Drugs targeting dynamin can restore cytoskeleton and focal contact alterations of urinary podocytes derived from patients with nephrotic syndrome
Introduction
The actin cytoskeleton is responsible for the maintenance of shape and function of podocytes.
Dynamic and constitutively active assembly and disassembly of actin fibers of the podocytic actin cytoskeleton are indispensable for normal kidney filter function. Actin and actin-related gene mutations like alpha actinin 4 (1), myosin IE (MYO1E), alpha 3 integrin (ITGA3), Rho GTPase activating protein 24 (ARHGAP24), anillin (ANLN) and CD2-associated protein (CD2AP) (2-4) can cause dysregulations in podocyte actin cytoskeleton leading to nephrotic syndrome, loss of podocytes and focal segmental glomerulosclerosis (FSGS) lesions.
Loss of podocytes into the urine (podocyturia) has been found in different glomerular diseases. Some of the urinary podocytes are fully viable, can be cultured, and continue to synthesize podocyte-specific proteins in vitro (5-8). We previously published that excretion of podocalyxin positive glomerular epithelial cells in the urine correlates with disease activity in different glomerular diseases (7,9).
Podocytes are highly differentiated post-mitotic cells without regenerative capacities. Therefore, once lost in the urine, they cannot be replaced.
However, damaged podocytes that are still attached display a remarkable capacity to recover their actin cytoskeleton and reform foot processes in vivo and in vitro. Podocyte foot processes are rich in microtubules and cortical actin that support their structural stability and podocytes are known to respond to a variety of vasoactive substances with changes of their cytoskeleton (10,11). Therefore, the podocyte actin cytoskeleton has been suggested as a new target for therapy of glomerular diseases. We recently described that Bis-T-23, which increases actin polymerization in injured podocytes, was able to reverse glomerular damage in diverse vertebrate models of kidney diseases (12). However, so far Bis-T-23 could not be used as a drug in humans and it is unclear if targeting dynamin would be successful in human disease. Here we report how the ex vivo treatment of podocytes that detached from the glomerular basement membrane in active human glomerular diseases respond to a treatment with drugs acting dynamin oligomerisation with cytoskeleton and focal adhesion recovery. In the future this form of personalized recovery assay might serve as a diagnostic tool to assess potential therapy option for podocyte foot process recovery.
Results
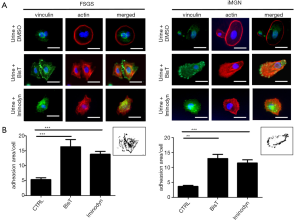
The urine sediments of two proteinuric patients, one with FSGS and one patient with idiopathic membranous glomerulonephropathy (iMGN) (see Table S1 for additional clinical information) were cultured overnight as described previously (7). Proteinuria at that time was 8.63 g/d for the patient with iMGN and 2.56 g/d for the patient with FSGS, retrospectively. We could detect podocalyxin positive (PDX+) cells in both sediments that were able to attach to collagen slides. On average, the FSGS patient excreted 88 PDX+ cells per mL urine and the iMGN patient excreted 40 PDX+ cells per mL urine. Staining of the urinary cells for phalloidin to detect actin filaments revealed that the pattern of actin expression was rearranged in both patients. Cells expressed actin in in ring structures that were located at the edge of the cells. Vinculin staining of the cells was used to detect and determine number and size of focal contacts (Figure 1A). We treated the urinary cells with Bis-T 23 (1 µL/mL) or Iminodyn (1 µL/mL) dissolved in DMSO or with DMSO alone for 1 h. We found that both, Bis-T-23 as well as Iminodyn were able to rearrange the actin cytoskeleton and increased vinculin expression in urinary cells from both patients (Figure 1).

Full table
Discussion
In many primary or secondary glomerular diseases podocytes detach from their basement membrane, are shed into the urine and leave FSGS lesions (13). The number of podocytes in the urine correlates with disease activity and even precedes proteinuria (14). Moreover, podocyturia is predominantly seen in active disease whereas proteinuria is present in active and chronic stages of glomerular diseases (7,15). Despite detachment during the disease process, urinary podocytes retain their ability to attach to tissue culture plates in vitro. Their ability to rearrange their cytoskeleton further indicates their viability (7-9). Podocyte loss is associated with dysregulation in the actin-driven membrane extensions (16) and dysregulation of the actin cytoskeleton upon podocyte injury is a highly dynamic process (17).
