
Efficacy and safety of tucidinostat in patients with advanced hormone receptor-positive human epidermal growth factor receptor 2-negative breast cancer: real-world insights
Highlight box
Key findings
• In hormone receptor-positive (HR+) human epidermal growth factor receptor 2-negative (HER2−) breast cancer, combination therapy with tucidinostat showed survival benefits with manageable safety. Postchemotherapy maintenance therapy demonstrated potential efficacy versus non-maintenance.
What is known and what is new?
• Randomized controlled studies have shown that tucidinostat plus exemestane significantly prolongs progression-free survival (PFS) in HR+/HER2− patients who have failed previous endocrine therapy and may be a promising new treatment option.
• We included unselected patients with HR+/HER2− advanced breast cancer and observed that tucidinostat, often given in later treatment lines, extended PFS by 4.37 months in the overall population. Notably, a subset undergoing maintenance therapy had significantly better PFS than did those who were not.
What is the implication, and what should change now?
• Tucidinostat-based combination therapy may be a treatment option for HR+/HER2− patients after endocrine therapy failure, warranting further exploration for developing an optimal therapeutic modality.
Introduction
Aromatase inhibitors (AIs) (1) and fulvestrant (2), with or without cyclin-dependent kinase 4/6 inhibitor (CDK4/6 inhibitor), are increasingly being used as first-line treatment for patients with hormone receptor-positive (HR+) human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer (ABC) (3-8). However, resistance to endocrine therapy (ET) is common in metastatic disease (9). Previous studies have suggested that endocrine resistance in BC is a complex process involving the dysregulation of multiple signaling pathways. Although the mechanisms are not fully understood, several known mechanisms include modifications in estrogen receptor (ER) signaling (e.g., ER downregulation and ESR1 mutation), alternative activation of growth signaling pathways, epigenetic reprogramming, aberrant cell cycle regulation, and changes in the tumor microenvironment (10-12). Therefore, exploration of drugs against these targets may help overcome endocrine resistance.
Tucidinostat (previously known as chidamide) is an orally administered, subtype-selective inhibitor of histone deacetylase (HDAC). It specifically targets HDAC1, HDAC2, HDAC3, and HDAC10, resulting in epigenetic reprogramming and inhibiting the growth of various tumor types (13-16). Tucidinostat was approved for relapsed or refractory (R/R) peripheral T-cell lymphoma, R/R adult T-cell leukemia-lymphoma and ABC, and is the only HDAC inhibitor approved to date for the treatment of solid tumors (14). In an exploratory study involving 20 patients with HR+ BC, the combination of tucidinostat and exemestane demonstrated a high clinical benefit rate (CBR) of 87.5% and good tolerability. The median progression-free survival (PFS) reached 7.6 months (17). Following the initial study, the Chidamide and Exemestane (ACE) study was conducted, involving 365 patients from 22 cancer centers in China. The patients received tucidinostat or placebo combined with exemestane. The median PFS increased by 3.6 months in the tucidinostat group compared with the placebo group, with a PFS of 7.4 and 3.8 months, respectively (P=0.033) (18). The updated results of ACE study showed the PFS extension did not translate into overall survival (OS) benefit (19). These findings suggest that tucidinostat plus exemestane may represent a promising new treatment option for patients who have experienced treatment failure with prior ET (20).
Despite CDK4/6 inhibitors emerging as the standard first- and second-line treatment, and because CDK4/6 inhibitors were not approved in China during the ACE enrollment period, very few patients had previously received such agents and the ACE study could not definitively determine whether tucidinostat can overcome endocrine resistance after progression following CDK4/6 inhibitor therapy. We noted that the ACE trial had enrolled only patients with adequate organ function and Eastern Cooperative Oncology Group (ECOG) performance scores of 0 or 1 who had received only one prior line of systemic therapy for metastatic disease; meanwhile, the trial excluded patients with active brain metastases and severe medical comorbidities. We planned to conduct a real-world study to address these limitations of the ACE trial by providing data on the efficacy of tucidinostat in different lines of treatment and to determine the optimal therapeutic modality (21). We present this article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1913/rc).
