incision which improves the surface of the skin paddle (42,43).
This method’s main drawback is the sacrifice of the largest human muscle, a motor for the normal shoulder joint biomechanics (44). For most of the patients, the harvest of the LD muscle is very well tolerated (45). However, moderate to severe shoulder functional loss and disability was found in around 10% of the patients with chronic pain and partial loss of function (especially for flexion and abduction) (44,46). Hence, we consider it should be avoided, if possible, for patients in a wheelchair and the most athletic ones. The most common complication is donor site seroma. The main way to prevent this morbidity is quilting sutures with the addition of fibrin sealant at the time of wound closure (47).
In our experience, candidates are considered for this flap when they are not admissible for DIEP reconstruction (previous abdominoplasty or liposuction, refusal of the patient to use the abdomen, insufficient abdominal skin or fat) (48). We believe that the LD flap should, as much as possible, be combined with autologous fat rather than using a breast implant. Indeed, doing the latter would cumulate the disadvantages of each technique: scars, potential shoulder pain or loss of function from LD flaps and rupture, capsular retraction, ALCL from breast implants. Several variations had been developed from that flap: muscle-sparing LD flap, thoracodorsal artery perforation (TDAP) flap and extended LD flap. The extended LD flap involves harvesting the fat tissue related to the muscle under the Scarpa fascia. This technique has the advantage of bringing more volume to the reconstruction, but it tends to give an asymmetrical aspect of the back in comparison with muscle-sparring LD flap (which consists of harvesting only of strip of muscle around the descending branch) (49,50).
We prefer to perform a dorsal decubitus harvest with an oblique skin paddle and an autologous fat graft 3 months after the initial surgery. Harvesting the LD flap and immediate fat transfer into the flap is associated with a high-fat necrosis rate and does not eliminate the need for further fat grafting (51).
Flaps from the thigh
Two flaps can be harvested from the thigh region: the transverse upper gracilis (TUG) flap and the profunda artery perforator (PAP) flap. They are mainly chosen when the abdomen and the back cannot be used (because of a history of previous surgeries, lack of available tissue or patient refusal).
The TUG flap (also called transverse myocutaneous gracilis flap) is harvested from the inner thigh and consists of the gracilis muscle (Figure 5) and a skin paddle, while the PAP flap is harvested from the inner and posterior aspects of the upper thigh. They are vascularized by the artery of the gracilis coming from the profunda femoris artery for the TUG flap and a branch of the profunda femoris artery for the PAP flap.
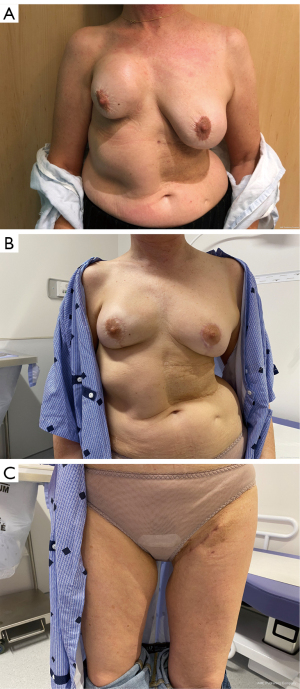
The harvest of the gracilis muscle has no consequence for walking function because it is an accessory adductor contributing only 11% of the adduction motion (52). The most common complications of these two thigh flaps are a sensory disturbance of the medial or posterior thigh observed in 24–75%, followed by wound dehiscence (range, 0.9–8.3%), and contour deformity (range, 0–5.2%) (53). Moreover, in a few cases, TUG flaps can lead to labial spreading (53,54). To avoid the contour deformity and the labial spreading, the superior incision should be placed 1 to 2 cm below the inguinal crease (55). Indeed, their pedicles remain quite shorter compared to the DIEP flap (average of 9.4 cm for PAP, 6.4 cm for TUG versus 15 cm for DIEP) and smaller caliber of artery (average of 2.0 mm for PAP, 1.5 cm for TUG versus 2.1 cm for DIEP) (56,57). In addition, the volume brought to the breast is small to moderate. Moreover, they do not allow a large resurfacing of the breast because of a limited-size skin paddle. Bilateral TUG flaps to reconstruct one breast were reported with the aim of adding volume. In that case, vascular connections are made in the internal mammary vessels, anterograde for one flap and retrograde for the second one (58).
The TUG flap and PAP flap may be the preferred initial option in alternative to DIEP flap depending on volume offered by the donor site, on the surgeon’s experience and if the volume of the breast to reconstruct is small to moderate. However, in our practice we use the TUG flap only when necessary. Regarding the PAP flap, we tend not to use it because of its firm texture, unlike the fat of the TUG flap, which is soft, allowing a more natural texture, in addition to the short pedicle and small vessel caliber of the PAP flap.
