
Design of 225Ac-PSMA for targeted alpha therapy in prostate cancer
Introduction
This overview describes the essential components in the design of a targeted alpha therapy (TAT) compound.
The basic known elements required for prostate cancer treatment are described in Section 2. The historical narrative description of the clinical use of 225Ac-prostate-specific membrane antigen (PSMA) therapy has been presented in Section 3, which includes both global and own experience. In Section 3, the clinical outcome of this TAT is presented as good biochemical responses and increased progression-free survivals (PFSs) and limited toxicities in clinical studies.
Prostate radioligand therapy (PRLT) using alpha-emitting PSMA derivatives
PRLT
PRLT in this context involves the systemic intravenous administration of a specific radiopharmaceutical composed of α-emitting or β-emitting radionuclide chelated to a small molecule for the purpose of delivering cytotoxic radiation to prostate cancer cells (1).
PSMA
PSMA, also known as folate hydrolase I or glutamate carboxypeptidase II, is a type II transmembrane protein, which is anchored in the cell membrane of prostate epithelial cells. These enzymes have normal physiologic function depending on their location in the human body. PSMA is highly expressed on prostate epithelial cells and strongly up-regulated in prostate cancer. The PSMA expression levels are directly correlated to androgen independence, metastasis, and prostate cancer progression (2). Thus, PSMA is a promising molecular target for diagnosis and therapy of metastatic prostate cancer at present (2).
PSMA-617
PSMA-617, a DOTA derivative of the Glu-urea-Lys motif, has been developed in the German Cancer Research Center (DKFZ), Heidelberg, Germany, for the treatment of patients with metastatic prostate cancer (3). Radionuclides are linked with a macrocyclic chelator 1,4,7,10-tetraaza-cyclododecane-1,4,7,10-tetraacetic acid, known with the acronym DOTA (Figure 1).
PSMA-I&T
PSMA I&T is a third-generation derivative of PSMA-compounds which has been used for imaging (68Ga-PSMA I&T) and therapy (177Lu-/225Ac-PSMA I&T) (4-6). Synonym for DOTAGA-(l-y)fk(Sub-KuE) (7). The macrocyclic linker molecule 1,4,7,10-tetraazacyclododececane,1-(glutaric acid)-4,7,10-triacetic acid is known with the acronym DOTAGA (Figure 1).
Actinium-225 (225Ac)
225Ac, an alpha emitter, has been labelled to PSMA ligands as 225Ac-PSMA for TAT. 225Ac has a half-life of 9.9 days and decays to produce four α-particles with an energy of 5.8–8.4 MeV, with a tissue range of up to 85 µm (8). Alpha particles are attractive anti-tumor agents as they have a high linear energy transfer (LET) and relatively short tissue length and are able to produce double strand DNA damage whilst minimizing toxicity to adjacent tissue. Thus alpha particle emission can be considered advantageous as compared to β particle emission which mainly results in single strand DNA breaks with a relatively long tissue path length which contributes to its toxicity profile (9).
Relative biological effectiveness (RBE)
RBE is the ratio of biological effectiveness of one type of ionizing radiation relative to another, given the same amount of absorbed energy: here β- and α-emission, between the 177Lu and 225Ac (as biological consequence of different ionisation-densities along a particle-tract). The RBE is an empirical value that varies depending on the type of ionizing radiation, the energies involved, the biological effects being considered such as cell death, and the oxygen concentration, etc. RBE was 5 (for 225Ac/177Lu) in an experimental neuroendocrine tumor model (10).
Clinical experience—a review & own experience
Bismuth-213 (213Bi) for PSMA-TAT
213Bi is a mixed α- and β-emitter with a half-life of 45.6 min and it is produced from 225Ac decay (Figure 2). Small molecule PSMA-I&T induced more double-strand breaks than the nanobody in nonclinical studies (11), where TAT with 213Bi labeled antibody (J591), small molecule inhibitor PSMA-I&T or nanobody (JVZ-008) were compared; they demonstrated tumor targeting and tumor growth inhibition in nude mice with PSMA-overexpressing xenografts (11). Dosimetry calculations with 213Bi-PSMA-617 and 225Ac-PSMA-617 demonstrated the superiority of 225Ac as compared to short-lived 213Bi as the radionuclide label for PSMA-617 (12). Probably therefore, there is only one single patient case reported to date on the use of 213Bi-PSMA-617 (12). The patient was treated with two cycles of 213Bi-PSMA-617 with a cumulative activity of 592 MBq. The serum prostate-specific antigen (PSA) concentration decreased from 237 down to 43 µg/L as sign of biochemical response (13). Also, the short half-life of 213Bi, the currently limited supply of 225Ac/213Bi radionuclide generators and their high cost makes this radionuclide less suitable for routine clinical therapeutic applications.
225Ac for PSMA-TAT
225Ac has a half-life of 9.9 days and decays to produce four alpha particles with an energy of 5.8–8.4 MeV, with a tissue range of up to 85 µm (3). 225Ac-PSMA-617 was first synthesized and investigated in vitro at JRC Karlsruhe in 2013, stimulated by initial reports on encouraging clinical results with the beta emitter labelled analogue 177Lu-PSMA-617. The combination of 225Ac with the ligand PSMA-617 seemed favorable due to matching decay characteristics of the alpha emitter and pharmacokinetics of the ligand. The rapid tumor uptake of PSMA-617, an extended tumor retention time and rapid clearance of unbound ligand favor its combination with a relatively long-lived and highly cytotoxic radionuclide such as 225Ac. Importantly, the high degree of internalization of the ligand allows to retain the daughter nuclides of 225Ac at the target site and harness their alpha emissions for added therapeutic efficacy, whilst minimizing toxicity to untargeted organs. In addition, the chemical properties of trivalent Ac(III) allow for stable complexation with DOTA-chelated ligands such as PSMA-617. For routine clinical application, a reliable, microwave-based protocol for synthesis of 225Ac-PSMA-617 was developed (14).
Clinical application of 225Ac-PSMA TAT as last line of therapy in patients with metastatic castration-resistant prostate cancer (mCRPC) has demonstrated an excellent response in chemotherapy naive patients, although most clinical studies report it as third-line therapy or after a failure of 177Lu-PRLT. Widespread application of 225Ac-PSMA TAT is hampered by its the currently limited supply of the radionuclide, a critical issue that is currently being addressed worldwide through development of additional production methods and facilities. There is a clinical review in the literature about preclinical and clinical studies with 225Ac-PSMA compounds (15). We have listed an updated Table 1 with our own data. The clinical findings are described in a more detailed manner in the following.
