
Vismodegib as an adjuvant treatment for periorbital basal cell carcinoma: a case report and review of literature
Highlight box
Key findings
• Neoadjuvant vismodegib, is a promising treatment to shrink the periocular basal cell carcinoma (BCC) lesion prior to any surgical intervention.
What is known and what is new?
• Neoadjuvant vismodegib has shown a promising clinical outcome in the treatment of BCC.
• Neoadjuvant vismodegib, followed by surgery excision, such as Mohs micrographic surgery, has shown a promising clinical and aesthetic outcome in the treatment of periorbital BCC.
What is the implication, and what should change now?
• The cases of periocular BCC and mainly those with extended lesions can benefit from early treatment with vismodegib prior to any surgical intervention.
Introduction
Basal cell carcinoma (BCC) is the most common cancer type throughout the world, with a slight male predominance (1). While 80% of BCCs are head and neck malignancies, 20% of them occur on the eyelids, and only 1.6–2.5% of them have orbital invasion which can lead to eyelid disfiguration, tissue destruction, vision loss, or even death (2,3). Treatment options for periorbital BCC include surgical excision, excision with frozen section control of margins, Mohs micrographic surgery, radiotherapy, imiquimod, exenteration, and neo-adjuvant therapies (4). For previously untreated patients, the recurrence rates for BCC after Mohs micrographic surgery and for recurrent tumors are 2.1% and 5%, respectively; however, periorbital BCC lesions have a higher rate of recurrence (3,4). Because functionality and aesthetics can be compromised with total surgical excision and radiation therapy intervention, neoadjuvant therapy is recommended for locally advance and metastatic BCC (5).
The search to collect relevant manuscripts was performed in PubMed and MEDLINE up to June 2023. The utilized keywords included “basal cell carcinoma”, “vismodegib”, “periorbital”, “peri-ocular”, “ocular”, and their combinations. The English abstract of non-English papers was used if available. No limitation for publication date was applied, and reference lists were searched and cross-matched for relevant articles. Twenty-one articles were selected after the initial literature review based on inclusion criteria of the vismodegib administration in periorbital BCC. Patel et al. (6), in a retrospective chart review, evaluated the successful response to sonidegib or vismodegib for the median of 40 weeks among 13 patients with 14 BCC lesions; however, we excluded this study because only one lesion was in the periorbital area. In addition, Sagiv et al. and Furdova et al.’s studies were excluded due to lack of data on treatment and follow up duration (7,8). At the end, we reviewed 19 studies including our patient (Table 1). We present this case in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1589/rc).
Table 1
| Author | Type of study | Metastatic | Recurrence before treatment | Number of patients, demographics | Treatment | Adverse event | Treatment duration | Outcome | Follow-up duration |
|---|---|---|---|---|---|---|---|---|---|
| Jabbehdari 2023 | Case report | No | Yes | 1 (1 m) patient, age: 50 years | Vismodegib + possible Mohs micrographic excision with oculoplastic reconstructive surgery | Dysgeusia, fatigue, dizziness, weight loss | 24 weeks | Responded well | 24 weeks |
| Kahana 2013 (3) | Case report | No | Yes | 1 (1 m) patient, age: 79 years | Vismodegib + surgical excision | Nausea, diarrhea and muscle spasms | 20 weeks | Responded well | 8 weeks |
| Shoji 2021 (9) | Photo essay | No | No | 1 (1 f) patient, age: 73 years | Vismodegib followed by radiotherapy | Not mentioned | 8 weeks | Responded well | 8 weeks |