Flaps from the gluteal region
Two flaps can be harvested in the gluteal region: the superior gluteal artery perforator (SGAP) and the inferior gluteal artery perforator (IGAP) (Figure 6) flaps which are based on the arteries of the same name, both originating from the internal iliac vessels. They are perforator flaps which means the muscles underneath are preserved. The SGAP flap was first described in 1995 by Allen and Tucker for autologous breast reconstruction (59).
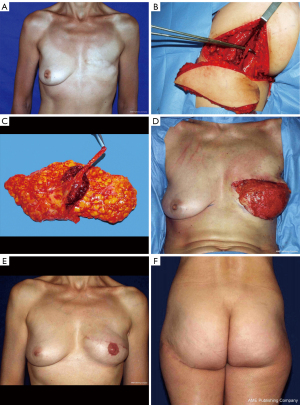
Those two flaps are not go-to flaps, but can constitute an option in case of unavailability of others. Indeed, they present many disadvantages: limited volume offered, an average-sized skin paddle (approximately 8 cm × 18 cm) (60), a short pedicle (5.6 cm) and a small diameter artery for IGAP (1.7 mm) (61), a loss of padding on thin patients, buttock asymmetry and contour deformity (62) and a low quality of tissue for a breast reconstruction because of the very firm texture. The SGAP flap presents a pedicle of a larger diameter (3.38 mm) and an average length of 9.1 cm (63). Additionally, postoperative pain over the proximal lateral thigh can occur in case of damage to the lateral femoral cutaneous nerve when undermining the flanks too aggressively for additional bulk, resulting in pain over the proximal lateral thigh (64). The buttock is a zone of tension, hence leading to scarring issues such as a widened scar, and it is associated with a frequent need for secondary donor-site revision procedures (63). Finally, a position change is required leading to an increased ischemia time. This donor site has been gradually put in the background and is rarely used in our University Hospital Center. The relatively low number of publications attests to this.
The free fasciocutaneous infragluteal (FCI) flap is another flap that can be elevated from the infragluteal crease (65) and vascularized by the descending branch of the inferior gluteal artery (66). The presence of the posterior cutaneous femoral nerve alongside the vascular pedicle allows for potential of a sensory flap transfer (67). However, the FCI flap is not commonly employed for breast reconstruction.
Breast reconstructions using the superficial body fat (Figures 7,8)
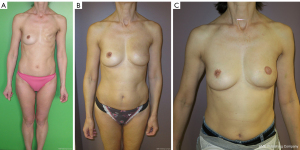
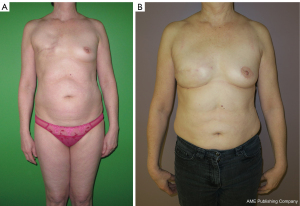
Historically, Czerny tempted the first case of autogenous breast reconstruction in 1895 using a large lipoma (3). Later, in 1941, Billings and May presented a case of adipose tissue transfer with its fascia with the idea that the fascia allowed for a better preservation of fat (68,69).
The liposuction technique was popularized by Illouz in the 1980s, which consists in breaking up and sucking out the fat tissue with a canula through small incisions. In 1987, Coleman standardized the procedure of autologous fat transfer increasing fat graft survival, making it more reliable (69-71). Even though some improvements have been added, it has remained the gold standard for lipofilling (72).
Autologous fat grafting consists in harvesting fat tissue and reinjecting it after purification into the breast as a graft. It can be used to complete a flap-based breast reconstruction (73) or exclusively for a delayed breast reconstruction. Several sessions may be necessary and, in that case, they should be separated by 3 months.
The main advantages of this technique are the minimal invasive aspect and the secondary benefits of the liposuction which is the improvement of the body contouring. It is important to inform the patients that fat grafting comes along with a 30% postoperative resorption of the volume injected (74). For a reconstruction with exclusively fat transfer, 4 to 5 sessions are necessary (74). For each session, the same volume of purified fat can be injected as the volume of the reconstructed breast.
Donor sites are chosen according to patients’ morphology depending on where the steatomeries are located. The most common donor sites are the abdomen, the flanks, the trochanteric areas and the inner thighs. The first step is the infiltration of the deep fat layer of the donor sites with adrenaline saline (1 mg of adrenaline: 1 L of saline) to diminish bleedings and make the fragmentation of fat easier. Then, fat cells are harvested by liposuction with a 4-mm canula by making crossed-tunnels into the infiltrated areas through 5-mm skin incisions. The collected fat is purified either by washing or by centrifugation, in the aim to separate the fat from the blood, the oil and the connective tissue. Then the fat is reinjected with 10 mL syringes and a 2.5-mm cannula with a blunt tip, in a retrograde fashion, into three planes: subdermal plane, deep plane (including in the pectoralis major) and intermediary plane. At the end of the injections, we would recommend doing a massage for a better distribution of the fat graft. For secondary breast reconstruction managed with exclusive fat grafting, blind fasciotomies should be performed throughout the procedure. In our experience, it is crucial to optimize the cosmetic outcomes to prepare recipient sites prior to surgery with massages and scar detachment maneuvers to relax the skin.