Table 1
| Clinical study | Compound | Patient number | Activity per cycle | Biochemical response/PSA50 | PFS/OS, months | Major toxicity |
|---|---|---|---|---|---|---|
| Kratochwil 2016 (16) | PSMA-617 | 2 | 100 kBq/kg | 100% (2/2) | – | Xerostomia |
| Kratochwil 2017 (17) | PSMA-617 | 14 | 50–200 kBq/kg | 44% (4/9) | NA/8.5 | Xerostomia |
| Kratochwil 2018 (18) | PSMA-617 | 40 | 100 kBq/kg | 63% (24/38) | NA/>12 | Xerostomia |
| Sathekge 2019 (19) | PSMA-617 | 1 | 8 MBq | 100% (1/1) | – | – |
| Sathekge 2019 (20) | PSMA-617 | 17 | 4–8 MBq | 88% (15/17) | – | Xerostomia |
| Sathekge 2020 (21) | PSMA-617 | 73 | 4–8 MBq | 70% (51/73) | 15.2/18.0 | Xerostomia |
| Sathekge 2022 (22) | PSMA-617 | 53 | 8 MBq | 91% (48/53) | 20/>55 | Xerostomia |
| Lawal 2022 (23) | PSMA-617 | 106 | 8 MBq | 80% (85/106) | 14.0/15.0 | Xerostomia |
| Sathekge 2023 (24) | PSMA-617 | 21 | 8 MBq | 86% (18/21) | >36/>36 | Xerostomia |
| Feuerecker 2021 (25) | PSMA-617 | 26 | 4–8 MBq | 65% (17/26) | 3.5/7.7 | Xerostomia, pancytopenia |
| Yadav 2020 (26) | PSMA-617 | 28 | 100 kBq/kg | 39% (11/28) | 12/17 | Xerostomia fatigue |
| van der Doelen 2021 (27) | PSMA-617 | 13 | 6–8 MBq | 69% (9/13) | NA/8.5 | Xerostomia |
| Ilhan 2021 (28) | PSMA-I&T | 1 | 8 MBq | 100% (1/1) | – | Xerostomia |
| Zacherl 2021 (29) | PSMA-I&T | 14 | 7.8 MBq | 50% (7/14) | – | Xerostomia |
| Sen 2021 (30) | PSMA-617 | 38 | 100 kBq/kg | 66% (25/38) | 8/12 | Xerostomia |
| Satapathy 2020 (31) | PSMA-617 | 11 | 100 kBq/kg | 45% (5/11) | – | Xerostomia |
| Khreish 2020 (32) | PSMA-617 | 20 | 5.3 (1.5–7.9) MBq† | 65% (13/20) | 4.7/11 | Xerostomia |
| Rosar 2021 (33) | PSMA-617 | 15 | 2.7±1.1 MBq‡ | 53% (8/15) | NA/19.4 | Xerostomia |
| Sanli 2021 (34) | PSMA-617 | 12 | 100 kBq/kg | 50% (6/12) | 4/10 | Xerostomia |
†, median (minimum-maximum). ‡, mean ± standard deviation. PSMA, prostate-specific membrane antigen; PSA, prostate-specific antigen; PFS, progression-free survival; OS, overall survival; NA, not available.
Clinical investigation of 225Ac-PSMA-617 was started in 2014 in collaboration of JRC Karlsruhe and University Hospital Heidelberg, Germany and first reported by Kratochwil et al. (16), describing a complete response in two patients with mCRPC who had failed multiple lines of previous therapy. Because of challenging clinical situations and extensive pretreatment patients were treated with 100 kBq/kg of 225Ac-PSMA-617 at every 8 weeks as salvage therapy after the presence of a PSMA-positive tumor phenotype had been validated by 68Ga-PSMA-11 positron emission tomography (PET)/computed tomography (CT) (16). The first patient was not suitable for 177Lu-PSMA-617 because of wide spread marrow disease and the second one progressed from 177Lu-PSMA therapy presenting with diffuse abdominal and liver disease (16). Both patients showed a complete response on the 68Ga-PSMA-11 PET/CT scan, and PSA declined below the measurable level (16). Salivary gland toxicity (xerostomia) was reported in both patients (16).
The second study with 14 mCRPC patients found that a treatment activity of 100 kBq/kg of body weight of 225Ac-PSMA-617 per cycle every 8 weeks was the most optimal when considering both efficacy (biochemical response) and tolerability (17). Severe xerostomia was the dose-limiting toxicity (17). Kratochwil et al. reviewed next the efficacy of 225Ac-PSMA-617 in a large cohort of 40 patients with advanced disease (18). All patients had mCRPC and had failed or were ineligible for conventional therapy; 70%, 85% and 60% of the patient cohort had had prior docetaxel, abiraterone and enzalutamide respectively (18). 68Ga-PSMA PET/CT and 99mTc-PSMA single photon emission computed tomography (SPECT)/CT imaging was used for patient selection with patients with limited disease selected for 177Lu-PSMA radioligand therapy (RLT) whilst those with diffuse uptake on imaging were treated with 225Ac-PSMA, those patients who demonstrated no tumor uptake on imaging were declined TAT (18). An activity of 100 kBq/kg 225Ac-PSMA-617 was administered 8 weekly for a minimum 3 and up to 5 cycles (18).
This study demonstrated a PSA decline of more than 50% in 63% of patients, with a median duration of tumor control of 9 months (18). The median overall survival (OS) was more than 12 months (18). Thirty-eight patients out of 40 survived at least 8 weeks with 63% of these patients demonstrating a PSA response >50% and 87% demonstrating any PSA response, a median OS and PFS of >12 and 7.0 months were demonstrated (18).
This standardized treatment protocol for 225Ac-PSMA-617 is routinely applied for salvage therapy of end-stage mCRPC patients in many studies (17-20).