| Ishai 2020 (10) | Randomized control trial | Locally advanced in 238 cases, metastatic BCC in 6 cases |
No | 244 (143 m/101 f) patients, median age: 72.0 (interquartile range, 60.0–82.0) years | Vismodegib, 64 patients (26.2%) had radiotherapy | Alopecia, muscle spasms, dysgeusia, weight loss, decreased appetite, asthenia, ageusia, nausea, fatigue, and diarrhea | 40 weeks | 22 participants (9.0%) died, 70 (28.7%) achieved complete response, and 94 (38.5%) achieved partial response. vismodegib was discontinued owing to an adverse event in 58 patients (23.8%) | 39 weeks |
| Sagiv 2019 (11) | Retrospective interventional study | No, locally advanced T4 tumor | Yes | 8 (8 m) patients, median age at presentation: 69 (range, 55–84) years | Vismodegib + eye-sparing surgery | Intolerable side effects such as fatigue, alopecia, changes in taste, loss of appetite and weight and muscle cramps | 56 weeks | All final surgical margins were negative for tumor | 72 weeks |
| Keserü 2017 (12) | Retrospective chart review | Periorbital BCC | No | 4 (1 m/3 f) patients, mean age: 87 years | Vismodegib | Most common: muscle spasms | 30 weeks | Complete clinical remission in 75% of patients and interim stabilization of tumor growth in 25% of patients | 68 weeks |
| Demirci 2015 (13) |
Retrospective chart review | No | Yes | 8 (6 m/2 f) patients, mean age: 70.62 years | Vismodegib in 6 patients, off-label neoadjuvant in 1 and adjuvant treatment in 1 patient | Muscle spasm (75%) followed by alopecia (50%), dysgeusia (25%), dysosmia, and episodes of diarrhea and constipation (13%) | 28 weeks | Orbital tumors in all 4 patients who received vismodegib as sole treatment showed partial response with a mean 83% shrinkage in tumor size after a median of 7 months of therapy. In the 2 patients receiving vismodegib as neoadjuvant or adjuvant therapies, there was complete response after a median of 7 months of therapy and no evidence of clinical recurrence after discontinuing therapy for a median of 15 months. The 2 patients with extensive periocular involvement experienced complete clinical response after a median 14 months of treatment | 52 weeks |
| Ojevwe 2015 (14) | Case report | No | No | 1 (1 m) patient, age: 31 years | Vismodegib | No side effect | 24 weeks | Significant reductions in the size of multiple BCCs | 24 weeks |
| Angnardo 2021 (15) | Case report | No, locally advanced | Yes | 1 (1 m) patient, age: 75 years | Vismodegib prior to surgical excision | Muscle cramps, loss of taste, and gastric distress | 15 weeks | Tumor successfully responded to vismodegib allowing surgical excision with clear margins | 48 weeks |
| Eiger-Moscovich 2019 (5) | Retrospective chart review | No, orbital and advanced periocular BCC | Yes | 21 (16 m/5 f) patients, median age: 76 years | Vismodegib | Muscle spasm (76%), followed by dysgeusia (57%), alopecia (47%), weight loss (47%) and decreased appetite (19%). The only grade 3 or 4 adverse event was hepatotoxicity (10%) | 36 weeks | Completed clinical response in 10 patients, partial in 10 patients, and stable in 1 patient. Eight patients discontinued treatment because of side effects. Five patients died, most from reasons unrelated to vismodegib therapy, except for 1 patient who died from possibly treatment-related sepsis | 68 weeks |
| Ozgur 2015 (16) | Retrospective interventional case series | Yes, 4 had metastasis | No | 12 (10 m/2 f) patients, median age: 64.5 years | Vismodegib | Muscle spasms (12 patients), weight loss (10 patients), dysgeusia (9 patients), alopecia (9 patients), decreased appetite (5 patients), and fatigue (4 patients). Five patients developed grade II adverse effects | 46 weeks | 3 patients had a complete response, 6 had a partial response, and 3 had stable disease at last follow-up | 50 weeks |