The complications for the donor sites include bruising, swelling, hematoma, paresthesia, infection, and contour irregularities. Damage of the underlying structures like intrapleural, intraperitoneal or intramuscular penetration of the cannula remains exceptional (72,75,76).
In the reconstructed breast during the follow-up, other complications may be diagnosed especially cytosteatonecrosis, oil cysts and infection. Higher rates of fat necrosis are found in higher injected volumes, multiple sessions, or following radiation (77). Moreover, post-lipofilling calcifications can be found in mammograms. Concerns had arisen because these calcifications can interfere with oncological surveillance. The data in the literature show that fat grafting is oncologically safe (no increased risk of local recurrence or new breast cancer), with no impairment in breast surveillance but possibly a slight increase in the incidence of nonroutine imaging and biopsies (78,79).
Nipple areola complex reconstruction
The reconstruction of a nipple-areolar complex (NAC) is the final step of the breast reconstruction, usually performed in a different operating time than the restoration of the volume. It should be discussed with the patient because a non-negligible percentage of patients refuse a NAC reconstruction even though they strongly desired the breast reconstruction. One of the reasons for this refusal is the addition of another surgery to this long process even if the NAC reconstruction is performed under local anesthesia. Moreover, restoring the volume of the breast allows a recovery of the social aspect of the breast while the reconstruction of the NAC plays a role in restoring a more intimate aspect of breast. The satisfaction of the patients after NAC reconstruction does not differ when compared to patients without the surgery with regards to the overall appearance in clothes but differ significantly in nude appearance and in overall satisfaction (80).
Several options are available regarding the reconstruction of the areola (81). Firstly, the NAC sharing techniques involve the harvest of a part of the contralateral NAC. In case of large contralateral areola or nipple, the excess can be used as a graft to reconstruct the NAC. This is a technique of choice since it allows the best color-match and texture. Also, a skin graft from upper tight or inguinal fold can be performed with a good color-match, but at the cost of a supplementary scar. Some depigmentation of the graft may occur. Finally, tattoos showcase a growing popularity (82). However, they can cause inflammatory complications (83,84). Moreover, the chromaticity can fade over time and another tattoo session may be necessary. Finally, the free nipple-areola graft is a technique employed in immediate breast reconstruction for skin-sparing mastectomies. (85). It is indicated in risk-reducing mastectomies and in certain oncological cases with oncologic safety (85-87). Better results are obtained when a circumareolar incision is possible. However, the position of the nipple-areola complex is difficult in case of large breasts or significant ptosis (88). In those cases, secondary areola pigmentation has its rightful place.
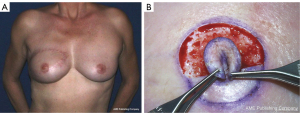
In addition to the NAC sharing technique (Figure 9), the nipple can be reconstructed with local random flaps folded in a three-dimension shape (Figure 10) (81). The flap is pedicled on the dermis and stays reliable. It is necessary to oversize the flap because its height usually decreases over time. Other techniques are available for nipple reconstruction: graft from the labia minores (but may lead to dyspareunia), ear lobule or cartilage grafting. In our practice, the most common flaps performed are Little’s flap and C-V flap.
Discussion
In this narrative review, the authors conducted a thorough search from the MEDLINE database to gather original articles in English about autogenous breast reconstruction, published from January 2010 to December 2022. The review encompasses a comprehensive range and cover the entire spectrum of autologous breast reconstruction.
In the beginning, breast reconstruction was mostly based on breast implants. Introduced into the market 1963, breast implants have been a controversial subject due to high rate of complications with early generation implants, such capsular contracture, silicone bleeding and rupture (89). The second-generation implants were developed in the 1980s with a reinforced multilayered silicone elastomer shell, but concerns about possible association with autoimmune reactions led to a moratorium on their cosmetic use between 1992 and 2006 (90). Despite this, breast augmentation remained popular, and more recently, there has been focus on breast implant-associated ALCL related to textured implants (91). BII has also gained attention on social media, but no causal relationship between breast implants and systemic diseases has been established based on current scientific evidence (92).
Faced with these many problems related to breast implants, autologous methods have emerged. Firstly, the pedicled TRAM flap with a transverse abdominal scar (93) was a very popular option. Then, the free version of the TRAM flap became the gold standard. Meanwhile, the popularity of the LD flap associated with an implant has been maintained from 1970s to nowadays. All of these techniques accumulate many problems: the abdominal weakening for the TRAM, the loss of function a large muscle for the LD. Moreover, the use of both LD flap and an implant concentrates the inconveniences of both autologous techniques including scarring and implants issues. A search of new donor sites with less morbidity was undergone. Since Taylor’s research on angiosomes, new donor sites have been described, using the abdomen, the back, the thighs and the buttocks. To put into perspectives the popularity of each autogenous techniques, the Figure 11 shows the number of publications referred in the PubMed database from January 2010 to December 2022. The DIEP flap and the LD flap appear to be the two dominant techniques. Because of its numerous advantages (Table 2), the DIEP flap is the gold standard to date without a doubt in autologous breast reconstruction.