Feuerecker et al. investigated 225Ac-PSMA-617 TAT in 26 patients who had failed a median of six lines of previous therapy for mCRPC, all had progressed after 177Lu-PSMA therapy (25). A PSA decline of >50% was demonstrated in 65% of the patients whilst 88% of the patients demonstrated any PSA reduction, however, no complete response was seen in the population (25). The median OS was 7.7 months [95% confidence interval (CI): 4.5–12.1 months] (25). Mild irreversible xerostomia was seen in all patients with 23% of patients refusing any further treatment due to severe xerostomia, 8% of the patients had to have their treatment discontinued to prevent further deterioration of marrow toxicity which had been pre-existing (25). Poor prognosis could be seen in the patients who had failed previous 177Lu-PSMA including the presence of liver metastases and higher Eastern Cooperative Oncology Group (ECOG) status (25).
Similar findings were described by Yadav et al. where 28 patients with mCRPC were enrolled to receive 225Ac-PSMA-617 TAT, 54% of these patients had failed 177Lu-PSMA therapy whilst 46% were 177Lu-PSMA therapy naïve (26). A comparison of the two groups, previous 177Lu-PSMA and 177Lu PSMA naïve, demonstrated a PSA decline of >50% and progression rate of 26.6% and 46% vs 53.8% and 22.3%, respectively (26). Interestingly there was no difference in median survival and OS between the two groups, 10 vs. 12 months and 16 vs. 17 months, respectively (26).
In South Africa, Sathekge et al. investigated 225Ac-PSMA in 17 patients who were chemotherapy naive (20). 225Ac-PSMA-617 was administered in 2 monthly intervals. An initial activity of 8 MBq was administered with response assessments determined using PSA and 68Ga-PSMA PET/CT imaging prior to subsequent cycles of 225Ac-PSMA. A “dynamic dose-reduction” was used where the subsequent activity of 225Ac-PSMA was reduced in patients who had demonstrated a response to the previous cycle, the mean administered activity was 7.4±1.5 MBq, with 3 of the 17 patients only receiving 2 cycles of therapy after having demonstrated an excellent response. A PSA decline >90% was seen in 82% of the patients at end of therapy, at median follow up of 13 months post initiation of treatment 82% of the patients were still alive with 50% of these patients in remission demonstrating undetectable serum PSA levels and the other 50% with stable disease. Grade 1–2 xerostomia was the most frequently noted side-effect with no discontinuation in therapy reported due to severe symptoms. Grade 4 nephrotoxicity was noted in a patient with only a single functional kidney who had poor renal functioning from baseline (20).
More recently, Sathekge et al. reported a follow up study in 53 mCRPC patients who received treatment with 225Ac-PSMA-617 directly post androgen deprivation therapy (ADT) (22). Remarkably, 48 patients (91%) had a PSA decline of at least 50% and a median OS of >55 months (median OS not yet reached at latest follow-up). These extremely favorable results warrant further studies of 225Ac-PSMA-617 therapy in comparison with current standard of care treatment options such as second line hormonal therapy or chemotherapy.
Sathekge et al., in the largest study population to date enrolled 73 men with mCRPC who failed standard therapy, 14 of these patients had prior 177Lu-PSMA therapy (21). At the end of 225Ac-PSMA-617 therapy 70% of the patients demonstrated a ≥50% PSA decline whilst 82% had any decline in PSA (21). Post therapy 68Ga-PSMA PET/CT images were negative in 29% of the patients (22). Median OS and PFS were determined to be 18 months (95% CI: 16.2–19.9 months) and 15.2 months (95% CI: 13.1–17.4 months) respectively, 13 patients had passed away whilst 23 patients had demonstrated disease progression (21). Factors found to be associated with higher OS and PFS included baseline PSA, PSA decline ≥50%, prior chemotherapy, prior radiation therapy, and Hb at baseline, whilst prior RLT with 177Lu-PSMA was associated with a poorer PFS (21).
Recently interesting preliminary findings in a retrospective series of 21 metastatic hormone-sensitive prostate cancer (mHSPC) patients that refused standard treatment options and were treated with 225Ac-PSMA-617 indicated that this could be an alternative efficacious treatment option for these patients (24). Since PSMA-617 crosses the blood-brain barrier and accumulates in cerebral metastases (35), a significant regression of cerebral metastases was demonstrated using 225Ac-PSMA-617 (19). Prostate cancer patients with brain metastases have limited treatment options and poor survival, and TAT with 225Ac-PSMA-617 may have substantial therapeutic potential for these patients. Also, encouraging response to TAT in a patient with advanced mCRPC showing progression after 10 cycles 177Lu-PSMA RLT has been reported (28). The patient received two cycles of 225Ac-PSMA-I&T after failure 177Lu-PSMA-617 and showed encouraging response (28). The main TAT-related side effect was grade 2 xerostomia (grade 2), which was already preexisting after 10 cycles of RLT. No TAT-related grade 3/4 hematological side effects were noted (28).
First patient exceeding 5-year complete remission after 225Ac-PSMA-TAT, has been reported in the literature (36). This patient received 3 cycles of mean 8.4-MBq (July/September/November 2014) 225Ac-PSMA-617 at PSA levels of 39.7, 7.7, and 0.32 µg/L, respectively. This patient developed chronic xerostomia, and with some delay, creatinine increased from 1.3 in October 2015 to 3.3 mg/dL in January 2019. This could partially be related to the renal radiation exposure of PSMA therapy, but also with concomitant cardiorenal syndrome, diabetes, and arterial hypertension (36).
PSMA-617 has been the main theragnostic agent which has been under review in TAT in mCRPC, however PSMA-I&T has been investigated in 177Lu-PSMA RLT and did not show any inferiority in the literature when compared to 177Lu-PSMA-617 (37). The first clinical data using 225Ac-PSMA-I&T showed highly comparable biochemical responses after 225Ac-PSMA-617 TAT (19,37).