| Papastefanou 2017 (17) | Case report | No | Yes | 1 (1 m) patient, age: 84 years | Vismodegib and eventually requiring orbital exenteration | Anorexia, weight loss, alopecia and muscle cramps | 36 weeks | Responded dramatically to vismodegib after 3 months but recurred after 9 months due to drug resistance, eventually requiring orbital exenteration |
72 weeks |
| Oliphant 2020 (18) | Retrospective chart review | No | Yes, 8/13 (62%) recurrent and 5/13 were primary | 13 (7 m/6 f) patients, mean age: 75 years | Six patients had further surgery after vismodegib, seven patients only vismodegib | Eleven out of 13 patients developed side effects, the most common being fatigue in six patients (46%) | 28 weeks | Complete response in 5/13 patients (38%) and a partial response in 8/13 patients (62%). Three patients developed recurrence (23%). Three patients (23%) ultimately underwent exenteration | 96 weeks |
| Su 2020 (19) | Case report | No, locally advanced periocular | Yes | 1 (1 f) patient, age: 63 years | Vismodegib therapy prior to surgery (Mohs micrographic excision with oculoplastic reconstructive surgery) | Fatigue, dysgeusia and weight loss | 84 weeks | The tumor size had reduced significantly. Negative margins and satisfactory cosmetic result | 96 weeks |
| Gill 2013 (20) | Prospective observational case series | No | Yes | 7 (5 m/2 f) patients, mean age: 71 (range, 43–100) years | Surgery prior to Vismodegib therapy | Adverse reactions occurred in 6 patients (86%) and included alopecia (29%), dysgeusia (29%), muscle cramps (29%), and anorexia (14%) | 11 weeks | Two patients (29%) demonstrated complete clinical regression, 2 (29%) demonstrated greater than 80% partial clinical regression, 2 (29%) demonstrated less than 35% partial clinical regression, and 1 (14%) progressed | 29.2 weeks |
| González 2019 (21) | Prospective case series | No | Yes, 3 cases had recurrent BCC | 8 (6 m/2 f) patients, mean age: 76 years | Vismodegib followed by Mohs surgery | The most common included dysgeusia (100%) and muscle spasms (100%). Weight loss was present in 75% of the patients with a mean loss of 12.6 pounds and hair loss was seen in 50%. Only 1 (12.5%) patient withdraw from treatment because of intolerable muscle spasms | 19.2 weeks | Seven patients (87.5%) had a complete response and 1 (12.5%) progressed. Mohs micrographic surgery allowed to confirm a complete histologic response in 5 of 6 (83.3%) cases, and 1 patient refused surgery. All 7 patients are disease free | 57.6 weeks |
| Kahana 2021 (22) | Open-label, nonrandomized phase IV trial | No | No | 34 (19 m/15 f) patients, mean age: 68.5 years | Vismodegib followed by surgery (19 patients underwent exenteration and 15 patients underwent globe-sparing surgery) | Dysgeusia (25, 74%), myalgia (23, 67%), and alopecia (16, 47%) | 9 weeks | A total of 56% (19) of patients demonstrated complete tumor regression by physical examination, and 47% (n=15) had complete regression by MRI/CT. A total of 79.4% (n=27) of patients underwent surgery, of which 67% (n=18) had no histologic evidence of disease, 22% (n=6) had residual disease with clear margins, and 11% (n=3) had residual disease extending to margins | 48 weeks |
| Paulsen 2016 (23) | Case report | No | Yes | 1 (1 m) patient, age: 63 years | Vismodegib followed by exenteration | Alopecia, muscle cramps and dysgeusia | 40 weeks | Complete response | 36 weeks |
| Wong 2017 (24) | Prospective study | No | Yes | 15 (9 m/6 f) patients, mean age: 74 years | Vismodegib followed by exenteration. In 3 cases | Alopecia, muscle cramps and dysgeusia, gastrointestinal problem, constipation | 42 weeks | 10 complete response, 3 partial response and 2 progression | 144 weeks |
m, male; f, female; BCC, basal cell carcinoma; MRI, magnetic resonance imaging; CT, computed tomography.