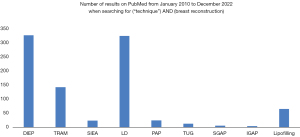
Table 2
| Region | Autologous tissue | Pedicle | Advantages | Disadvantages |
|---|---|---|---|---|
| Abdomen | TRAM | Pedicled: superior epigastric | Reliable; short operative time for the flap harvest | Weakening of the abdominal wall (hernia, abdominal bulge) |
| Free: deep inferior epigastric | ||||
| DIEP | Deep inferior epigastric | Preserve the rectus abdominis muscle and the strength of the abdominal wall | More time consuming; extensive dissection | |
| SIEA | Superficial inferior epigastric | Limited dissection; preserve the strength of the abdominal wall | Spasticity of the artery; inconsistency of the territory of vascularization; anastomosis of the superficial vein necessary | |
| Back | LD | Thoraco-dorsal | Reliable; short operative time for the flap harvest | Loss of function of a large muscle |
| LD-MS, TDAP | Muscle preservation; short operative time for the flap harvest; reliable | More time consuming | ||
| Extended LD | More volume brought for the breast; short operative time for the flap harvest; Reliable | Aspect of an empty back | ||
| Inner thigh | TUG | Artery of the gracilis | Short operative time for the flap harvest | Short pedicle |
| PAP | Perforator | – | Short pedicle | |
| Buttocks | IGAP | Inferior gluteal artery perforator | – | Short pedicle; position change required; firm tissue |
| SGAP | Superior gluteal artery perforator | Large diameter of the pedicle | Short pedicle; position change required; firm tissue | |
| Fat graft | Abdomen, flanks, inner thighs, trochanteric region | Not applicable | Improvement of the body contours; minimal scarring; short operative time | Multiple surgeries |
TRAM, transverse rectus abdominis myocutaneous; DIEP, deep inferior epigastric perforator; SIEA, superficial inferior epigastric artery; LD, latissimus dorsi; MS, muscle-sparing; TDAP, thoracodorsal artery perforation; TUG, transverse upper gracilis; PAP, profunda artery perforator; IGAP, inferior gluteal artery perforator; SGAP, superior gluteal artery perforator.
Conclusions
Autologous reconstructions provide very satisfactory, durable, and reliable results with relatively low complication rates. DIEP flaps, LD flaps and autologous fat grafting have arisen as the most common types of autogenous breast reconstructions. This article highlights the advantages and disadvantages of each technique in order to allow surgeons to offer the best possible information and to orient the patient towards the most suitable choice of reconstruction according to her physical characteristics and preferences.
Acknowledgments
Thanks to our mentors, Professor Marc Revol and the late Professor Jean Marie Servant of the School of Plastic Surgery at Hôpital St Louis de Paris, for inspiring this work and conceptualizing some of the topics discussed in this narrative review.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Johnny Ionut Efanov) for the series “The Modern Plastic and Reconstructive Surgeon – Collaborator, Innovator, Leader” published in Annals of Translational Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1471/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1471/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1471/coif). The series “The Modern Plastic and Reconstructive Surgeon – Collaborator, Innovator, Leader” was commissioned by the editorial office without any funding or sponsorship. M.A.D. is a consultant for Allergan, Johnson and Johnson, Establishment labsand and receives payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from them. M.A.D. participated in the safety board of Knight therapy and Activis med and receives payment for expert testimony from them. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Eltahir Y, Werners LLCH, Dreise MM, et al. Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures. Plast Reconstr Surg 2013;132:201e-209e. [Crossref] [PubMed]
- Liu D. New plastic surgery statistics and breast reconstruction trends. American Society of Plastic Surgeons. Tuesday March 14, 2017. [cited 2023 Jan 5]. Available online: https://www.plasticsurgery.org/news/blog/new-plastic-surgery-statistics-and-breast-reconstruction-trends