Zacherl et al. were the first to study a clinical cohort with PSMA-I&T in TAT (29). Fourteen patients who were either not eligible for or had failed conventional therapy were included in the study with 79% of these patients having received prior 177Lu-PSMA RLT, 18F-PSMA-1007 PET/CT was used to assess suitability for therapy (29). This group demonstrated a PSA decline ≥50% of 45% and any PSA decline of 73% in the subgroup of patients who had received prior 177Lu-PSMA RLT which is comparable with other groups which have investigated 225Ac-PSMA therapy in patients who have failed 177Lu-PSMA RLT (29). In this retrospective analysis of using 225Ac-PSMA-I&T fourteen patients were studied: Eleven had prior second-line antiandrogen treatment with abiraterone or enzalutamide, prior chemotherapy, and prior 177Lu-PSMA therapy (29). Patients were treated with two monthly intervals until they progressed or got intolerable side effects. Thirty-four cycles of 225Ac-PSMA-I&T were applied (median dose, 7.8 MBq; range, 6.0–8.5 MBq), with one cycle in two patients, two cycles in seven patients, four cycles in three patients, and five cycles in one patient. No acute toxicity was observed during hospitalization. Baseline PSA was 112 µg/L (range, 20.5–818 µg/L). The best PSA response after TAT (a PSA decline ≥50%) was observed in seven patients, and a PSA decline of any amount was observed in 11 patients (37). Three patients had no PSA decline at any time. A subgroup analysis of eleven patients with prior 177Lu-PSMA treatment showed any PSA decline in eight patients and a decline of at least 50% in five patients. After TAT, grade 3 anemia was observed in three of the 14 patients, with two of them presenting with grade 2 anemia already at baseline. Grade 3 leukopenia was observed in one patient. Eight patients with preexisting xerostomia after 177Lu-PSMA showed no worsening after TAT. Newly diagnosed grade 1 or 2 xerostomia after TAT was observed in five patients. One patient reported no xerostomia at all (37).
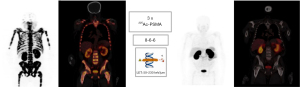
The imaging data of one of our own patients is shown in Figure 3. This prostatectomized, 67-year-old patient had been treated with ADT before receiving three cycles of 225Ac-PSMA (38). The widespread skeletal disease has fully disappeared visually. No hematologic toxicity was observed during the follow-up period.
Discussion
Radiochemical decay
Radiochemistry during 225Ac-decay may play a pivotal role in PRLT compound design. As shown in the decay scheme (Figure 2), there will be multiple intermediate radionuclides during the process. The daughter radionuclides 221Fr, 217At, 213Bi, 213Po, 209Tl, 209Pb and 209Bi differ from each other remarkably: for example, francium is an alkali metal with an oxidation state +1, astatine is a halogen with a most common oxidation state-1, bismuth is a post-transition metal with a most common oxidation state +3 and lead is a heavy metal with two common oxidation states +2 and +4. Due to their different chemical properties and the high recoil energy associated with alpha particle emission, it is expected that the daughter nuclides will not be retained within the DOTA or DOTAGA chelate. Therefore, in order to minimize toxicity to other organs from errant daughter nuclides, a high degree of internalization is pivotal for successful design of radiopharmaceuticals labelled with 225Ac.
213Bi-PSMA is inferior to 225Ac-PSMA because of its short half-life, which does not match well with the pharmacokinetics of PSMA-617. Tumor uptake is relatively too slow, while kidney toxicity is high during excretion of unbound ligand. This is mirrored in the dosimetry (12).
Treatment sequencing
As displayed by multiple 177Lu-PSMA clinical trials, there is a need to optimize the clinical trials. 225Ac-PSMA is mainly administered post-chemotherapy and with good clinical results. To this effect there are some ongoing clinical trials to determine the dose and the dose interval. There are also promising results that have been demonstrated in the de novo HSPC (21), Post-ADT (22) and chemonaive (20) studies have demonstrated excellent results. Randomized control trials are needed to determine the rightful place of each agent in the treatment sequence of mCRPC.
Radioresistance
Radioresistance as a result of mutations in the genes responsible for DNA repair has been thought to be the reason that some patients did not demonstrate a response to 225Ac-PSMA TAT despite demonstrating tumor PSMA expression as evidenced by intense tumor uptake of tracer on PSMA PET/CT imaging (39,40). A combination of 225Ac-PSMA TAT and poly (ADP-ribose)-polymerase (PARP) inhibitors, a DNA damage-repair-targeting molecule has been suggested for these patients to overcome the radioresistance (39,40).
Kratochwil et al. identified 10 patients out of 60 who presented with a poor response to 225Ac-PSMA-617, despite sufficient tumor uptake in PSMA PET/CT. From the seven nonresponding patients 7 CT-guided biopsies with histologic validation were taken.
Specimens were analyzed by next generation sequencing (NGS) interrogating 37 DNA damage-repair–associated genes.
A total of 15 whole-gene deletions, deleterious or presumably deleterious mutations affecting TP53 (n=3), CHEK2 (n=2), ATM (n= 2), and BRCA1, BRCA2, PALB2, MSH2, MSH6, NBN, FANCB, and PMS1 (n=1 each) were found from the tumor samples. The average number of deleterious or presumably deleterious mutations was 2.2 (range, 0–6) per patient. In addition, several variants of unknown significance in ATM, BRCA1, MSH2, SLX4, ERCC, and various FANC genes were detected (40).
Patients with resistance to PSMA-TAT despite PSMA positivity frequently harbor mutations in DNA damage-repair and checkpoint genes findings encourage future combinatory treatments PSMA-TAT and PARP inhibitors, because these target DNA damage-repair.
However, in another study with 177Lu-PSMA and/or 225Ac-PSMA seventeen patients out of 40 patients had a tumor with a pathogenic DNA damage repair aberration (41). These patients had an equal response to PSMA therapy as compared to those without pathological DNA damage repair anomalies, such as PFS (5.9 vs. 6.4 months), ≥50% PSA response (59% vs. 65%,) or OS (11.1 vs. 10.7 months) (41).
225Ac-PSMA TAT toxicity profile
Salivary glands
In the clinical setting, several studies reported toxicity related to TAT with 225Ac-PSMA-617/PSMA-I&T (Table 1). Figure 4 represents the toxicity profiles in the largest reported study with 225Ac-PSMA-617 (21). Xerostomia is a common side effect that causes 10–25% of patients to stop TAT with 225Ac-PSMA (16-20,22,37). Xerostomia should, therefore, be prevented.