Case presentation
A 50-year-old Caucasian male was referred to the oculoplastic clinic for the evaluation of an ulcerated pearly plaque with rolled borders lesion and multilocular fungating hemorrhagic characteristics located on the left lower eyelid in periorbital area (Figure 1A). He also had another lesion located on his superior chest, in the midline area, which was evaluated by the dermatology team. Both lesions had been growing for over a year and bled frequently. The periorbital lesion was originally excised a few years ago, following which, recurrence led to the need for Mohs micrographic surgery. Shave biopsies indicated that the lesion on the lower eyelid was nodular type BCC, while the one on his chest was morpheaform type BCC. The chest lesion was excised, but due to the location of the periorbital lesion, the patient was referred to the oculoplastic clinic. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
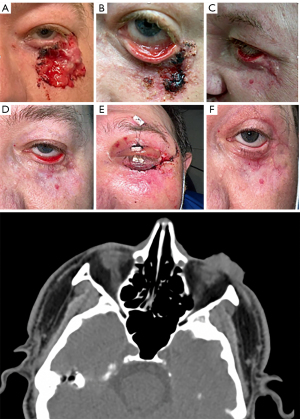
The patient had a past medical history of thalamic stroke, myocarditis, and medically controlled hypertension. There was no past medical history of trauma, cancer, infectious, inflammatory, or autoimmune conditions. Reviews of systems as well as family and social history were otherwise unremarkable. On exam, a large 3 cm left lower eyelid exophytic lesion extending into the lateral canthus and into the fornix of the lower lid, approaching bulbar conjunctiva with no lacrimal system involvement was noted (Figure 1A). The lesion felt mobile over the periosteum. Extraocular movements were intact, and pupils were round and reactive with no relative afferent pupillary defect. Intraocular pressure by applanation was 14 mmHg in both eyes. Anterior and posterior segment examinations were unremarkable.
A CT scan showed a plaque-like heterogeneous mass involving the skin and subcutaneous fat on the lower eyelid as well as extension into the upper left malar cheek
(Figure 1). Labs at initiation showed slightly low hemoglobin and hematocrit levels (12.5 g/dL, 38.8 L/L).
The patient was given treatment options that included undergoing Mohs micrographic surgery and/or having a course of vismodegib. The patient decided to take vismodegib 150 mg orally daily. A plan of treatment with vismodegib, followed by Mohs micrographic surgery and reconstruction, was made.
Twelve weeks after starting vismodegib, the tumor had shrunk significantly, and the patient reported less discomfort and decreased bleeding of the lesion (Figure 1B). Physical examination at this point revealed a 2.5 cm left lower eyelid exophytic lesion, with much less protrusion, extending into the lateral canthus and into the fornix of the lower lid, still approaching bulbar conjunctiva. Further examination noted a cicatricial ectropion with conjunctival injection. The patient reported loss of taste, loss of weight, fatigue, and dizziness as side effects of vismodegib, but because his symptoms were mild, he was instructed to continue the vismodegib treatment. Six months after initiation, the daily oral 150 mg vismodegib was discontinued, and pulse dosing of 150 mg vismodegib for 10 days at the beginning of each month was started. Monitoring of lesion with potential scouting biopsy continued which did not indicate the recurrence of BCC (Figure 1C). Due to the cicatricial ectropion of left lower lid (Figure 1D), rotation flap, full thickness skin graft to anterior lamellar, and adjacent tissue transposition (Tenzel) to correct ectropion was done
(Figure 1E). The biopsy during ectropion repair was obtained to exclude the residual cancerous tissue. The external photo of the left eye at the latest visit shows the improvement of ectropion with telangiectatic vessels in the periorbital area in the area of treated BCC (Figure 1F).
Nineteen articles, including seven case reports, one photo essay, one randomized control trial, two retrospective interventional case series, four retrospective chart reviews, three prospective observational case series, and one open-label nonrandomized trial, were selected to review based on inclusion of the periorbital BCC treated with vismodegib. A total of 382 patients, including our patient, were selected from the literature review (Figure 2A). Of them, 232 (60.73%) were males, and 150 (39.27%) were females (Figure 2B). Only ten cases were metastatic, and BCC lesions among patients in 13 studies were results of recurrence BCC (Figure 2C). All patients showed at least one side effect of treatment with vismodegib, including dysgeusia, fatigue, dizziness, weight loss, nausea, diarrhea, muscle spasms, and loss of appetite. Of the 382 patients, 244 patients were only treated with vismodegib; 65 cases underwent radiotherapy following vismodegib; 24 had orbital exenteration; and 47 patients had surgical excision, eye-sparing surgery, and Mohs micrographic surgery after vismodegib therapy. The mean of treatment duration among all patients was 35.71 weeks (Figure 3A), and the mean of follow up was 49.19 weeks (Figure 3B). In addition, 167 patients had complete response, 131 had partial response, and 26 patients had progression or death (Figure 3C). A higher number of complete responses was reported among those who had combination therapy with vismodegib and other interventions, other than oral vismodegib therapy.


