Is it time to break the fast?—a paradigm shift in clinical lipidology
Introduction
Cardiovascular risk assessment traditionally includes a fasting blood sample for determination of the lipid panel, including total plasma (or serum) cholesterol, triglycerides (TG), HDL-C and calculated (or directly measured) LDL-C levels. Some laboratories also include plasma apolipoprotein (apo) B and apo A-I. It has been known for centuries that ingestion of a fatty meal will increase the concentrations of circulating TG-rich lipoproteins and more so, in conditions such as obesity, type 2 diabetes mellitus and familial disorders of TG metabolism (1). This led to the recommendation to analyze lipid levels only after an overnight fast, in order to measure lipids in a steady state situation. It is a longstanding misunderstanding that not only TGs but also cholesterol levels are affected postprandially. Many investigators have repeatedly shown that even after an oral fat loading test, plasma cholesterol levels do not change, and this also accounts for HDL-C levels which are only minimally affected by fat ingestion (2,3). Moreover, accumulating evidence strongly supports the use of non-fasting lipids for the evaluation of cardiovascular risk (4,5). Despite this overwhelming quantity of data, some laboratories for clinical chemistry still send people away when asked to measure plasma lipids if the subjects are not fasting. The European Atherosclerosis Society and the European Federation of Clinical Chemistry and Laboratory Medicine have released a joint consensus statement proposing to use non-fasting lipid samples to determine lipid profiles (6). This report will lead to a paradigm shift in clinical lipidology and it will simplify cardiovascular risk assessment in clinical practice.
Methods to study TG metabolism
Lipid metabolism is dynamic with a high turnover rate and with many different enzymes, receptors and proteins involved. This complex system comprises both cholesterol and TG metabolism, which have distinctive regulators. Due to the dynamic changes of this system influenced by so many environmental and genetic factors (7), studies on TG metabolism have been carried out in the setting of strictly controlled metabolic ward conditions, but less sophisticated methods have also been introduced using daytime capillary TG profiles optionally in combination with one single venous sample (8).
Hypertriglyceridemia, remnant cholesterol and postprandial hyperlipemia
The postprandial state is characterized by accumulation of intestinally derived chylomicrons and very low-density lipoproteins (VLDL) from the liver. Each chylomicron carries a single apo B-48 molecule as the structural protein and is lipolyzed in the systemic circulation resulting in the generation of free fatty acids, which are readily taken up by cells or bound to albumin. In this process, the ubiquitous, endothelial-bound lipoprotein lipase (LPL) and apo CII on the lipoproteins as co-factor are essential. Subsequently, the smaller chylomicron remnants, which are enriched with cholesterol by enzymes like cholesterol-ester transfer protein (CETP), are taken up by the liver where they are degraded. In parallel to this process, the liver synthesizes and secretes VLDL particles. The assembly of VLDL is almost identical to chylomicrons except that apo B-100 is the structural protein of VLDL. The human liver lacks the post-translational editing complex necessary to change the apo B-100 mRNA into the smaller apo B-48 (9). Chylomicrons and VLDL compete for the same lipolytic pathway, ultimately resulting in the formation of remnants, with LDL-C being the end product in the case of VLDL (10).
The remnant cholesterol has been causally linked to cardiovascular disease (11). Therefore, it is not surprising that patients with coronary artery disease (CAD) frequently show postprandial hyperlipidemia with prolonged postprandial TG peaks (Figure 1A) (12-14). TGs and remnant cholesterol are directly related to each other showing a correlation coefficient of 0.99. Therefore, hypertriglyceridemia reflects impaired metabolism and accumulation of triglyceride-rich lipoproteins and their remnants as a result of impaired postprandial lipemia. Monogenic causes for hypertriglyceridemia such as LPL deficiency and apo CII deficiency are well known causes of severe hypertriglyceridemia, but the vast majority of the hypertriglyceridemia is polygenic with the co-existence of environmental stressors and co-morbidity, such as poor diet, obesity, insulin resistance and diabetes mellitus (Figure 1B) (15).