Modification of the administered activity of 225Ac-PSMA-617 and the number of cycles of TAT may decrease the side effects while still achieving response (21,42). Sialendoscopy with dilatation, saline irrigation, and steroid injection (prednisolone) have been investigated in patients with some but limited success (32). Eleven men with mCRPC underwent sialendoscopy, dilatation, saline irrigation and steroid injection of both submandibular and both parotid glands before or after every cycle of 225Ac-PSMA-617 TAT (43). Sialendoscopy and steroid injection were performed by a senior otolaryngologist. General quality of life and specific xerostomia were evaluated, before and 3 months after the intervention. In all 11 patients, both parotid and both submandibular glands were affected by radiation sialadenitis and sialendoscopy was performed (43). Sialendoscopy with dilatation, saline irrigation and steroid injection had beneficial effects on salivary gland function and quality of life in patients undergoing 225Ac-PSMA-617 RLT. However, even with sialadenoscopic support after multiple cycles of TAT, salivary gland function was reduced and xerostomia was present. Therefore, not only inflammation but also the direct effect of radiation is a putative cause of dry mouth (43). A case report in one patient describes the potential beneficial effects of intraparenchymal injections of botulinum toxin before 225Ac-PSMA-617 TAT (44). External cooling of the salivary gland using ice packs causes vasoconstriction and therefore was expected to reduce PSMA radioligand uptake (42).
However, the relative contributions of salivary gland cooling and the reduced 225Ac-PSMA-617 activity in minimizing xerostomia severity remain unclear. Therefore, effective methods to reduce salivary toxicity are needed.
Kidneys
PSMA has normal physiological uptake in kidneys and it is predominantly excreted via urinary pathway 225Ac-PSMA-617, there is concern about possible radiation toxicity to the kidneys that may cause acute and long-term effects (45,46). There is a report describing the kidney function deterioration in a patient with only one functional kidney after 225Ac-PSMA-617 (22) and another report about chronic kidney disease in two patients with mCRPC after 225Ac-PSMA-617 (47). Until now, retention times of PSMA ligands either in kidneys or in tumor cells have not yet been evaluated systematically (38). If PSMA on the surface of cancer cells is not sufficiently internalized after binding of the ligand, TAT with 225Ac with multiple unstable daughters might be suboptimal and toxic (38). It has also been speculated that the radioactive daughters of 225Ac, but not 225Ac-PSMA-617, can accumulate in the tubular cells and irradiate the kidneys, leading to renal injury (47). In 14 patients receiving 225Ac-PSMA-I&T, only one patient showed grade 1 nephrotoxicity (32).
Hematologic toxicity
The hematologic toxicity in 14 patients receiving 225Ac-PSMA-I&T was mild, single patients demonstrated grade 3 anemia and grade 3 leucopenia. The short path length of alpha particles (47–85 µm) may explain the low hematological toxicity seen in patients treated with 225Ac-PSMA, even if the marrow infiltrated by tumor cells (26). Baseline myelosuppression may be a contributor to increased severity of hematological toxicity (35). Baseline image findings of diffuse widespread marrow involvement have also been found to be predictors for hematological toxicity (35).
Recently a study of 106 patients treated with 225Ac-PSMA has been reported (23). They received, in average four treatment cycles (range, 1–9). Abnormal baseline hematologic parameters were seen in 98 patients (92.5%) (23). One patient had grade 4 thrombocytopenia, grade 3 anemia, leukopenia, or thrombocytopenia were seen in six patients (5.6%). In spite of this, 85 patients (80.2%) achieved PSA response, and the median PFS and OS of the study population were 14.0 months and 15.0, respectively (23).
General safety measures
Though PSMA is highly expressed in prostate cancer cells, physiological expression of PSMA is seen in the lacrimal glands, salivary glands, gastrointestinal tract, and renal tubular cells (48). Binding to these non-malignant-tissue PSMA expressing sites is contributing to the side effects that are seen with 225Ac-PSMA therapy. Probably, therefore also xeropthalmia has been reported after 225Ac-PSMA-TAT (49). Safety measures that may be adopted to reduce the risk of developing nephrotoxicity include baseline screening e.g., with 99mTc-MAG3 scintigraphy for obstructive renal pathology and correction where feasible and co-administration of normal saline with the radioligand. Patients with poor baseline renal function may be at risk of developing severe nephrotoxicity (20). Most patients have demonstrated limited nephrotoxicity following therapy with 225Ac-PSMA. However, it is known that with radionuclide therapy radiation induced renal injury may develop at a delayed stage and thus be missed in the reported cohorts.
Salivary gland toxicity is the most common toxicity from TAT with 225Ac-PSMA. Symptoms from xerostomia may range from mild symptoms to severe symptoms without requiring dietary changes to severe symptoms requiring nasogastric feeding or total parenteral nutrition. In a case series salivary gland toxicity was the dose limiting factor as patients refused any further treatment with 225Ac-PSMA due to intolerable xerostomia (18). Salivary gland toxicity is dose dependent, even irreversible severe xerostomia may develop when high cumulative activity is administered. To improve salivary gland toxicity, Sathekge et al. used a “dynamic de-escalation” where 8 MBq of 225Ac-PSMA was the initial activity. If the patient demonstrated a good response after the first cycle then the subsequent activity was reduced by 2 MBq, this process is then repeated again prior to the third cycle down to 4 MBq. This approach resulted in patients reporting only grade II xerostomia and no need for withdrawal of treatment due to salivary gland toxicity (20). Tandem administration of a reduced 225Ac-PSMA and full dose 177Lu-PSMA has also been used as an alternative approach to reduce the severity of salivary gland toxicity without compromising PSA response (42).
Dosimetric aspects
Dosimetry studies have demonstrated that the salivary glands receive the highest absorbed dose of the non-target organs (17,50). The mechanism of PSMA uptake in the salivary glands has not been fully understood, to date several interventions have been reviewed to improve patient quality of life, however these attempts at preventing salivary gland toxicity have been unsuccessful. These strategies have been discussed earlier and in order to predict the grade of salivary gland toxicity the absorbed radiation dose should be measured.
RBE
A starting point for clinical dosimetry calculations has been the RBE (RBE =5) found in an experimental study in a mouse model using immunohistochemical γH2AX-foci formation as an indicator for the amount of DNA double strand breaks (10). The response to internal radiotherapy between α- and β-emission (225Ac/177Lu) as a biological consequence of different ionization-densities along a particle-track was measured in somatostatin expressing AR42J cells which were incubated with octreotate analogs 225Ac-DOTATOC and 177Lu-DOTATOC up to 48 h. The cell viability was analyzed using the common MTT assay. DNA double strand breaks were quantified by immunofluorescence staining of γH2AX-foci and cell cycle was analyzed by flow cytometry. In vivo uptake of both radiolabeled somatostatin-analogues into subcutaneously growing AR42J tumors and the number of cells displaying γH2AX-foci were measured (10).