Discussion
Mohs micrographic surgery is superior to wide local excision with frozen section control of margins and other current options in the treatment of locally advanced or metastatic periorbital BCC. However, radiation therapy is the most useful approach as salvage palliative therapy to control the symptoms (2-4).
Oral vismodegib, a novel Food and Drug Administration (FDA)-approved hedgehog pathway inhibitor, has been used recently with the purpose of decreasing tumor size in patients who cannot tolerate radiotherapy or surgery, or who have recurrent locally advanced or metastatic disease (4,25). The FDA approval decision was made based on a noncomparative clinical study, taking into consideration the rarity of this condition and the lack of treatment options for locally advanced or metastatic BCC lesions. The hedgehog pathway is important in embryogenesis due to its crucial role in cell proliferation and differentiation; however, in adults, its activity is limited to certain groups of cells, mostly stem cells, skin cells, and hair follicles (10).
In 2018, an expert panel established guidelines for treatment of BCC, according to the American Joint Committee on Cancer (AJCC) TNM staging. They recommended surgical excision for eyelid tumors at stages T1, T2a, and T2b. Further, they recommended that noninvasive T3a tumors should be considered for radiotherapy, and tumors staged invasive T3a, T3b, and T4 that are not amenable and not appropriate for radical local therapy should be defined as locally advanced BCC and assessed for treatment with vismodegib (5,7,11). When any tumors regardless of their size invade the orbit, they might have worse outcomes compared to a larger tumor with no orbital invasion. Therefore, relying only on TNM staging for periorbital BCC lesions is not enough (26). Invasion of the BCC and eyelid deformity should be considered in staging of the disease and the treatment plan.
Two landmark studies, the phase II study ERIVANCE BCC and The SafeTy Events in VIsmodEgib study (STEVIE) confirmed in clinical practice, the tolerability, safety, and efficacy of this drug in those with metastatic or locally advanced BCC (25,27,28). Due to the wide range of side effects and health risks associated with vismodegib usage, oral vismodegib therapy can be used with the purpose of tumor shrinkage for further surgical interventions. In addition, it has been reported that vismodegib as a neoadjuvant therapy before Mohs micrographic surgery could decrease the number of future Mohs reconstructive surgeries (21,27). The results of our literature review showed the promising role of vismodegib in shrinking the size of BCC prior to any reconstructive surgery.
Our case demonstrated the risk of margin-control excision of periorbital BCC and the further challenge of Mohs surgery after a reconstructive surgery when the residual cancerous cells were possibly seeded in the surrounding non-contiguous area. Vismodegib was started for the patient which shrunk the initial lesion. However, we continued to monitor the lesion with scouting biopsy to find any residual cancerous tissue. The biopsy during ectropion repair has also obtained to exclude the residual cancerous tissue. The residual cancer can be found and the standard histologic techniques might miss the residual cluster of cancer cells (29). Our patient did not show any residual cancerous cells during follow-up exams as the VISMONEO trial confirmed no peripheral islands of tumor in the locally advanced periorbital BCC treated with vismodegib (30).
Our patient reports willingness to continue this medication, although experiencing some mild (grade 1) side effects (according to the Common Terminology Criteria for Adverse Events) (11). Adverse effects are common for vismodegib, and our results showed that all patients in all studies showed at least one side effect. In the STEVIE study, 98% had at least one adverse event (27).