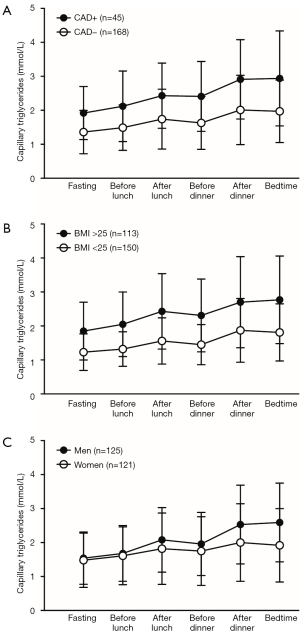
In type 2 diabetes mellitus, the classic diabetic dyslipidemia characterized by elevated fasting TG levels and lower HDL-C (16) also includes postprandial hyperlipidemia (17,18). Patients with Familial Combined Hyperlipidemia (FCH) show changing lipoprotein phenotypes with sometimes increased LDL-C and/or TGs. Several studies, with both self-measurements of capillary TGs and fat loading tests, have shown that patients with FCH have persistent postprandial hypertriglyceridemia compared with normolipidemic subjects (19,20). In fact, this is not surprising due to the fact that these subjects usually have fasting hypertriglyceridemia. However, FCH subjects with relatively lower fasting TGs may also show postprandial hyperlipidemia due to the overproduction of VLDL, which is the main metabolic defect in this disorder. These changing and variable phenotypes are due to numerous small-effect genetic variants in combination with environmental stressors. Therefore, it has recently been suggested that delineation of FCH and familial hypertriglyceridemia is neither feasible nor clinically relevant (15). Some evidence also points at disturbed postprandial hyperlipidemia in the case of Familial Hypercholesterolemia (FH), which is a disorder characterized by elevated LDL-C levels due to a reduced LDL receptor activity (19,21).
TGs: daytime rise and fall and its effect on LDL-C
Previous guidelines advised to determine the lipid profile in the fasting state due to a postprandial increase in TGs and concomitant decrease in LDL-C, which is usually obtained using the Friedewald equation [LDL-C = TC − HDL-C − (TG/2.2)]. In a real life setting, circulating TG rise approximately 0.5 mmol/L in women and 1.0 mmol/L in men with the greatest increase at the end of the day due to dinner (Figure 1C). The absolute increase in TG seems similar between subjects with different levels of triglyceridemia and fasting TG are therefore the best predictor for non-fasting TG. It was also believed that TG variability increases in the non-fasting state, but the intra-individual variability of TG is not affected much throughout the day (22).
Large epidemiological studies have shown that LDL-C levels are 0.2–0.6 mmol/L lower when measured two hours postprandially in comparison to fasting measurements (3,4). One cross-sectional study with 209.180 subjects demonstrated that fasting had only a minor effect on LDL-C (2). In this study, the variation of LDL-C between fasting and non-fasting samples was 10%. LDL-C is approximately 0.2 mmol/L lower one to six hours after food intake when compared to fasting samples. This will probably reflect the routine measurements since it is supposed that most lipid profiles are measured during office hours (3).
However, these data regarding non-fasting LDL-C are based on epidemiological and cross-sectional studies comparing different subjects with differing fasting times. There are no direct comparisons within subjects between fasting and non-fasting lipid profiles as guidance for lipid lowering therapy (23). It is unknown to which extent a non-fasting LDL-C is affected to such extent that it will result in clinically relevant differences. In theory, a non-fasting LDL-C can result in a false measurement or reaching LDL-C treatment targets. Current European guidelines, suggest that LDL-C above 1.8 mmol/L in secondary prevention should be followed by intensification of lipid lowering therapy (24,25). Therefore, falsely lower non-fasting LDL-C levels could potentially result in insufficient lipid lowering treatment. This problem is addressed in the consensus statement by Nordestgaard et al. and judged as probably non-significant in clinical practice (6). We have previously recommended calculating LDL-C in the fasting state to determine lipid lowering intensity in order to prevent undertreatment (7).
As suggested in the consensus statement, non-HDL-C and apo B can be considered as alternative parameters for LDL-C to guide lipid lowering therapy and CVD risk assessment, since they take all atherogenic lipoproteins into account. Prospective studies and meta-analyses have consistently demonstrated that non-HDL-C and apo B are least as good as or superior to LDL-C in the prediction of CVD during lipid-lowering therapy (7). Another advantage of non-HDL-C and apo B over LDL-C is the fact that apo B measurements are not influenced by the prandial state and non-HDL-C only slightly (6).
The new guideline advises to measure a fasting lipid profile under certain circumstances. For example, when non-fasting TG are above 5 mmol/L or in patients under treatment for hypertriglyceridemia. However, the daytime increase in TG is relatively similar for subjects with normal TG and those with hypertriglyceridemia (12). Therefore, subjects with non-fasting TG above 5 mmol/L will always have elevated fasting TG, which makes additional sampling in the fasting state unnecessary.
The authors propose to consider non-fasting TGs above 2 mmol/L (175 mg/dL) to be inadequate, whereas fasting TGs should be lower than 1.7 mmol/L (150 mg/dL). These are very concrete targets, which are easily applicable in clinical practice. We now await the next consensus statement indicating the intervention strategies to reduce TG levels. Most clinicians will hold on to lifestyle recommendations and in more severe cases the use of fibrates or nicotinic acid. The clinical relevance of these recommendations in relation to cardiovascular disease is still not clear since recent trials using these drugs have not been conclusive.
Acknowledgements
None.