225Ac-DOTATOC resulted in ED50 values of 14 kBq/mL after 48 h, whereas 177Lu-DOTATOC displayed ED50 values of 10 MBq/mL. The number of DNA double strand breaks grew with increasing concentration of 225Ac-DOTATOC and similarly with 177Lu-DOTATOC when applying a factor of 700-fold higher activity compared to 225Ac (10).
Dose finding
The clinical dosimetry basis and dose finding study was performed by using the isotopes 225Ac and 177Lu by Kratochwil et al. (17). A dosimetry estimate was calculated on the basis of time–activity curves derived from serially obtained 177Lu-PSMA-617 scans extrapolated to the physical half-life of 225Ac, assuming instant decay of unstable daughter nuclides. Salvage therapies empirically conducted with 50 (n=4), 100 (n=4), 150 (n=2), and 200 kBq/kg (n=4) of 225Ac-PSMA-617 were evaluated retrospectively regarding toxicity and treatment response. Eight of 14 patients received further cycles in either 2- or 4-month intervals with identical or de-escalated activities.
From this study, the following dosimetry estimates were observed for 1 MBq of 225Ac-PSMA-617 assuming a relative biologic effectiveness of 5: 2.3 Sv for salivary glands, 0.7 Sv for kidneys, and 0.05 Sv for red marrow that is composed of 99.4% α-, 0.5% β-, and 0.1% photon radiation, respectively. The absorbed radiation dose estimates in the salivary glands were 17.2 Sv/7.4 MBq (RBE=5) for 225Ac-PSMA-617, 17.1 Gy/3.7 GBq for 131I-“PSMA” (MP-1095) and 10.2 Gy/7.4 GBq for 177Lu-PSMA-617, respectively (16). The absorbed radiation dose estimates in the kidneys were 5.48 Sv/7.4 MBq for 225Ac-PSMA-617, 5.37 Gy/3.7 GBq for 131I-MP-1095 and 5.55 Gy/7.4 GBq for 177Lu-PSMA-617, respectively (13). The absorbed radiation dose estimates in the red marrow were 0.37 Sv/7.4 MBq for 225Ac-PSMA-617, 1.15 Gy/3.7 GBq for 131I--MP-1095 and 0.22 Gy/7.4 GBq for 177Lu-PSMA-617, respectively (16). We see from this data that mean dose for salivary glands is approximately 70% higher with 225Ac-PSMA-617 than with 177Lu-PSMA-617. The red marrow dose is also 70 % higher, whereas there is no major difference in the kidney’s doses.
In clinical application, severe xerostomia became the dose-limiting toxicity if treatment activity exceeded 100 kBq/kg per cycle. At 100 kBq/kg, the duration of PSA decline was less than 4 months, but if therapy was repeated every 2 months patients experienced additive antitumor effects. Treatment activities of 50 kBq/kg were without toxicity but induced insufficient antitumor response in these high-tumor-burden patients. Remarkable antitumor activity by means of objective radiologic response or tumor marker decline was observed in 9 of 11 evaluable patients.
For advanced-stage patients, a treatment activity of 100 kBq/kg of 225Ac-PSMA-617 per cycle repeated every 8 weeks presents a reasonable trade-off between toxicity and biochemical response. This rationale is shown in Figure 5 (17).
Gamma imaging
The clinical dosimetry for 225Ac is cumbersome as discussed earlier. Even though dosimetry in clinical radionuclide therapy practice is mandatory according to EU guidelines, there are no tools available for clinical practice. One attempt has been shown in the literature utilizing gamma emissions from the 225Ac decay chain (440 keV, 25.9%; 218 keV, 11.4%) (44). However, recommended low therapeutic activities (4–8 MBq) limit the clinical applicability of SPECT, although initial attempts for 225Ac imaging exist. Gosewisch et al. (51) reported a mCRPC patient (65 years), whose imaging of the abdomen was performed at 24 h p.i. after therapeutic activity of 8.1 MBq 225Ac-PSMA-I&T on a SPECT/CT camera (γ-energy 440 keV; window 20%).
Final absorbed dose assessment was performed by combining the single 225Ac image with the effective half-life information determined from a previous 177Lu-PSMA-I&T imaging sequence. This resulted in an absorbed dose of 0.18 and 0.17 Sv(RBE =5)/MBq for the left and right kidney, respectively, compared with 0.27 and 0.24 Gy/GBq for the preceding 177Lu cycle (6.2 GBq). A comparison with the pre-therapy 18F-PSMA-I&T PET/CT demonstrates that 225Ac SPECT imaging for this patient was able to locate a small lesion in the right hip. The 225Ac-absorbed dose was determined as 0.26 Sv(RBE =5)/MBq, compared with 0.35 Gy/GBq for 177Lu-PSMA-I&T (51).
Future research could compare alpha therapy and combine it with external beam radiotherapy (52), because the dosimetry is well understood with a low uncertainty. Alpha therapy requires extensive radiobiology modelling (with assumptions) and correlative data from other modalities as shown here. Anyway, multiple studies have been performed and even dose prediction is possible (50).
Conclusions
The future potential of 225Ac-PSMA therapy and TAT of cancer in general is enormous. Prospective clinical trials comparing 225Ac-PSMA therapy to the standard of care are required to define the best place for TAT in the treatment regime of prostate cancer. Novel vectors with pharmacokinetics matching the half-life of 225Ac targeting further cancer entities are highly needed. The clinical experience with TAT to date has shown great success and physicians dedicated to theragnostics are anxiously waiting for new applications.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1842/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1842/coif). K.K. serves as an unpaid editorial board member of Annals of Translational Medicine from July 2023 to June 2025. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol 2017;71:630-42. [Crossref] [PubMed]
- Haberkorn U, Eder M, Kopka K, et al. New Strategies in Prostate Cancer: Prostate-Specific Membrane Antigen (PSMA) Ligands for Diagnosis and Therapy. Clin Cancer Res 2016;22:9-15. [Crossref] [PubMed]
- Kratochwil C, Giesel FL, Eder M, et al. [177Lu]Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur J Nucl Med Mol Imaging 2015;42:987-8.