Methods
Patients and eligibility criteria
This was a single-center, real-world study. We included all eligible patients who received tucidinostat therapy with histologically or cytologically confirmed HR+ HER2− ABC at West China Hospital, Sichuan University, between August 2020 and May 2023. These patients had previously experienced treatment failure with ET in the adjuvant or metastatic setting and were treated with a combination of tucidinostat and other agents. This study did not impose restrictions on menopausal status or prior lines or regimens. Clinical assistants conducted regular follow-ups on these patients through the Breast Cancer Information Management System (BCIMS), regularly documenting patients’ clinical and pathological characteristics, diagnoses, treatment courses, efficacy assessments, and dates of death or last follow-up. Each patient’s data was verified in our hospital electronic medical records. The study was reviewed and approved by the Clinical Test and Biomedical Ethics Committee of West China Hospital, Sichuan University (No. 2023[no. 1005]). Relevant investigations were conducted in accordance with the principles stated in the Declaration of Helsinki (as revised in 2013). In accordance with the national legislation and institutional requirements, written informed consent of the patients for participation was not required for this retrospective study.
Therapeutic regimen
Tucidinostat was orally administered with single cycle consisting of a dose of 30 mg, taken 30 minutes after a meal, twice a week, and for a duration of 4 weeks. In case of drug intolerance, a reduction in dosage or necessary delay in drug administration was permitted. Other agents were administered at their standard doses as per the respective guidelines. The treatment was continued until disease progression, occurrence of unacceptable adverse events (AEs), or loss to follow-up.
Evaluation of efficacy and monitoring of AEs
The efficacy was evaluated in accordance with the Response Evaluation Criteria in Solid Tumors 1.1, which entails the selection of appropriate imaging examinations based on the patient’s tumor metastatic site(s). These evaluations were conducted every 2–3 months. It was recommended that the patients undergo weekly blood counts and biochemical laboratory examinations every 4 weeks. AEs were recorded and graded according to the Common Terminology Criteria for Adverse Events 4.0.
Outcomes
The primary outcomes were the investigator-assessed PFS, defined as the time from the initiation of tucidinostat administration to the first documented disease progression or death for any reason, and CBR, defined as the proportion of patients who achieved a partial response (PR) or stable disease (SD) for at least 6 months during the treatment. The secondary outcome included OS (defined as the time from the initiation of tucidinostat administration to death for any reason) and the frequency and severity of AEs.
Statistical analysis
Categorical variables are described using median, frequency, and proportion. The PFS and OS were estimated using the Kaplan-Meier method and compared with the log-rank test. Univariate Cox regression analysis was employed to assess the influencing factors for PFS. Hazard ratio (HR) and 95% confidence interval (CI) were calculated using the Cox regression model. Patients who had not experienced any event in term of PFS at their last tumor assessment were considered censored. All statistical analyses were performed using R version 4.2.3 software (The R Foundation for Statistical Computing), and P<0.05 (both sides) was considered statistically significant.
Results
Patient characteristics
From August 2020 to May 2023, a total of 50 patients were eligible for the study, among whom 3 were lost to follow-up, with 47 patients eventually being included. Of these 47 patients, 6 were still undergoing treatment at the time of data cutoff, while 41 patients had discontinued the treatment. Of the 41 patients who had terminated the treatment, 37 experienced disease progression and 22 died. The median follow-up time was 18.20 months (95% CI, 14.70–27.70).
The baseline characteristics of the patients are shown in Table 1. All patients had estrogen receptor (ER)-positive tumors, and 85.11% of the patients had ER expression levels greater than 50%. Visceral metastasis was present in 80.85% of the patients, while 19.15% of the patients had brain metastasis. The median line of tucidinostat therapy was 3 (range, 1–9), with 65.96% of the patients having received 3 or more lines of tucidinostat. Around 32% of the patients exhibited primary resistance to ET, while 63.83% had previously received any treatment with CDK4/6 inhibitors. Among the patients, 53.19% were treated with tucidinostat plus fulvestrant, and 38.30% were treated with tucidinostat plus AIs. Of particular note, 10.64% of the patients with rapidly progressing visceral metastases received tucidinostat plus ET as maintenance treatment after achieving disease control with chemotherapy.