The large GTPase dynamin regulates actin cytoskeleton formation (18). Cleavage of dynamin by a cytoplasmic form of cathepsin L leads to reorganization of the podocyte actin cytoskeleton, podocyte failure and proteinuria. Dynamin mutants resistant to cathepsin L cleavage can restore podocyte function and resolve proteinuria (19,20). The double-knockout of dynamin 1 and dynamin 2 in mouse podocytes leads to podocyte effacement (21).
Bis-T-23 and Iminodyn prolong dynamin ring lifetime and facilitate the formation of filamentous actin (22). With these small molecules actin-dependent dynamin oligomerisation of the cytoskeleton can be targeted.
We previously demonstrated that Bis-T-23 restored the normal ultrastructure of podocyte foot processes and lowered proteinuria in different renal disease models (12). Treating cultured mouse podocytes with Bis-T-23 promotes stress fiber formation and focal adhesion maturation in a dynamin-dependent manner (20,23).
Here we report the response of excreted vital podocytes from two patients with different forms of active glomerular disease to two small molecules targeting dynamin. These lost podocytes showed rearrangements of their cytoskeleton and redistribution of vinculin and actin fibers. Normally, vinculin is expressed in focal adhesions of podocytes where it transmits outside-in and inside-out signalling to modulate actin polymerization, cell morphology and motility (24).
We found that Bis-T-23 and Iminodyn were both able to induce actin and vinculin rearrangement and recovery in these injured cells. This is the first experimental evidence that ameliorating alterations in actin cytoskeleton might be beneficial in humans with nephrotic diseases. Together with our previous publications in different vertebrate models these findings underline the potential for dynamin targeting drugs as a novel treatment concept for human glomerular diseases. Moreover, podocyturia may serve as a noninvasive marker of active glomerular damage and might drive therapeutic treatment decisions. The treatment response of lost podocytes ex vivo with substances that restore their cytoskeleton might serve as a tool to estimate a potential recovery rate in the course of the diseases process in response to therapy.
Methods
Spot urine samples of patients were centrifuged at 1,200 rpm for 8 min, the supernatant was removed and the pellet was suspended in a sterile HDF solution (137 mM NaCl, 5 mM KCl, 5.5 mM glucose, 4 mM NaHCO and 0.2% EDTA). After additional centrifugation at 1,200 rpm for 8 min, the pellet was re-suspended in DMEM/F-12 medium containing 10% FCS, 0.5 U/L penicillin and 0.5 mg/dL streptomycin. The re-suspended pellet was seeded equally in a 24-well dish containing collagen I coated cover slides. Samples were incubated at 37 °C with 5% CO overnight. The following day, medium of the slides was added with 1 µL/mL BisT, 1 µL/mL Iminodyn or 0.1% DMSO for 1 h.