Concerns regarding treatment with vismodegib at this stage would be progression to more severe adverse effects as well as resistance. After initial response to treatment, resistance due to the acquisition of a mutation in the SMO gene, has been noted in previous studies. It has been also reported that many mutations in this gene had the potential to decrease sensitivity to vismodegib, with complete resistance observed when the E518 residue was altered. Some mutations can inhibit binding while others can cause conformational rearrangements that obstruct binding (27). The patient stated “I am very happy with the final outcome, it’s amazing that I don’t have any periorbital irritation or bleeding after treatment.”
Conclusions
The promising role of vismodegib in decreasing the size of tumors, prior to any surgical interventions, makes vismodegib a good option in the treatment of locally advanced or metastatic periorbital BCC.
Acknowledgments
The authors would like to thank the patient for his consent to the publication of this case.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1589/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1589/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1589/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Saleh GM, Desai P, Collin JR, et al. Incidence of eyelid basal cell carcinoma in England: 2000-2010. Br J Ophthalmol 2017;101:209-12. [Crossref] [PubMed]
- Leibovitch I, McNab A, Sullivan T, et al. Orbital invasion by periocular basal cell carcinoma. Ophthalmology 2005;112:717-23. [Crossref] [PubMed]
- Kahana A, Worden FP, Elner VM. Vismodegib as eye-sparing adjuvant treatment for orbital basal cell carcinoma. JAMA Ophthalmol 2013;131:1364-6. [Crossref] [PubMed]
- Shi Y, Jia R, Fan X. Ocular basal cell carcinoma: a brief literature review of clinical diagnosis and treatment. Onco Targets Ther 2017;10:2483-9. [Crossref] [PubMed]
- Eiger-Moscovich M, Reich E, Tauber G, et al. Efficacy of Vismodegib for the Treatment of Orbital and Advanced Periocular Basal Cell Carcinoma. Am J Ophthalmol 2019;207:62-70. [Crossref] [PubMed]
- Patel AD, Ravichandran S, Kheterpal M. Hedgehog inhibitors with and without adjunctive therapy in treatment of locally advanced basal cell carcinoma. Int J Dermatol 2022;61:118-24. [Crossref] [PubMed]
- Sagiv O, Ding S, Ferrarotto R, et al. Impact of Food and Drug Administration Approval of Vismodegib on Prevalence of Orbital Exenteration as a Necessary Surgical Treatment for Locally Advanced Periocular Basal Cell Carcinoma. Ophthalmic Plast Reconstr Surg 2019;35:350-3. [Crossref] [PubMed]
- Furdova A, Lukacko P. Periocular Basal Cell Carcinoma Predictors for Recurrence and Infiltration of the Orbit. J Craniofac Surg 2017;28:e84-7. [Crossref] [PubMed]
- Shoji MK, Pirakitikulr N, Tran AQ, et al. Basal cell carcinoma with extensive periorbital involvement response to vismodegib. Orbit 2021;40:543. [Crossref] [PubMed]
- Ben Ishai M, Tiosano A, Fenig E, et al. Outcomes of Vismodegib for Periocular Locally Advanced Basal Cell Carcinoma From an Open-label Trial. JAMA Ophthalmol 2020;138:749-55. [Crossref] [PubMed]
- Sagiv O, Nagarajan P, Ferrarotto R, et al. Ocular preservation with neoadjuvant vismodegib in patients with locally advanced periocular basal cell carcinoma. Br J Ophthalmol 2019;103:775-80. [Crossref] [PubMed]
- Keserü M, Green S, Dulz S. Vismodegib Therapy for Periocular Basal Cell Carcinoma. Klin Monbl Augenheilkd 2017;234:64-9. [Crossref] [PubMed]
- Demirci H, Worden F, Nelson CC, et al. Efficacy of Vismodegib (Erivedge) for Basal Cell Carcinoma Involving the Orbit and Periocular Area. Ophthalmic Plast Reconstr Surg 2015;31:463-6. [Crossref] [PubMed]
- Ojevwe FO, Ojevwe CD, Zacny JP, et al. Treatment of multiple unresectable basal cell carcinomas from Gorlin-Goltz syndrome: a case report. Anticancer Res 2015;35:1777-81.