- Homsy A, Rüegg E, Montandon D, et al. Breast Reconstruction: A Century of Controversies and Progress. Ann Plast Surg 2018;80:457-63. [Crossref] [PubMed]
- Olivari N. The latissimus flap. Br J Plast Surg 1976;29:126-8. [Crossref] [PubMed]
- Dziubek M, Laurent R, Bonapace-Potvin M, et al. Silicone particles in capsules around breast implants: Establishment of a new pathological methodology to assess the number of particles around breast implants. Ann Chir Plast Esthet 2023;68:19-25. [Crossref] [PubMed]
- Israeli R, Feingold RS. Acellular dermal matrix in breast reconstruction in the setting of radiotherapy. Aesthet Surg J 2011;31:51S-64S. [Crossref] [PubMed]
- Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg 2010;125:429-36. [Crossref] [PubMed]
- Liu AS, Kao HK, Reish RG, et al. Postoperative complications in prosthesis-based breast reconstruction using acellular dermal matrix. Plast Reconstr Surg 2011;127:1755-62. [Crossref] [PubMed]
- Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg 1987;40:113-41. [Crossref] [PubMed]
- Mavrogenis AF, Markatos K, Saranteas T, et al. The history of microsurgery. Eur J Orthop Surg Traumatol 2019;29:247-54. [Crossref] [PubMed]
- Toyserkani NM, Jørgensen MG, Tabatabaeifar S, et al. Autologous versus implant-based breast reconstruction: A systematic review and meta-analysis of Breast-Q patient-reported outcomes. J Plast Reconstr Aesthet Surg 2020;73:278-85. [Crossref] [PubMed]
- Casarrubios JM, Francés M, Fuertes V, et al. Oncological outcomes of lipofilling in breast reconstruction: a matched cohort study with 250 patients. Gland Surg 2021;10:914-23. [Crossref] [PubMed]
- Hartrampf CR, Scheflan M, Black PW. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg 1982;69:216-25. [Crossref] [PubMed]
- Scheflan M, Dinner MI. The transverse abdominal island flap: part I. Indications, contraindications, results, and complications. Ann Plast Surg 1983;10:24-35. [Crossref] [PubMed]
- Danino A, Ichinose M, Yoshimoto S, et al. Position of venous outflow: a crucial aspect in flaps with 2 opposed pedicles. Application to the cutaneous delay phenomenon. Experimental study with rats. Ann Chir Plast Esthet 1999;44:627-33.
- Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg 1989;42:645-8. [Crossref] [PubMed]
- Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 1994;32:32-8. [Crossref] [PubMed]
- Bond ES, Soteropulos CE, Yang Q, et al. The Impact of Prior Abdominal Surgery on Complications of Abdominally Based Autologous Breast Reconstruction: A Systematic Review and Meta-Analysis. J Reconstr Microsurg 2021;37:566-79. [Crossref] [PubMed]
- Parrett BM, Caterson SA, Tobias AM, et al. DIEP flaps in women with abdominal scars: are complication rates affected? Plast Reconstr Surg 2008;121:1527-31. [Crossref] [PubMed]
- Doval AF, Lamelas AM, Daly LT, et al. Deep Inferior Epigastric Artery Perforator Flap Breast Reconstruction in Women With Previous Abdominal Incisions: A Comparison of Complication Rates. Ann Plast Surg 2018;81:560-4. [Crossref] [PubMed]
- Chang EI, Liu J. Prospective Evaluation of Obese Patients Undergoing Autologous Abdominal Free Flap Breast Reconstruction. Plast Reconstr Surg 2018;142:120e-125e. [Crossref] [PubMed]
- Lee KT, Mun GH. Effects of Obesity on Postoperative Complications After Breast Reconstruction Using Free Muscle-Sparing Transverse Rectus Abdominis Myocutaneous, Deep Inferior Epigastric Perforator, and Superficial Inferior Epigastric Artery Flap: A Systematic Review and Meta-analysis. Ann Plast Surg 2016;76:576-84. [Crossref] [PubMed]
- Fu A, Liu C. Is Pregnancy Following a TRAM or DIEP Flap Safe? A Critical Systematic Review and Meta-analysis. Aesthetic Plast Surg 2021;45:2618-30. [Crossref] [PubMed]
- Laurent R, Schoucair R, Danino MA. DIEP flap in breast reconstruction: A morbidity study of bilateral versus unilateral reconstruction. Ann Chir Plast Esthet 2023;68:300-7. [Crossref] [PubMed]
- Taghizadeh R, Moustaki M, Harris S, et al. Does post-mastectomy radiotherapy affect the outcome and prevalence of complications in immediate DIEP breast reconstruction? A prospective cohort study. J Plast Reconstr Aesthet Surg 2015;68:1379-85. [Crossref] [PubMed]
