Isolated idiopathic aortitis with an unusually thickened aortic wall: case report
Introduction
Aortitis includes a broad range of disorders involving inflammation of the aorta. While most forms of aortitis can be linked to a specific cause, patients with idiopathic aortitis (IDA), are asymptomatic and usually diagnosed after surgical removal. The specific pathophysiology is not well understood, but can be strongly associated with tobacco smoking, young age at presentation, and family history of aortic aneurysm (1).
Wall thickening is the most common physical characteristic of aortitis, and the inflammation can affect any layer of the aorta. The normal wall thickness of the aorta is less than 4 mm and can be as thick as 9 mm (2). Few studies document a correlation between wall thickness and the severity of aortitis. This paper presents a unique case of severe aortic aneurysm associated with an abnormal thickening of the ascending aorta.
Case presentation
A previously healthy 53-year-old African-American man was admitted to the hospital with progressive cough and unintentional weight loss over the past 6 months. The patient had a history of tobacco and cocaine use 15 years ago. His father and brother both died of sudden cardiac death. before the age of 50 years. His daughter had a cerebral and abdominal aneurysm. His physical examination was within normal limits.
His initial laboratory workup was only abnormal for an increased platelet count of 563,000 per cm, an elevated sedimentation rate of 83 mm/h, and an increased C-reactive protein of 131 mg/L. Cardiac enzymes were also within normal limits. An electrocardiogram was normal. A chest X-ray showed a widening of the mediastinum, and the heart was normal in size.
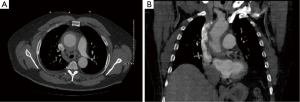
An initial non-contrast computed tomography (CT) scan of the chest performed in the emergency department revealed a dilation of the ascending aorta measuring 6.9 cm × 6.5 cm in diameter. A CT angiogram showed a thickened aortic wall (Figure 1). The luminal diameter was 4.9 cm × 4.9 cm, and the descending thoracic aorta was unremarkable with no evidence of an aortic dissection. Magnetic resonance angiography (MRA) of the chest showed a rind of abnormal signal intensity in the ascending thoracic aorta and proximal aortic arch.
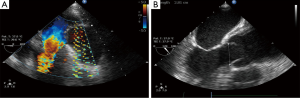
A transthoracic echocardiogram showed a left ventricular ejection fraction of 65%; mild to moderate aortic valve regurgitation; and severe aortic root dilation with severe thickening of the aortic wall. Color Doppler imaging showed no flow within the thickened aortic wall. Transesophageal echocardiography showed moderate aortic valvular regurgitation and dilation of the aortic root (Figure 2), incomplete closure of the aortic leaflets (Figure 3), and an aortic aneurysm (Figure 4). Coronary angiography showed no coronary artery disease.

A rheumatologic work-up for aortitis including p- and c-antineutrophil cytoplasmic antibodies, Lyme antibody screen, total protein electrophoresis, and immunoglobulin G subclass analysis was negative. A work-up for infectious causes of aortitis was also negative.
The patient remained stable with no increase in symptoms throughout his hospitalization. He was discharged from the hospital on labetalol and lisinopril and scheduled to have aortic surgery 6 weeks after hospitalization, he was rehospitalized before his scheduled surgery for vague chest pain. A repeat CT angiogram showed no change from the prior one. Another full laboratory work-up showed no changes. He underwent an ascending hemiarch aortic replacement with a 32-mm Hemashield graft, modified Bentall root replacement, and 27-mm aortic valve replacement with deep hypothermic circulatory arrest. He had an unremarkable postoperative course and was discharged from the hospital to be seen in the clinic in 2 weeks.
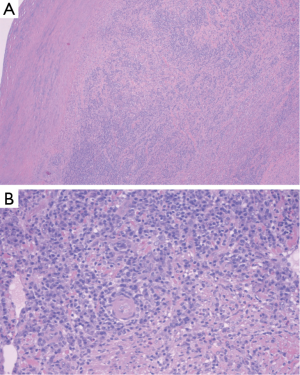
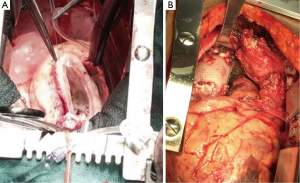
Histologic examination of the specimen showed dense infiltrate of plasma cells in all layers of the aortic wall, neutrophils, occasional eosinophils, and lymphoid follicles with reactive germinal centers (Figure 5). Blood vessels in the aortic wall were thickened and showed endothelial proliferation. There were no granulomas, and no areas of necrosis. Figure 6 shows the gross pathology of the thickened aortic wall and the surgical repair after placement of the graft.