- Hooijman EL, Chalashkan Y, Ling SW, et al. Development of [225Ac]Ac-PSMA-I&T for Targeted Alpha Therapy According to GMP Guidelines for Treatment of mCRPC. Pharmaceutics 2021;13:715.
- Ruigrok EAM, Tamborino G, de Blois E, et al. In vitro dose effect relationships of actinium-225- and lutetium-177-labeled PSMA-I&T. Eur J Nucl Med Mol Imaging 2022;49:3627-38. [Crossref] [PubMed]
- Nava-Cabrera M, Azorín-Vega E, Oros-Pantoja R, et al. Comparison between (177)Lu-iPSMA and (225)Ac-iPSMA dosimetry at a cellular level in an animal bone metastasis model. Appl Radiat Isot 2021;176:109898. [Crossref] [PubMed]
- Weineisen M, Schottelius M, Simecek J, et al. 68Ga- and 177Lu-Labeled PSMA I&T: Optimization of a PSMA-Targeted Theranostic Concept and First Proof-of-Concept Human Studies. J Nucl Med 2015;56:1169-76. [Crossref] [PubMed]
- Morgenstern A, Apostolidis C, Kratochwil C, et al. An Overview of Targeted Alpha Therapy with (225)Actinium and (213)Bismuth. Curr Radiopharm 2018;11:200-8. [Crossref] [PubMed]
- Sgouros G. Dosimetry, Radiobiology and Synthetic Lethality: Radiopharmaceutical Therapy (RPT) With Alpha-Particle-Emitters. Semin Nucl Med 2020;50:124-32. [Crossref] [PubMed]
- Graf F, Fahrer J, Maus S, et al. DNA double strand breaks as predictor of efficacy of the alpha-particle emitter Ac-225 and the electron emitter Lu-177 for somatostatin receptor targeted radiotherapy. PLoS One 2014;9:e88239. [Crossref] [PubMed]
- Nonnekens J, Chatalic KL, Molkenboer-Kuenen JD, et al. (213)Bi-Labeled Prostate-Specific Membrane Antigen-Targeting Agents Induce DNA Double-Strand Breaks in Prostate Cancer Xenografts. Cancer Biother Radiopharm 2017;32:67-73. [Crossref] [PubMed]
- Kratochwil C, Schmidt K, Afshar-Oromieh A, et al. Targeted alpha therapy of mCRPC: Dosimetry estimate of (213)Bismuth-PSMA-617. Eur J Nucl Med Mol Imaging 2018;45:31-7. [Crossref] [PubMed]
- Sathekge M, Knoesen O, Meckel M, et al. (213)Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 2017;44:1099-100. [Crossref] [PubMed]
- Ucar B. Synthesis and characterization of natural lanthanum labelled DOTA-Peptides for simulating radioactive Ac-225 labeling. Appl Radiat Isot 2019;153:108816. [Crossref] [PubMed]
- Juzeniene A, Stenberg VY, Bruland ØS, et al. Preclinical and Clinical Status of PSMA-Targeted Alpha Therapy for Metastatic Castration-Resistant Prostate Cancer. Cancers (Basel) 2021;13:779. [Crossref] [PubMed]
- Kratochwil C, Bruchertseifer F, Giesel FL, et al. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J Nucl Med 2016;57:1941-4. [Crossref] [PubMed]
- Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with (225)Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J Nucl Med 2017;58:1624-31. [Crossref] [PubMed]
- Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with (225)Ac-PSMA-617: Swimmer-Plot Analysis Suggests Efficacy Regarding Duration of Tumor Control. J Nucl Med 2018;59:795-802. [Crossref] [PubMed]
- Sathekge MM, Bruchertseifer F, Lawal IO, et al. Treatment of brain metastases of castration-resistant prostate cancer with (225)Ac-PSMA-617. Eur J Nucl Med Mol Imaging 2019;46:1756-7. [Crossref] [PubMed]
- Sathekge M, Bruchertseifer F, Knoesen O, et al. (225)Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging 2019;46:129-38. [Crossref] [PubMed]
- Sathekge M, Bruchertseifer F, Vorster M, et al. Predictors of Overall and Disease-Free Survival in Metastatic Castration-Resistant Prostate Cancer Patients Receiving (225)Ac-PSMA-617 Radioligand Therapy. J Nucl Med 2020;61:62-9. [Crossref] [PubMed]
- Sathekge M, Bruchertseifer F, Vorster M, et al. mCRPC Patients Receiving (225)Ac-PSMA-617 Therapy in the Post-Androgen Deprivation Therapy Setting: Response to Treatment and Survival Analysis. J Nucl Med 2022;63:1496-502. [Crossref] [PubMed]
- Lawal IO, Morgenstern A, Vorster M, et al. Hematologic toxicity profile and efficacy of [225Ac]Ac-PSMA-617 α-radioligand therapy of patients with extensive skeletal metastases of castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 2022;49:3581-92.
- Sathekge M, Bruchertseifer F, Vorster M, et al. (225)Ac-PSMA-617 radioligand therapy of de novo metastatic hormone-sensitive prostate carcinoma (mHSPC): preliminary clinical findings. Eur J Nucl Med Mol Imaging 2023;50:2210-8. [Crossref] [PubMed]
- Feuerecker B, Tauber R, Knorr K, et al. Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-resistant Prostate Cancer After Failure of Lutetium-177-PSMA. Eur Urol 2021;79:343-50. [Crossref] [PubMed]
- Yadav MP, Ballal S, Sahoo RK, et al. Efficacy and safety of (225)Ac-PSMA-617 targeted alpha therapy in metastatic castration-resistant Prostate Cancer patients. Theranostics 2020;10:9364-77. [Crossref] [PubMed]
- van der Doelen MJ, Mehra N, van Oort IM, et al. Clinical outcomes and molecular profiling of advanced metastatic castration-resistant prostate cancer patients treated with (225)Ac-PSMA-617 targeted alpha-radiation therapy. Urol Oncol 2021;39:729.e7-729.e16. [Crossref] [PubMed]
- Ilhan H, Gosewisch A, Böning G, et al. Response to 225Ac-PSMA-I&T after failure of long-term 177Lu-PSMA RLT in mCRPC. Eur J Nucl Med Mol Imaging 2021;48:1262-3. [Crossref] [PubMed]
- Zacherl MJ, Gildehaus FJ, Mittlmeier L, et al. First Clinical Results for PSMA-Targeted α-Therapy Using 225Ac-PSMA-I&T in Advanced-mCRPC Patients. J Nucl Med 2021;62:669-74.