- Shechter S, Arad E, Inbal A, et al. DIEP Flap Breast Reconstruction Complication Rate in Previously Irradiated Internal Mammary Nodes. J Reconstr Microsurg 2018;34:399-403. [Crossref] [PubMed]
- Prantl L, Moellhoff N, von Fritschen U, et al. Effect of Radiation Therapy on Microsurgical Deep Inferior Epigastric Perforator Flap Breast Reconstructions: A Matched Cohort Analysis of 4577 Cases. Ann Plast Surg 2021;86:627-31. [Crossref] [PubMed]
- Gundlapalli VS, Ogunleye AA, Scott K, et al. Robotic-assisted deep inferior epigastric artery perforator flap abdominal harvest for breast reconstruction: A case report. Microsurgery 2018;38:702-5. [Crossref] [PubMed]
- Selber JC. The Robotic DIEP Flap. Plast Reconstr Surg 2020;145:340-3. [Crossref] [PubMed]
- Lee MJ, Won J, Song SY, et al. Clinical outcomes following robotic versus conventional DIEP flap in breast reconstruction: A retrospective matched study. Front Oncol 2022;12:989231. [Crossref] [PubMed]
- Kurlander DE, Le-Petross HT, Shuck JW, et al. Robotic DIEP Patient Selection: Analysis of CT Angiography. Plast Reconstr Surg Glob Open 2021;9:e3970. [Crossref] [PubMed]
- Coroneos CJ, Heller AM, Voineskos SH, et al. SIEA versus DIEP Arterial Complications: A Cohort Study. Plast Reconstr Surg 2015;135:802e-807e. [Crossref] [PubMed]
- Lipa JE. Breast reconstruction with free flaps from the abdominal donor site: TRAM, DIEAP, and SIEA flaps. Clin Plast Surg 2007;34:105-21; abstract vii. [Crossref] [PubMed]
- Sacks JM. Handbook of Reconstructive Flaps. Plast Reconstr Surg 2022;149:792-3. [Crossref] [PubMed]
- Holmström H. The free abdominoplasty flap and its use in breast reconstruction. An experimental study and clinical case report. Scand J Plast Reconstr Surg 1979;13:423-27. [Crossref] [PubMed]
- Arnez ZM, Khan U, Pogorelec D, et al. Breast reconstruction using the free superficial inferior epigastric artery (SIEA) flap. Br J Plast Surg 1999;52:276-9. [Crossref] [PubMed]
- Wang XL, Liu LB, Song FM, et al. Meta-analysis of the safety and factors contributing to complications of MS-TRAM, DIEP, and SIEA flaps for breast reconstruction. Aesthetic Plast Surg 2014;38:681-91. [Crossref] [PubMed]
- Bricout N. Reconstruction mammaire différée par lambeau de grand dorsal. EMC - Gynécologie-Obstétrique 2005;2:409-21.
- Muhlbauer W, Olbrisch R. The latissimus dorsi myocutaneous flap for breast reconstruction. Chir Plastica 1977;4:27-34.
- Mathes SJ, Nahai F. Classification of the vascular anatomy of muscles: experimental and clinical correlation. Plast Reconstr Surg 1981;67:177-87.
- Spear SL, Clemens MW. Latissimus dorsi flap breast reconstruction. In: Neligan PC, Grotting JC. editors. Plastic Surgery. 3rd ed. Philadelphia, PA: Saunders (Elsevier); 2012:370-92.
- Binder JP, Cuminet J, Revol M, et al. Oblique latissimus dorsi myocutaneous flap in breast reconstruction. Ann Chir Plast Esthet 2003;48:167-72. [Crossref] [PubMed]
- Boa O, Servant JM, Revol M, et al. Dorsal decubitus positioning: a novel method to harvest the latissimus dorsi flap for massive upper extremity defect reconstruction. Tech Hand Up Extrem Surg 2011;15:166-71. [Crossref] [PubMed]
- Umar M, Jahangir N, Hughes M, et al. Incidence of shoulder functional morbidity following ipsilateral mastectomy and latissimus dorsi flap reconstruction. Acta Orthop Traumatol Turc 2019;53:448-51. [Crossref] [PubMed]
- Hammond DC. Latissimus dorsi flap breast reconstruction. Clin Plast Surg 2007;34:75-82; abstract vi-vii. [Crossref] [PubMed]
- S Steffenssen MCW. Kristiansen AH, Damsgaard TE. A Systematic Review and Meta-analysis of Functional Shoulder Impairment After Latissimus Dorsi Breast Reconstruction. Ann Plast Surg 2019;82:116-27. [Crossref] [PubMed]
- Shin IS, Lee DW, Lew DH. Efficacy of quilting sutures and fibrin sealant together for prevention of seroma in extended latissimus dorsi flap donor sites. Arch Plast Surg 2012;39:509-13. [Crossref] [PubMed]
- Sood R, Easow JM, Konopka G, et al. Latissimus Dorsi Flap in Breast Reconstruction: Recent Innovations in the Workhorse Flap. Cancer Control 2018;25:1073274817744638. [Crossref] [PubMed]
- Delay E, Jorquera F, Pasi P, et al. Autologous latissimus breast reconstruction in association with the abdominal advancement flap: a new refinement in breast reconstruction. Ann Plast Surg 1999;42:67-75. [Crossref] [PubMed]