Table 1
| Characteristics | Number (%) or median [range] |
|---|---|
| Age (years) | 54 [32–78] |
| ≤50 | 19 (40.43) |
| >50 | 28 (59.57) |
| Menopausal status | |
| Premenopause | 16 (34.04) |
| Postmenopause | 31 (65.96) |
| Hormone receptor | |
| ER >50% | 40 (85.11) |
| PR ≥20% | 23 (48.94) |
| Metastatic sites | |
| Visceral | 38 (80.85) |
| Liver | 24 (51.06) |
| Lung | 21 (44.68) |
| Brain | 9 (19.15) |
| Non-visceral | 9 (19.15) |
| Bone only | 6 (12.77) |
| Lines of tucidinostat therapy | 3 [1–9] |
| 1 | 1 (2.13) |
| 2 | 15 (31.91) |
| 3–4 | 19 (40.43) |
| ≥5 | 12 (25.53) |
| Maintenance therapy | |
| Yes | 5 (10.64) |
| No | 42 (89.36) |
| Endocrine resistance | |
| Primary resistance | 15 (31.91) |
| Secondary resistance | 32 (68.09) |
| Prior ET drugs† | |
| Tamoxifen or toremifene | 18 (38.30) |
| AI | 45 (95.74) |
| Fulvestrant | 24 (51.06) |
| Prior targeted therapy for metastatic disease | |
| Any CDK4/6 inhibitor | 30 (63.83) |
| Everolimus | 1 (2.13) |
| Endocrine partner of tucidinostat | |
| Toremifene | 1 (2.13) |
| Letrozole or anastrozole | 9 (19.15) |
| Exemestane | 9 (19.15) |
| Fulvestrant | 25 (53.19) |
| Chemotherapy | 3 (6.38) |
†, primary endocrine resistance, disease progression within 6 months of advanced first-line endocrine therapy; secondary endocrine resistance, disease progression >6 months of advanced first-line endocrine therapy; previous ET drugs, including endocrine therapies used in the adjuvant or metastatic setting. ER, estrogen receptor; PR, rogesterone receptor; ET, endocrine therapy; AI, aromatase inhibitor; CDK, cyclin-dependent kinase.
Efficacy and outcomes
Of the 43 patients who were evaluated for response, 2 patients achieved a PR, 9 patients had SD for a duration of less than 6 months, and 16 patients had SD for longer than 6 months. The CBR at 6 months was 41.86% (Table 2).
Table 2
| Best response | Values |
|---|---|
| PR, n (%) | 2 (4.65) |
| SD for <6 months, n (%) | 9 (20.93) |
| SD for ≥6 months, n (%) | 16 (37.21) |
| PD, n (%) | 16 (37.21) |
| CBR, % | 41.86 |
PR, partial response; SD, stable disease; PD, progressive disease; CBR, clinical benefit rate (defined as PR and SD for ≥6 months).
The overall population had a median PFS of 4.43 months (95% CI, 2.77–10.53) (Figure 1) and a median OS of 19.57 months (95% CI, 12.83–not reached) (Figure 2). The PFS of patients receiving tucidinostat, after excluding those with brain metastases, was 5.47 months (95% CI, 3.40–11.23). In subgroup analyses, we found that patients undergoing maintenance therapy demonstrated a significantly longer PFS than did those treated with the non-maintenance therapy (14.13 vs. 3.93 months; P=0.01). In addition, the patients who had received prior treatment with CDK4/6 inhibitors had a median PFS of 3.40 months, while the untreated patients had a median PFS of 5.47 months (P=0.9). Table 3 presents the PFS of different patient subgroups.
Table 3
| Variables | PFS (95% CI) (months) | P value |
|---|---|---|
| Maintenance therapy | 0.01* | |
| Yes | 14.13 (11.90–NA) | |
| No | 3.93 (2.73–6.27) | |
| Lines of tucidinostat therapy | 0.03* | |
| 1–2 | 6.27 (3.93–NA) | |
| ≥3 | 3.40 (2.70–10.53) | |
| Combination therapy based on tucidinostat | 0.04* | |
| Letrozole or anastrozole | 3.33 (2.73–NA) | |
| Exemestane | 2.20 (1.63–NA) | |
| Fulvestrant | 6.27 (4.43–14.13) | |
| Brain metastasis | 0.04* | |
| No | 5.47 (3.40–11.23) | |
| Yes | 2.62 (2.23–NA) | |
| Prior CDK4/6 inhibitor | 0.9 | |
| No | 5.47 (4.37–16.30) | |
| Yes | 3.40 (2.73–11.90) | |
*, the difference was statistically significant. PFS, progression-free survival; CI, confidence interval; CDK, cyclin-dependent kinase.