- Sen I, Thakral P, Tiwari P, et al. Therapeutic efficacy of (225)Ac-PSMA-617 targeted alpha therapy in patients of metastatic castrate resistant prostate cancer after taxane-based chemotherapy. Ann Nucl Med 2021;35:794-810. [Crossref] [PubMed]
- Satapathy S, Mittal BR, Sood A, et al. Health-Related Quality-of-Life Outcomes with Actinium-225-Prostate-Specific Membrane Antigen-617 Therapy in Patients with Heavily Pretreated Metastatic Castration-Resistant Prostate Cancer. Indian J Nucl Med 2020;35:299-304. [Crossref] [PubMed]
- Khreish F, Ebert N, Ries M, et al. (225)Ac-PSMA-617/(177)Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: pilot experience. Eur J Nucl Med Mol Imaging 2020;47:721-8. [Crossref] [PubMed]
- Rosar F, Krause J, Bartholomä M, et al. Efficacy and Safety of [225Ac]Ac-PSMA-617 Augmented [177Lu]Lu-PSMA-617 Radioligand Therapy in Patients with Highly Advanced mCRPC with Poor Prognosis. Pharmaceutics 2021;13:722.
- Sanli Y, Kuyumcu S, Simsek DH, et al. 225Ac-Prostate-Specific Membrane Antigen Therapy for Castration-Resistant Prostate Cancer: A Single-Center Experience. Clin Nucl Med 2021;46:943-51. [Crossref] [PubMed]
- Puttemans J, Lahoutte T, D'Huyvetter M, et al. Beyond the Barrier: Targeted Radionuclide Therapy in Brain Tumors and Metastases. Pharmaceutics 2019;11:376. [Crossref] [PubMed]
- Rathke H, Bruchertseifer F, Kratochwil C, et al. First patient exceeding 5-year complete remission after (225)Ac-PSMA-TAT. Eur J Nucl Med Mol Imaging 2021;48:311-2.
- Fendler WP, Rahbar K, Herrmann K, et al. (177)Lu-PSMA Radioligand Therapy for Prostate Cancer. J Nucl Med 2017;58:1196-200. [Crossref] [PubMed]
- Sathekge MM, Bruchertseifer F, Vorster M, et al. Global experience with PSMA-based alpha therapy in prostate cancer. Eur J Nucl Med Mol Imaging 2021;49:30-46.
- Filippi L, Palumbo B, Bagni O, et al. DNA Damage Repair Defects and Targeted Radionuclide Therapies for Prostate Cancer: Does Mutation Really Matter? A Systematic Review. Life (Basel) 2022;13:55. [Crossref] [PubMed]
- Kratochwil C, Giesel FL, Heussel CP, et al. Patients Resistant Against PSMA-Targeting α-Radiation Therapy Often Harbor Mutations in DNA Damage-Repair-Associated Genes. J Nucl Med 2020;61:683-8. [Crossref] [PubMed]
- Privé BM, Slootbeek PHJ, Laarhuis BI, et al. Impact of DNA damage repair defects on response to PSMA radioligand therapy in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 2022;25:71-8. [Crossref] [PubMed]
- Langbein T, Chaussé G, Baum RP. Salivary Gland Toxicity of PSMA Radioligand Therapy: Relevance and Preventive Strategies. J Nucl Med 2018;59:1172-3. [Crossref] [PubMed]
- Rathke H, Kratochwil C, Hohenberger R, et al. Initial clinical experience performing sialendoscopy for salivary gland protection in patients undergoing (225)Ac-PSMA-617 RLT. Eur J Nucl Med Mol Imaging 2019;46:139-47. [Crossref] [PubMed]
- Baum RP, Langbein T, Singh A, et al. Injection of Botulinum Toxin for Preventing Salivary Gland Toxicity after PSMA Radioligand Therapy: an Empirical Proof of a Promising Concept. Nucl Med Mol Imaging 2018;52:80-1.
- Chatalic KL, Heskamp S, Konijnenberg M, et al. Towards Personalized Treatment of Prostate Cancer: PSMA I&T, a Promising Prostate-Specific Membrane Antigen-Targeted Theranostic Agent. Theranostics 2016;6:849-61. [Crossref] [PubMed]
- Gallyamov M, Meyrick D, Barley J, et al. Renal outcomes of radioligand therapy: experience of (177)lutetium-prostate-specific membrane antigen ligand therapy in metastatic castrate-resistant prostate cancer. Clin Kidney J 2020;13:1049-55.
- Pelletier K, Côté G, Fallah-Rad N, et al. CKD After 225Ac-PSMA617 Therapy in Patients With Metastatic Prostate Cancer. Kidney Int Rep 2021;6:853-6. [Crossref] [PubMed]
- Kinoshita Y, Kuratsukuri K, Landas S, et al. Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J Surg 2006;30:628-36. [Crossref] [PubMed]
- de Medeiros RB, Grigolon MV, Araújo TP, et al. Metastatic castration-resistant prostate cancer (mCRPC) treated with 225Ac-PSMA-617. Case report. Braz J Oncol 2019; [Crossref]
- Belli ML, Sarnelli A, Mezzenga E, et al. Targeted Alpha Therapy in mCRPC (Metastatic Castration-Resistant Prostate Cancer) Patients: Predictive Dosimetry and Toxicity Modeling of (225)Ac-PSMA (Prostate-Specific Membrane Antigen). Front Oncol 2020;10:531660. [Crossref] [PubMed]
- Gosewisch A, Schleske M, Gildehaus FJ, et al. Image-based dosimetry for 225Ac-PSMA-I&T therapy using quantitative SPECT. Eur J Nucl Med Mol Imaging 2021;48:1260-1. [Crossref] [PubMed]
- Sarnelli A, Belli ML, Azzali I, et al. Alpha-Emitter Radiopharmaceuticals and External Beam Radiotherapy: A Radiobiological Model for the Combined Treatment. Cancers (Basel) 2022;14:1077. [Crossref] [PubMed]