- Kim H, Wiraatmadja ES, Lim SY, et al. Comparison of morbidity of donor site following pedicled muscle-sparing latissimus dorsi flap versus extended latissimus dorsi flap breast reconstruction. J Plast Reconstr Aesthet Surg 2013;66:640-6. [Crossref] [PubMed]
- Escandón JM, Escandón L, Ahmed A, et al. Breast reconstruction using the Latissimus Dorsi Flap and Immediate Fat Transfer (LIFT): A systematic review and meta-analysis. J Plast Reconstr Aesthet Surg 2022;75:4106-16. [Crossref] [PubMed]
- Deutinger M, Kuzbari R, Paternostro-Sluga T, et al. Donor-site morbidity of the gracilis flap. Plast Reconstr Surg 1995;95:1240-4. [Crossref] [PubMed]
- Lakhiani C, DeFazio MV, Han K, et al. Donor-Site Morbidity Following Free Tissue Harvest from the Thigh: A Systematic Review and Pooled Analysis of Complications. J Reconstr Microsurg 2016;32:342-57. [Crossref] [PubMed]
- Dayan JH, Allen RJ Jr. Lower Extremity Free Flaps for Breast Reconstruction. Plast Reconstr Surg 2017;140:77S-86S. [Crossref] [PubMed]
- Buchel EW, Dalke KR, Hayakawa TE. The transverse upper gracilis flap: Efficiencies and design tips. Can J Plast Surg 2013;21:162-6. [Crossref] [PubMed]
- Jo T, Kim EK, Eom JS, et al. Comparison of transverse upper gracilis and profunda femoris artery perforator flaps for breast reconstruction: A systematic review. Microsurgery 2020;40:916-28. [Crossref] [PubMed]
- Boucher F, Brosset S, Shipkov H, et al. An anatomic study of deep inferior epigastric artery diameters at the origin from external iliac and at the lateral border of rectus abdominis muscle by computed tomographic angiography from autologous breast reconstruction patients. Ann Chir Plast Esthet 2020;65:70-6. [Crossref] [PubMed]
- Christopoulos G, Khoury A, Sergentanis TN, et al. Bilateral Transverse Upper Gracilis Flaps for Unilateral Breast Reconstruction: A 4-Year Retrospective Study of the "2-in-1" Technique and a Systematic Review With Meta-analysis. Ann Plast Surg 2022;89:400-7. [Crossref] [PubMed]
- Allen RJ, Tucker C Jr. Superior gluteal artery perforator free flap for breast reconstruction. Plast Reconstr Surg 1995;95:1207-12. [Crossref] [PubMed]
- Granzow JW, Levine JL, Chiu ES, et al. Breast reconstruction with gluteal artery perforator flaps. J Plast Reconstr Aesthet Surg 2006;59:614-21. [Crossref] [PubMed]
- Hidaka T, Mori H, Shimizu H, et al. Comparison of Lumbar Artery and Superior Gluteal Artery Perforator Flaps for Breast Reconstruction: Multislice CT-Based Anatomical Study. Ann Plast Surg 2022;89:e39-44. [Crossref] [PubMed]
- LoTempio MM, Allen RJ. Breast reconstruction with SGAP and IGAP flaps. Plast Reconstr Surg 2010;126:393-401. [Crossref] [PubMed]
- Martineau J, Kalbermatten DF, Oranges CM. Safety and Efficacy of the Superior Gluteal Artery Perforator (SGAP) Flap in Autologous Breast Reconstruction: Systematic Review and Meta-Analysis. Cancers (Basel) 2022;14:4420. [Crossref] [PubMed]
- Opsomer D, Vyncke T, Ryx M, et al. Comparing the Lumbar and SGAP Flaps to the DIEP Flap Using the BREAST-Q. Plast Reconstr Surg 2020;146:276e-282e. [Crossref] [PubMed]
- Zaussinger M, Tinhofer IE, Hamscha U, et al. A Head-to-Head Comparison of the Vascular Basis of the Transverse Myocutaneous Gracilis, Profunda Artery Perforator, and Fasciocutaneous Infragluteal Flaps: An Anatomical Study. Plast Reconstr Surg 2019;143:381-90. [Crossref] [PubMed]
- Struckmann V, Peek A, Wingenbach O, et al. The free fasciocutaneous infragluteal (FCI) flap: Outcome and patient satisfaction after 142 breast reconstructions. J Plast Reconstr Aesthet Surg 2016;69:461-9. [Crossref] [PubMed]
- Windhofer C, Brenner E, Moriggl B, et al. Relationship between the descending branch of the inferior gluteal artery and the posterior femoral cutaneous nerve applicable to flap surgery. Surg Radiol Anat 2002;24:253-7. [Crossref] [PubMed]
- Billings E Jr, May JW Jr. Historical review and present status of free fat graft autotransplantation in plastic and reconstructive surgery. Plast Reconstr Surg 1989;83:368-81. [Crossref] [PubMed]
- Costanzo D, Romeo A, Marena F. Autologous Fat Grafting in Plastic and Reconstructive Surgery: An Historical Perspective. Eplasty 2022;22:e4.
- Coleman SR. Structural fat grafting. Aesthet Surg J 1998;18:386, 388.