Footnote
Provenance: This is a Guest Editorial commissioned by Executive Editor Zhi-De Hu (Department of Laboratory Medicine, General Hospital of Ji’nan Military Region, Ji’nan, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Erkelens DW, de Bruin TW, Castro Cabezas M. Tulp syndrome. Lancet 1993;342:1536-7. [Crossref] [PubMed]
- Sidhu D, Naugler C. Fasting time and lipid levels in a community-based population: a cross-sectional study. Arch Intern Med 2012;172:1707-10. [Crossref] [PubMed]
- Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation 2008;118:2047-56. [Crossref] [PubMed]
- Bansal S, Buring JE, Rifai N, et al. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007;298:309-16. [Crossref] [PubMed]
- Mora S, Rifai N, Buring JE, et al. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation 2008;118:993-1001. [Crossref] [PubMed]
- Nordestgaard BG, Langsted A, Mora S, et al. Fasting Is Not Routinely Required for Determination of a Lipid Profile: Clinical and Laboratory Implications Including Flagging at Desirable Concentration Cutpoints-A Joint Consensus Statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem 2016;62:930-46. [Crossref] [PubMed]
- de Vries M, Klop B, Castro Cabezas M. The use of the non-fasting lipid profile for lipid-lowering therapy in clinical practice - point of view. Atherosclerosis 2014;234:473-5. [Crossref] [PubMed]
- Su JW, Nzekwu MM, Cabezas MC, et al. Methods to assess impaired post-prandial metabolism and the impact for early detection of cardiovascular disease risk. Eur J Clin Invest 2009;39:741-54. [Crossref] [PubMed]
- Innerarity TL, Young SG, Poksay KS, et al. Structural relationship of human apolipoprotein B48 to apolipoprotein B100. J Clin Invest 1987;80:1794-8. [Crossref] [PubMed]
- Klop B, Wouter Jukema J, Rabelink TJ, et al. A physician's guide for the management of hypertriglyceridemia: the etiology of hypertriglyceridemia determines treatment strategy. Panminerva Med 2012;54:91-103. [PubMed]
- Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet 2014;384:626-35. [Crossref] [PubMed]
- Klop B, Cohn JS, van Oostrom AJ, et al. Daytime triglyceride variability in men and women with different levels of triglyceridemia. Clin Chim Acta 2011;412:2183-9. [Crossref] [PubMed]
- Przybycień K, Kornacewicz-Jach Z, Torbus-Lisiecka B, et al. Is abnormal postprandial lipemia a familial risk factor for coronary artery disease in individuals with normal fasting concentrations of triglycerides and cholesterol? Coron Artery Dis 2000;11:377-81. [Crossref] [PubMed]
- van Wijk JP, Halkes CJ, De Jaegere PP, et al. Normalization of daytime triglyceridemia by simvastatin in fasting normotriglyceridemic patients with premature coronary sclerosis. Atherosclerosis 2003;171:109-16. [Crossref] [PubMed]
- Staniak HL, Salgado Filho W, Miname MH, et al. Association between postprandial triglycerides and coronary artery disease detected by coronary computed tomography angiography. Atherosclerosis 2014;233:381-6. [Crossref] [PubMed]
- Lewis GF, Xiao C, Hegele RA. Hypertriglyceridemia in the genomic era: a new paradigm. Endocr Rev 2015;36:131-47. [Crossref] [PubMed]
- Schofield JD, Liu Y, Rao-Balakrishna P, et al. Diabetes Dyslipidemia. Diabetes Ther 2016;7:203-19. [Crossref] [PubMed]
- van Wijk JP, de Koning EJ, Castro Cabezas M, et al. Rosiglitazone improves postprandial triglyceride and free fatty acid metabolism in type 2 diabetes. Diabetes Care 2005;28:844-9. [Crossref] [PubMed]
- van Wijk JP, Halkes CJ, Erkelens DW, et al. Fasting and daylong triglycerides in obesity with and without type 2 diabetes. Metabolism 2003;52:1043-9. [Crossref] [PubMed]
- Pavlidis AN, Kolovou GD, Anagnostopoulou KK, et al. Postprandial metabolic heterogeneity in men with primary dyslipidaemia. Arch Med Sci 2010;6:879-86. [Crossref] [PubMed]
- Delawi D, Meijssen S, Castro Cabezas M. Intra-individual variations of fasting plasma lipids, apolipoproteins and postprandial lipemia in familial combined hyperlipidemia compared to controls. Clin Chim Acta 2003;328:139-45. [Crossref] [PubMed]
- Kolovou GD, Anagnostopoulou KK, Pilatis ND, et al. Heterozygote men with familial hypercholesterolaemia may have an abnormal triglyceride response post-prandially. Evidence for another predictor of vascular risk in familial hypercholesterolaemia. Int J Clin Pract 2005;59:311-7. [Crossref] [PubMed]
- Khera AV, Mora S. Fasting for lipid testing: Is it worth the trouble? Arch Intern Med 2012;172:1710-2. [Crossref]
- Catapano AL, Reiner Z, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias. Atherosclerosis 2011;217:3-46. [Crossref] [PubMed]
- Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol 2016;23:NP1-96. [Crossref] [PubMed]