Discussion
The inflammatory insult in aortitis leads to vascular remodeling and variable symptomatology. Unlike other types of aortitis, IDA has an indolent course. Retrospective studies show that approximately greater than half of patients with IDA have no prior history or symptoms of vasculitis (3), and most cases of IDA are diagnosed incidentally on histological examination after surgical removal (4,5).
Imaging plays an essential role in differentiating the subclasses of aortitis. The most commonly used imaging modality for IDA or any type of aortitis is CT. Angiography of the aorta was previously considered the gold standard but now is widely replaced by CT angiography as it characterizes the tissues outside the lumen. A typical CT scan finding for IDA shows perianeurysmal fibrosis with a thickened wall. There is also an associated aortic aneurysm which is a common complication of IDA (5).
As there are no specific symptoms of IDA, a thorough diagnostic work-up is critical in addition to obtaining a thorough history and physical examination. IDA is associated with formation of aneurysms in other areas of the body, and there are recommendations for full body CT to identify these at the time of diagnosis (6). A full rheumatologic workup should also be undertaken to rule out other types of aortitis. The treatment for IDA is resection, and the long- term prognosis is generally good without complications. The role for glucocorticoids in aortitis is not well understood at this time, but some studies show that it can have some benefit in preventing recurrence of other aneurysms (7).
This case is unique for several reasons. First, the patient in our case report had a wall thickness of 1.5 cm which is larger than the upper range in IDA (0.9 cm) (8-10). In addition, despite an abnormally thickened wall, the patient presented with non-specific symptoms. Even though the patient returned with vague chest pain a few weeks earlier than the anticipated date of surgery, his work-up was unchanged from his initial hospitalization.
Our patient had a strong family history of cardiovascular disease which may suggest a hereditary component. Both his father and brother died from sudden cardiac death before the age of 50 years, and his daughter had 2 aneurysms. Some studies of abdominal aneurysms have shown familial associations (6,7). Our case of IDA involved only the ascending aorta, and there are no studies that suggest a hereditary pattern for IDA. Finally, isolated aortitis is a rare subset of aortitis and is poorly understood. At times, it is histologically compared to giant cell arteritis (GCA) (11). GCA is characterized by patchy areas of medial necrosis with infiltration of lymphocytes and plasma cells (1,2). Our patient did not have any areas of necrosis.
In conclusion, aortitis encompasses a broad range of disease patterns that needs extensive diagnostic work-up. Our patient who had minimal symptoms had severe aortitis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: This patient moved to an unidentified location. There is no way this case report shows the identity of the person involved.
References
- Wang H, Li L, Wang L, et al. Comparison of clinical and pathological characteristics of isolated aortitis and Takayasu arteritis with ascending aorta involvement. J Clin Pathol 2012;65:362-6. [Crossref] [PubMed]
- Miller DV, Isotalo PA, Weyand CM, et al. Surgical pathology of noninfectious ascending aortitis: a study of 45 cases with emphasis on an isolated variant. Am J Surg Pathol 2006;30:1150-8. [Crossref] [PubMed]
- Gornik HL, Creager MA. Aortitis. Circulation 2008;117:3039-51. [Crossref] [PubMed]
- García-Martínez A, Prieto-González S, Arguis Giménez P, et al. Aortitis and aortic aneurysm in systemic vasculitis. In: Grundmann R. editor. Etiology, Pathogenesis and Pathophysiology of Aortic Aneurysms and Aneurysm Rupture, Barcelona, Spain: In Tech, 2011: 135-58.
- Rojo-Leyva F, Ratliff NB, Cosgrove DM 3rd, et al. Study of 52 patients with idiopathic aortitis from a cohort of 1,204 surgical cases. Arthritis Rheum 2000;43:901-7. [Crossref] [PubMed]
- Litmanovich DE, Yıldırım A, Bankier AA. Insights into imaging of aortitis. Insights Imaging 2012;3:545-60. [Crossref] [PubMed]
- Hellmann DB, Grand DJ, Freischlag JA. Inflammatory abdominal aortic aneurysm. JAMA 2007;297:395-400. [Crossref] [PubMed]
- Tang T, Boyle JR, Dixon AK, et al. Inflammatory abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2005;29:353-62. [Crossref] [PubMed]
- Slobodin G, Nakhleh A, Rimar D, et al. Increased aortic wall thickness for the diagnosis of aortitis: a computed tomography-based study. Int J Rheum Dis 2016;19:82-6. [Crossref] [PubMed]
- Erbel R, Eggebrecht H. Aortic dimensions and the risk of dissection. Heart 2006;92:137-42. [Crossref] [PubMed]
- Liang KP, Chowdhary VR, Michet CJ, et al. Noninfectious ascending aortitis: a case series of 64 patients. J Rheumatol 2009;36:2290-7. [Crossref] [PubMed]