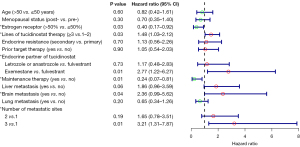
Univariate Cox regression analysis revealed that maintenance therapy after chemotherapy, tucidinostat in first or second line, tucidinostat plus fulvestrant, an ER expression level of >50%, one metastatic site, and no brain metastasis were favorable factors for PFS. Age, menopausal status, primary or secondary resistance, and prior treatment with CDK4/6 inhibitors did not show any significant association with PFS (Figure 3). Due to the small sample size, a multivariate analysis was not conducted.
After the progression of tucidinostat, more than half (55.81%) of patients chose chemotherapy and 11.63% chose to change to another CDK4/6 inhibitor and endocrine drugs, as detailed in Table S1.
Safety
Among the 47 patients, the incidence of all-grade AEs was 44.68%, with a 19.15% incidence of grade ≥3 events. The most common AE was thrombocytopenia (all grades: 31.91%; grades 3–4: 14.89%), followed by neutropenia (all grades: 10.64%; grades 3–4: 6.38%) and anemia (grades 1–2: 6.38%). Non-hematologic AEs were less common, with systemic reactions including fatigue (all grades: 6.38%; grade 3: 2.13%) and grade 1–2 pain (6.38%). Gastrointestinal reactions included nausea and vomiting (all grades: 8.51%; grade 3: 2.13%), and grade 1–2 mucositis (2.13%). Other events included grade 1–2 pruritic rash (2.13%), and grade 1–2 nasal hemorrhage (2.13%). The treatment was discontinued in 4 patients (8.51%): in one case due to grade 3 vomiting, in another case due to grade 3 fatigue and grade 2 thrombocytopenia, and in two cases due to grade 3–4 thrombocytopenia. Four patients (8.51%) had their dose reduced due to hematologic AEs. There were no reports of treatment-related death (Table 4).
Table 4
| Adverse events | All grades | Grades 1–2 | Grades 3–4 |
|---|---|---|---|
| All events, n (%) | 21 (44.68) | 12 (25.53) | 9 (19.15) |
| Myelosuppression, n (%) | |||
| Thrombocytopenia | 15 (31.91) | 8 (17.02) | 7 (14.89) |
| Neutropenia | 5 (10.64) | 2 (4.26) | 3 (6.38) |
| Anemia | 3 (6.38) | 3 (6.38) | 0 |
| General reaction, n (%) | |||
| Fatigue | 3 (6.38) | 2 (4.26) | 1 (2.13) |
| Pain | 3 (6.38) | 3 (6.38) | 0 |
| Gastrointestinal reactions, n (%) | |||
| Nausea/vomiting | 4 (8.51) | 3 (6.38) | 1 (2.13) |
| Oral mucositis | 1 (2.13) | 1 (2.13) | 0 |
| Pruritic rash, n (%) | 1 (2.13) | 1 (2.13) | 0 |
| Nasal hemorrhage, n (%) | 1 (2.13) | 1 (2.13) | 0 |
Discussion
The emergence of novel targeted therapies including HDAC inhibitors that address the mechanisms of resistance opens new avenues for the management of HR+ HER2− BC (9,22). This study enrolled unselected patients with HR+ HER2− ABC, and we noticed that tucidinostat was more commonly administered in later lines of treatment. Despite this, we observed a prolongation of PFS by 4.37 months in the overall population. Importantly, within our study, a small subgroup of patients received maintenance therapy after achieving disease control with chemotherapy, and we observed a significantly longer PFS in those patients than in the patients who received non-maintenance tucidinostat. For patients with rapid tumor progression, and significant symptoms, chemotherapy is often necessary for short-term tumor control and symptom relief. After disease control is achieved, switching to maintenance endocrine therapy is a reasonable option, similar to the treatment strategy employed in the Fulvestrant After First-line Chemotherapy (FANCY) study (23). In the FANCY study, which enrolled 58 patients with tumor responses or disease control after chemotherapy (responders), the median PFS with first-line maintenance therapy using fulvestrant was 16.1 months, whereas the median PFS calculated from the initiation of first-line chemotherapy was 19.5 months. Moreover, endocrine therapy drugs were well tolerated and compared with chemotherapy, they significantly improved the patients’ quality of life.