- Coleman SR. Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg 1995;19:421-5. [Crossref] [PubMed]
- Simonacci F, Bertozzi N, Grieco MP, et al. Autologous fat transplantation for breast reconstruction: A literature review. Ann Med Surg (Lond) 2016;12:94-100. [Crossref] [PubMed]
- Sinna R, Delay E, Garson S, et al. Breast fat grafting (lipomodelling) after extended latissimus dorsi flap breast reconstruction: a preliminary report of 200 consecutive cases. J Plast Reconstr Aesthet Surg 2010;63:1769-77. [Crossref] [PubMed]
- Delay E, Meruta AC, Guerid S. Indications and Controversies in Total Breast Reconstruction with Lipomodeling. Clin Plast Surg 2018;45:111-7. [Crossref] [PubMed]
- Wederfoort JLM, Hebels SA, Heuts EM, et al. Donor site complications and satisfaction in autologous fat grafting for breast reconstruction: A systematic review. J Plast Reconstr Aesthet Surg 2022;75:1316-27. [Crossref] [PubMed]
- De Decker M, De Schrijver L, Thiessen F, et al. Breast cancer and fat grafting: efficacy, safety and complications-a systematic review. Eur J Obstet Gynecol Reprod Biol 2016;207:100-8. [Crossref] [PubMed]
- Shamoun F, Asaad M, Hanson SE. Oncologic Safety of Autologous Fat Grafting in Breast Reconstruction. Clin Breast Cancer 2021;21:271-7. [Crossref] [PubMed]
- Sayyed AA, Perez-Alvarez IM, Singh T, et al. Review of Autologous Fat Grafting in Postmastectomy Reconstruction Patients: Nonroutine Diagnostics and Oncologic Safety. Plast Reconstr Surg Glob Open 2022;10:e4579. [Crossref] [PubMed]
- Hanson SE, Kapur SK, Hwang RF, et al. Autologous fat grafting in breast reconstruction: implications for follow-up and surveillance. Gland Surg 2021;10:487-93. [Crossref] [PubMed]
- Wellisch DK, Schain WS, Noone RB, et al. The psychological contribution of nipple addition in breast reconstruction. Plast Reconstr Surg 1987;80:699-704. [Crossref] [PubMed]
- Sisti A, Grimaldi L, Tassinari J, et al. Nipple-areola complex reconstruction techniques: A literature review. Eur J Surg Oncol 2016;42:441-65. [Crossref] [PubMed]
- Kuruvilla AS, Gopman JM, Cham S, et al. Nipple-areolar tattoo: Comprehensive review of history, theory, technique, and outcomes. J Plast Reconstr Aesthet Surg 2022;75:544-9. [Crossref] [PubMed]
- Dréno B. Tattoo and sarcoidosis reaction. Bull Acad Natl Med 2020;204:611-5. [Crossref] [PubMed]
- Bazex J, Arné JL, Lambert D. Uveitis and tattoos. Bull Acad Natl Med 2020;204:616-21. [Crossref] [PubMed]
- Fansa H, Linder S. Autologous Breast Reconstruction with Free Nipple-Areola Graft after Circumareolar (Skin Reducing) Mastectomy. J Pers Med 2022;12:1588. [Crossref] [PubMed]
- Jakub JW, Peled AW, Gray RJ, et al. Oncologic Safety of Prophylactic Nipple-Sparing Mastectomy in a Population With BRCA Mutations: A Multi-institutional Study. JAMA Surg 2018;153:123-9. [Crossref] [PubMed]
- Margenthaler JA, Gan C, Yan Y, et al. Oncologic Safety and Outcomes in Patients Undergoing Nipple-Sparing Mastectomy. J Am Coll Surg 2020;230:535-41. [Crossref] [PubMed]
- Kim H, Park SJ, Woo KJ, et al. Comparative Study of Nipple-Areola Complex Position and Patient Satisfaction After Unilateral Mastectomy and Immediate Expander-Implant Reconstruction Nipple-Sparing Mastectomy Versus Skin-Sparing Mastectomy. Aesthetic Plast Surg 2019;43:313-27. [Crossref] [PubMed]
- Barr S, Bayat A. Breast implant surface development: perspectives on development and manufacture. Aesthet Surg J 2011;31:56-67. [Crossref] [PubMed]
- Spear SL, Parikh PM, Goldstein JA. History of breast implants and the food and drug administration. Clin Plast Surg 2009;36:15-21. v. [Crossref] [PubMed]
- Loch-Wilkinson A, Beath KJ, Knight RJW, et al. Breast Implant-Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand: High-Surface-Area Textured Implants Are Associated with Increased Risk. Plast Reconstr Surg 2017;140:645-54. [Crossref] [PubMed]
- Magnusson MR, Cooter RD, Rakhorst H, et al. Breast Implant Illness: A Way Forward. Plast Reconstr Surg 2019;143:74S-81S. [Crossref] [PubMed]
- Jones G. The pedicled TRAM flap in breast reconstruction. Clin Plast Surg 2007;34:83-104; abstract vii. [Crossref] [PubMed]


