
Dendritic cell vaccination combined with irreversible electroporation for treating pancreatic cancer—a narrative review
Introduction
Despite the advancement of immunotherapy during the last decade, pancreatic ductal adenocarcinoma (PDAC) was not able to reflect similar benefits observed in several cancers. The recent preclinical studies suggested that dendritic cell (DC)-based immunotherapy may inhibit growth and improve survival (1-4); however, immunosuppressive tumor microenvironment (TME) remains a challenge for researchers (5-12).
Thermal ablation techniques were utilized to sensitize the tumors to chemotherapeutic or immunotherapeutic agents by destructing TME and facilitating systemic immune response in solid cancers (13). However, these ablation therapies demonstrated limited success and led to high morbidity in PDAC patients (14,15). In contrast, irreversible electroporation (IRE) ablation, facilitating lethal nanopores via delivery of strong shorth electrical pulses, destroys TME and promotes an antitumor immune response leading to promising therapeutic responses (16). Based on the distance from the origin, the underlying structures of the tissues underwent either temporary or permanent ablation reflecting the therapeutic efficacy which is clinically challenging (17). While ultrasound (US) and computed tomography (CT) imaging techniques are utilized to monitor dynamic changes following IRE ablation (18,19), magnetic resonance imaging (MRI) provides superior efficacy associated with soft-tissue contrast. Previous studies suggested that MRI can detect rapid tumor changes in TME following IRE ablation therapy (20,21). Traditional imaging techniques lack precision in identifying TME changes; however, data-driven artificial intelligence (AI) strategies enhance the detection of imaging biomarkers from MRI data in which these quantitative imaging biomarkers correlate with therapeutic response indicators (19,22). Recent studies emphasized the potential benefit of AI-derived quantitative models for the differentiation of IRE ablation regions allowing immediate assessment (22,23).
In this review, we focused on the combination of IRE ablation with DC vaccine immunotherapy as a potent therapeutic strategy for PDAC and the role of AI-driven MRI biomarkers in the early assessment of the combination therapy. We present this article in accordance with the Narrative Review reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1882/rc).
Methods
We performed a literature search on PubMed and Web of Science databases to identify papers related to DC vaccine therapy and IRE ablation as therapeutic strategies against PDAC, published up to February 20, 2023. Our search terms were “pancreatic ductal adenocarcinoma”, “PDAC”, “immunotherapy”, “IRE”, “irreversible electroporation”, “DC vaccine”, “dendritic cell”, “radiomics”, “machine learning”, “artificial intelligence”, and “AI” (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | February 20th, 2023 |
| Databases and other sources searched | PubMed and Web of Science |
| Search terms used | “Pancreatic ductal adenocarcinoma”, “Pancreatic Cancer”, “PDAC”, “immunotherapy”, “IRE”, “Irreversible Electroporation”, “DC vaccine”, “dendritic cell”, “radiomics”, “machine learning”, “artificial intelligence”, “AI” |
| Timeframe | From 1972 until February 20th, 2023 |
| Inclusion and exclusion criteria | No restrictions on study type or language |
| Selection process | Search conducted by Zigeng Zhang and Guangbo Yu, with consensus by all authors |
PDAC, pancreatic ductal adenocarcinoma; IRE, irreversible electroporation; DC, dendritic cell, AI, artificial intelligence.
DC vaccination combined with IRE ablation
Treatment for advanced PDAC
Despite advanced treatments, the prognosis of PDAC remains grim with a 5-year survival rate under 10% (24). Nearly 80% of cases are inoperable at diagnosis, resulting in 5-year OS rates below 5% (25). Standard treatments like chemotherapy and radiotherapy offer limited success, with median survival under 10 months for unresectable PDAC (26-28). Although immunotherapy holds potential, immunosuppressive nature of PDAC TME hinders its efficacy (29). DCs, which can trigger specific immune responses, have been tested for PDAC (30). However, immunosuppressive TME restricts effective immune response. There’s a pressing need for innovative strategies to enhance treatments like DC vaccination for PDAC.