Second, among endocrine partners, we found that the efficacy of fulvestrant combined with tucidinostat was superior to that of AIs. The possible mechanism underlying this result involves a mutation of the ligand-binding domain (LBD) of ESR1 following aromatase inhibitor treatment in ABC. Compared with ESR1 wild type (ESR1-WT), patients with ESR1 mutations (ESR1-MUT) experience a short PFS when treated with exemestane. However, they still benefit from fulvestrant (24). Under the pressure of multiple-line treatments, there may be multiple mechanisms of resistance beyond ESR1 mutations. Therefore, the combination of fulvestrant and tucidinostat may be a favorable choice.
Most patients included in this study had previously received CDK4/6 inhibitors. Compared with CDK4/6 inhibitor–naïve patients, those who had previously received CDK4/6 inhibitors showed similar PFS in our study. This in line with a previous study which specifically investigated the efficacy of tucidinostat after CDK4/6 inhibitor treatment. The findings revealed a median PFS of 2.0 months and a median OS of 14 months. Multivariate analysis showed that patients with only one metastatic site and those who received sequential tucidinostat therapy after CDK4/6 inhibitor progression were more likely to benefit from tucidinostat combined with ET (25).
There is no optimal recommendation for patients after failure of CDK4/6 inhibitor in later lines of therapy (26). In a real-world study of exemestane plus everolimus, there was no significant difference in PFS between patients who had or had not received prior CDK4/6 inhibitor treatment, with PFS durations of 3.6 and 4.2 months, respectively (27). In another study, alpelisib with fulvestrant demonstrated a median PFS of 3.7 months for patients with PIK3CA mutations after treatment with CDK4/6 inhibitors and AIs (28). A multicenter real-world study was conducted to assess treatment strategies and regimen selection following CDK4/6 inhibition. In total, 200 patients were enrolled, with most patients (73.5%) receiving subsequent chemotherapy and the rest (26.5%) receiving ET, including tucidinostat or everolimus plus exemestane. The median PFS in the ET + targeted therapy group was not inferior to that in the chemotherapy group (4.6 vs. 5.6 months; P=0.669) (29). Tucidinostat-based therapy has shown similar efficacy to the mTOR inhibitors, PIK3CA inhibitors, or chemotherapy, making it a viable option following CDK4/6 inhibitors. However, the conclusions drawn from a multicenter retrospective study recently did not strongly support tucidinostat. This study included HR+/HER2− ABC patients who experienced disease progression during palbociclib treatment. It aimed to compare the treatment efficacy of abemaciclib versus tucidinostat, revealing a significant extension in PFS within the abemaciclib group compared to the tucidinostat group (5.0 vs. 2.0 months; P<0.001) (30). The inherent heterogeneity in the patient population, such as the slightly higher proportion of patients non-sensitive to prior palbociclib and the lower use of fulvestrant in tucidinostat group, might partially explain the rapid disease progression observed in tucidinostat group (31,32).
Regarding the next treatment option after the progression of tucidinostat, for patients with germline BRCA1/2 mutations, PARP inhibitors (such as olaparib) are recommended (33). In cases with low HER2 expression, consideration may be given to trastuzumab deruxtecan in later lines of therapy (34). Additionally, Sacituzumab govitecan may be considered for later lines (35). The choice of single-agent or combination chemotherapy remains among the available options (36).
Our study has a number of limitations. It was a single-center retrospective study with a small sample size and a relatively short follow-up duration. Variations in patients’ prior treatments could have influenced the outcomes observed. The low incidence of AEs reported in this study was due to incomplete records in the clinic.