- Angnardo L, Humeda Y, Alexandraki I, et al. Vismodegib as Eye-Sparing Neoadjuvant Treatment for Locally Advanced Periocular Basal Cell Carcinoma. J Drugs Dermatol 2021;20:552-4. [Crossref] [PubMed]
- Ozgur OK, Yin V, Chou E, et al. Hedgehog Pathway Inhibition for Locally Advanced Periocular Basal Cell Carcinoma and Basal Cell Nevus Syndrome. Am J Ophthalmol 2015;160:220-227.e2. [Crossref] [PubMed]
- Papastefanou VP, René C. Secondary Resistance to Vismodegib After Initial Successful Treatment of Extensive Recurrent Periocular Basal Cell Carcinoma with Orbital Invasion. Ophthalmic Plast Reconstr Surg 2017;33:S68-70. [Crossref] [PubMed]
- Oliphant H, Laybourne J, Chan K, et al. Vismodegib for periocular basal cell carcinoma: an international multicentre case series. Eye (Lond) 2020;34:2076-81. [Crossref] [PubMed]
- Su MG, Potts LB, Tsai JH. Treatment of periocular basal cell carcinoma with neoadjuvant vismodegib. Am J Ophthalmol Case Rep 2020;19:100755. [Crossref] [PubMed]
- Gill HS, Moscato EE, Chang AL, et al. Vismodegib for periocular and orbital basal cell carcinoma. JAMA Ophthalmol 2013;131:1591-4. [Crossref] [PubMed]
- González AR, Etchichury D, Gil ME, et al. Neoadjuvant Vismodegib and Mohs Micrographic Surgery for Locally Advanced Periocular Basal Cell Carcinoma. Ophthalmic Plast Reconstr Surg 2019;35:56-61. [Crossref] [PubMed]
- Kahana A, Unsworth SP, Andrews CA, et al. Vismodegib for Preservation of Visual Function in Patients with Advanced Periocular Basal Cell Carcinoma: The VISORB Trial. Oncologist 2021;26:e1240-9. [Crossref] [PubMed]
- Paulsen JF, Øregaard JS, Nielsen AL, et al. Vismodegib and surgery combined - effective treatment of locally advanced basal cell carcinoma. Acta Oncol 2016;55:1492-4. [Crossref] [PubMed]
- Wong KY, Fife K, Lear JT, et al. Vismodegib for Locally Advanced Periocular and Orbital Basal Cell Carcinoma: A Review of 15 Consecutive Cases. Plast Reconstr Surg Glob Open 2017;5:e1424. [Crossref] [PubMed]
- Dirix L, Rutten A. Vismodegib: a promising drug in the treatment of basal cell carcinomas. Future Oncol 2012;8:915-28. [Crossref] [PubMed]
- Unsworth SP, Heisel CJ, Kahana A. A New Paradigm in the Treatment of Advanced Periocular Basal Cell Carcinoma? Am J Ophthalmol 2019;206:215-6. [Crossref] [PubMed]
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med 2012;366:2171-9. [Crossref] [PubMed]
- Basset-Séguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: Primary analysis of STEVIE, an international, open-label trial. Eur J Cancer 2017;86:334-48. [Crossref] [PubMed]
- Unsworth SP, Tingle CF, Heisel CJ, et al. Analysis of residual disease in periocular basal cell carcinoma following hedgehog pathway inhibition: Follow up to the VISORB trial. PLoS One 2022;17:e0265212. [Crossref] [PubMed]
- Bertrand N, Guerreschi P, Basset-Seguin N, et al. Vismodegib in neoadjuvant treatment of locally advanced basal cell carcinoma: First results of a multicenter, open-label, phase 2 trial (VISMONEO study): Neoadjuvant Vismodegib in Locally Advanced Basal Cell Carcinoma. EClinicalMedicine 2021;35:100844. [Crossref] [PubMed]