Slides were then fixed at −20 °C for 10 min using ice-cold methanol and permeableized using 0.1% Triton. After blocking with 10% donkey serum, immunofluorescent staining was done with primary antibodies overnight at 4 °C followed by incubation in secondary antibody for 1 h at room temperature. Finally, slides were mounted on glass slides using Vecta Shield with DAPI (Vector laboratories, Burlingame, CA, USA). Primary antibodies used were vinculin mouse anti-vinculin (V9131, sigma Aldrich) and rabbit anti-phalloidin, (Alexa Fluor 546, Invitrogen). We quantified vinculin positive adhesions per cell using Image J software.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kaplan JM, Kim SH, North KN, et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 2000;24:251-6. [Crossref] [PubMed]
- Brown EJ, Schlöndorff JS, Becker DJ, et al. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet 2010;42:72-6. [Crossref] [PubMed]
- Mele C, Iatropoulos P, Donadelli R, et al. MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N Engl J Med 2011;365:295-306. [Crossref] [PubMed]
- Kim JM, Wu H, Green G, et al. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science 2003;300:1298-300. [Crossref] [PubMed]
- Hara M, Yanagihara T, Kihara I. Urinary podocytes in primary focal segmental glomerulosclerosis. Nephron 2001;89:342-7. [Crossref] [PubMed]
- Vogelmann SU, Nelson WJ, Myers BD, et al. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol 2003;285:F40-8. [Crossref] [PubMed]
- Achenbach J, Mengel M, Tossidou I, et al. Parietal epithelia cells in the urine as a marker of disease activity in glomerular diseases. Nephrol Dial Transplant 2008;23:3138-45. [Crossref] [PubMed]
- Mueller-Deile J, Kümpers P, Achenbach J, et al. Podocalyxin-positive glomerular epithelial cells in urine correlate with a positive outcome in FSGS. J Nephrol 2012;25:802-9. [Crossref] [PubMed]
- Garovic VD, Wagner SJ, Turner ST, et al. Urinary podocyte excretion as a marker for preeclampsia. Am J Obstet Gynecol 2007;196:320.e1-7. [Crossref] [PubMed]
- Mundel P, Heid HW, Mundel TM, et al. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol 1997;139:193-204. [Crossref] [PubMed]
- Shirato I, Sakai T, Kimura K, et al. Cytoskeletal changes in podocytes associated with foot process effacement in Masugi nephritis. Am J Pathol 1996;148:1283-96. [PubMed]
- Schiffer M, Teng B, Gu C, et al. Pharmacological targeting of actin-dependent dynamin oligomerization ameliorates chronic kidney disease in diverse animal models. Nat Med 2015;21:601-9. [Crossref] [PubMed]
- Sato Y, Wharram BL, Lee SK, et al. Urine podocyte mRNAs mark progression of renal disease. J Am Soc Nephrol 2009;20:1041-52. [Crossref] [PubMed]
- Yu D, Petermann A, Kunter U, et al. Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol 2005;16:1733-41. [Crossref] [PubMed]
- Nakamura T, Ushiyama C, Suzuki S, et al. Urinary podocytes for the assessment of disease activity in lupus nephritis. Am J Med Sci 2000;320:112-6. [Crossref] [PubMed]
- Reiser J, Sever S. Podocyte biology and pathogenesis of kidney disease. Annu Rev Med 2013;64:357-66. [Crossref] [PubMed]
- Seiler MW, Rennke HG, Venkatachalam MA, et al. Pathogenesis of polycation-induced alterations ("fusion") of glomerular epithelium. Lab Invest 1977;36:48-61. [PubMed]
- Sever S, Chang J, Gu C. Dynamin rings: not just for fission. Traffic 2013;14:1194-9. [Crossref] [PubMed]
- Sever S, Altintas MM, Nankoe SR, et al. Proteolytic processing of dynamin by cytoplasmic cathepsin L is a mechanism for proteinuric kidney disease. J Clin Invest 2007;117:2095-104. [Crossref] [PubMed]
- Gu C, Chang J, Shchedrina VA, et al. Regulation of dynamin oligomerization in cells: the role of dynamin-actin interactions and its GTPase activity. Traffic 2014;15:819-38. [Crossref] [PubMed]
- Soda K, Balkin DM, Ferguson SM, et al. Role of dynamin, synaptojanin, and endophilin in podocyte foot processes. J Clin Invest 2012;122:4401-11. [Crossref] [PubMed]
- Hill T, Odell LR, Edwards JK, et al. Small molecule inhibitors of dynamin I GTPase activity: development of dimeric tyrphostins. J Med Chem 2005;48:7781-8. [Crossref] [PubMed]
- Gu C, Lee HW, Garborcauskas G, et al. Dynamin Autonomously Regulates Podocyte Focal Adhesion Maturation. J Am Soc Nephrol 2016. pii: ASN.2016010008.
- Blattner SM, Kretzler M. Integrin-linked kinase in renal disease: connecting cell-matrix interaction to the cytoskeleton. Curr Opin Nephrol Hypertens 2005;14:404-10. [Crossref] [PubMed]