Conclusions
Based on limited available data, tucidinostat demonstrated modest efficacy in different lines of treatment for patients with HR+/HER2− ABC. In certain patients, maintenance therapy after chemotherapy may lead to long-term benefits in terms of PFS, warranting further exploration for developing an optimal therapeutic modality and combination therapy strategies for tucidinostat.
Acknowledgments
We would like to thank all of the patients who were enrolled in this study.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1913/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1913/dss
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1913/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1913/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was reviewed and approved by the Clinical Test and Biomedical Ethics Committee of West China Hospital, Sichuan University (No. 2023[no. 1005]). Relevant investigations were conducted in accordance with the principles stated in the Declaration of Helsinki (as revised in 2013). In accordance with the national legislation and institutional requirements, written informed consent of the patients for participation was not required for this retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mouridsen H, Gershanovich M, Sun Y, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol 2001;19:2596-606. [Crossref] [PubMed]
- Robertson JFR, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 2016;388:2997-3005. [Crossref] [PubMed]
- Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 2016;375:1925-36. [Crossref] [PubMed]
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med 2016;375:1738-48. [Crossref] [PubMed]
- Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019;5:5. [Crossref] [PubMed]
- Wang T, Shen G, Li J, et al. Second-line Endocrine Therapy of Hormone Receptor-positive/HER2- negative Advanced Breast Cancer: A Systematic Review and Network Meta-analysis. Curr Cancer Drug Targets 2023;23:718-30. [Crossref] [PubMed]
- Giaquinto AN, Sung H, Miller KD, et al. Breast Cancer Statistics, 2022. CA Cancer J Clin 2022;72:524-41. [Crossref] [PubMed]
- Yuan Y, Zhang S, Yan M, et al. Chemotherapy or endocrine therapy, first-line treatment for patients with hormone receptor-positive HER2-negative metastatic breast cancer in China: a real-world study. Ann Transl Med 2021;9:831. [Crossref] [PubMed]
- Augereau P, Patsouris A, Bourbouloux E, et al. Hormonoresistance in advanced breast cancer: a new revolution in endocrine therapy. Therapeutic Advances in Medical Oncology 2017;9:335-46. [Crossref] [PubMed]
- Saatci O, Huynh-Dam KT, Sahin O. Endocrine resistance in breast cancer: from molecular mechanisms to therapeutic strategies. J Mol Med (Berl) 2021;99:1691-710. [Crossref] [PubMed]
- Hanker AB, Sudhan DR, Arteaga CL. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell 2020;37:496-513. [Crossref] [PubMed]
- Garcia-Martinez L, Zhang Y, Nakata Y, et al. Epigenetic mechanisms in breast cancer therapy and resistance. Nat Commun 2021;12:1786. [Crossref] [PubMed]
- Ning ZQ, Li ZB, Newman MJ, et al. Chidamide (CS055/HBI-8000): a new histone deacetylase inhibitor of the benzamide class with antitumor activity and the ability to enhance immune cell-mediated tumor cell cytotoxicity. Cancer Chemother Pharmacol 2012;69:901-9. [Crossref] [PubMed]
- Sun Y, Hong JH, Ning Z, et al. Therapeutic potential of tucidinostat, a subtype-selective HDAC inhibitor, in cancer treatment. Front Pharmacol 2022;13:932914. [Crossref] [PubMed]
- Raha P, Thomas S, Thurn KT, et al. Combined histone deacetylase inhibition and tamoxifen induces apoptosis in tamoxifen-resistant breast cancer models, by reversing Bcl-2 overexpression. Breast Cancer Res 2015;17:26. [Crossref] [PubMed]
- Zhou Q, Atadja P, Davidson NE. Histone deacetylase inhibitor LBH589 reactivates silenced estrogen receptor alpha (ER) gene expression without loss of DNA hypermethylation. Cancer Biol Ther 2007;6:64-9. [Crossref] [PubMed]
- Zhang Q, Wang T, Geng C, et al. Exploratory clinical study of chidamide, an oral subtype-selective histone deacetylase inhibitor, in combination with exemestane in hormone receptor-positive advanced breast cancer. Chin J Cancer Res 2018;30:605-12. [Crossref] [PubMed]
- Jiang Z, Li W, Hu X, et al. Tucidinostat plus exemestane for postmenopausal patients with advanced, hormone receptor-positive breast cancer (ACE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:806-15. [Crossref] [PubMed]
- Zhang Q, Li W, Hu X, et al. Tucidinostat plus exemestane for postmenopausal patients with advanced, hormone receptor-positive breast cancer: a long-term safety and overall survival update from the randomised, double-blind, placebo-controlled, phase 3 trial. Transl Breast Cancer Res 2023;4:18. [Crossref] [PubMed]
- Wang C, Lin Y, Zhu H, et al. Efficacy and Safety Profile of Histone Deacetylase Inhibitors for Metastatic Breast Cancer: A Meta-Analysis. Front Oncol 2022;12:901152. [Crossref] [PubMed]
- Raphael MJ, Gyawali B, Booth CM. Real-world evidence and regulatory drug approval. Nat Rev Clin Oncol 2020;17:271-2. [Crossref] [PubMed]
- Gennari A, André F, Barrios CH, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol 2021;32:1475-95. [Crossref] [PubMed]
- Xu F, Zheng Q, Xia W, et al. A Phase II Study of Fulvestrant 500 mg as Maintenance Therapy in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Patients with Advanced Breast Cancer After First-Line Chemotherapy. Oncologist 2021;26:e742-e8. [Crossref] [PubMed]
- Brett JO, Spring LM, Bardia A, et al. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res 2021;23:85. [Crossref] [PubMed]
- Zhou J, Wu X, Zhang H, et al. Clinical outcomes of tucidinostat-based therapy after prior CDK4/6 inhibitor progression in hormone receptor-positive heavily pretreated metastatic breast cancer. Breast 2022;66:255-61. [Crossref] [PubMed]
- Breast Cancer Guidelines Of Chinese Society Of Clinical Oncology (CSCO)(2023)[DB/OL]. Available online: http://www.csco.org.cn/
- Cook MM, Al Rabadi L, Kaempf AJ, et al. Everolimus Plus Exemestane Treatment in Patients with Metastatic Hormone Receptor-Positive Breast Cancer Previously Treated with CDK4/6 Inhibitor Therapy. Oncologist 2021;26:101-6. [Crossref] [PubMed]
- Turner S, Chia S, Kanakamedala H, et al. Effectiveness of Alpelisib + Fulvestrant Compared with Real-World Standard Treatment Among Patients with HR+, HER2-, PIK3CA-Mutated Breast Cancer. Oncologist 2021;26:e1133-e42. [Crossref] [PubMed]
- Li Y, Li W, Gong C, et al. A multicenter analysis of treatment patterns and clinical outcomes of subsequent therapies after progression on palbociclib in HR+/HER2- metastatic breast cancer. Ther Adv Med Oncol 2021;13:17588359211022890. [Crossref] [PubMed]
- Yuan Y, Zhang S, Wang T, et al. Efficacy and safety of abemaciclib-based therapy versus tucidinostat-based therapy after progression on palbociclib in patients with HR+HER2− metastatic breast cancer. Translational Breast Cancer Research 2023;4:10.
- Neven P, Dullens L, Han S, et al. Navigating next-generation HR+/HER2− metastatic breast cancer therapies: a critical commentary on abemaciclib vs. tucidinostat after palbociclib progression. Transl Breast Cancer Res 2023;4:31.
- Balanchivadze N, Robert NJ. Abemaciclib-based therapy versus tucidinostat-based therapy in patients with HR+HER2− metastatic breast cancer after palbociclib progression: insights and challenges from a comparative cohort study in China. Transl Breast Cancer Res 2023;4:32.
- Robson ME, Tung N, Conte P, et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol 2019;30:558-66. [Crossref] [PubMed]
- Modi S, Jacot W, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med 2022;387:9-20. [Crossref] [PubMed]
- Rugo HS, Bardia A, Marmé F, et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. The Lancet 2023;402:1423-33. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Metastatic Breast Cancer(2023 Version 4). Available online: http://www.nccn.org