Clinical challenge in assessing treatment response to cancer immunotherapy
Evaluating the response to cancer immunotherapy using imaging poses a significant challenge. Conventional imaging biomarkers of treatment response, which are based on changes in tumor size (31,32), such as immune-RECIST (iRECIST), immune-modified RECIST (imRECIST), and immune-related response criteria (irRC), often fall short in accurately detecting novel patterns of immunotherapy responses (33-35). These existing methods don’t account for tumor heterogeneity; are susceptible to inaccurate assessments due to pseudoprogression and mixed immune-related response patterns; and are limited in assessing early responses or forecasting overall survival (OS).
IRE mitigates immune suppression and boosts immunotherapy against PDAC
A non-thermal method, IRE ablation, uses electric pulses to cause tumor cell death and reduce fibrosis in PDAC models and patients (36-39). It has been found to instigate a robust antitumor response by altering the TME and activating immune cells and has the potential to trigger apoptosis in the initial phases and reduce the presence of immune-suppressive cells (36-39). This impact of IRE might enhance the efficacy of immunotherapy by transitioning from an inherently immunosuppressive microenvironment to one with proinflammatory and anti-tumorigenic properties (40). In terms of cancer hallmarks, the treatment primarily influences cellular injury and regeneration. Prior to undergoing IRE treatment, patient-derived xenograft (PDX) tumors exhibited heightened cellular injury signaling, which subsequently decreased following the therapy. Moreover, there was an observed increase in regenerative processes and repaired signaling, particularly with higher dosages of IRE (41). Current research shows that IRE increases immune cells like NK1.1, CD8+ T cells, and CD11c+ DCs in PDAC mouse models, promoting broader immune responses (42-44). This technique also enhances tumor tissue permeability, aiding in the influx of immune cells for treatment. The immune boost from IRE might counter the immunosuppressive TME of PDAC, enhancing DC-vaccine therapy. Still, integrating IRE with DC vaccination for PDAC needs more research.
Clinical challenge for assessing changes in TME during IRE ablation
IRE, a predominantly non-thermal method, offers advantages over traditional thermal ablations like radiofrequency and cryoablation (45,46). It utilizes targeted electric pulses for electroporation to cause cell death by permanently disrupting cell membranes (45,46). Electroporation involves applying a brief electric field to the cell membrane, leading to the formation of nanoscale pores, which in turn enhances membrane permeability (47). Normally, these pores close shortly after the electric field is applied, a reversible electroporation (RE) process extensively employed to aid in gene transfer (8-10,48-50) and drug delivery (51,52). Nevertheless, if the electric field strength across the cell membrane is significantly high, the pores fail to reseal, disrupting cellular homeostasis and ultimately causing cell demise. There are challenges in using imaging to assess tumor responses post-IRE: Conventional imaging methods, such as US, CT, positron emission tomography (PET), and MRI, display changes due to electroporation, not specific TME changes from IRE. While these changes might hint at IRE responses in regular tissues, they’re not consistently seen in tumors (53). Previous imaging was practical for thermal ablation but might not be suited for detecting early non-thermal IRE effects. The core clinical hurdle with IRE is pinpointing TME changes and determining fully treated areas (IRE zone) from partially treated ones (RE zone). Such insights are vital for assessing treatment effectiveness and preserving healthy pancreatic tissue.
Identification of novel AI-derived imaging biomarkers for TME changes in response to IRE-immunotherapy
AI-derived new biomarkers from conventional MRI data can be used for tracking responses to cancer immunotherapy and IRE ablation. AI methods often excel over human efforts in cancer imaging, particularly in image segmentation and tracking tumor progress (54,55). Using radiomics and statistical methods, AI can derive detailed imaging features or biomarkers from radiological data (54,55). The goal is to correlate these AI-derived biomarkers from MRI with histological tumor markers and treatment results. Therefore, these AI markers will be able to accurately link imaging features to histological findings, providing insights into prognosis and treatment responses to IRE ablation, DC vaccination, or their combination in the future. Furthermore, AI can standardize evaluations across multiple institutions, reducing the potential variability introduced by subjective interpretations of clinicians (56,57). Consequently, the integration of AI into cancer immunotherapy has the potential to yield favorable results for patients.
Combined therapy of local IRE with DC immunotherapy
Surgery is the primary treatment for PDAC (5-12). Traditional thermal ablation methods for the pancreas are discouraged due to high risks and complications (58-63). In contrast, non-thermal IRE ablation effectively causes tumor cell death, reduces fibrosis, and releases tumor neoantigens, facilitating the migration of immune cells to the tumor (29,64,65). IRE demonstrated the ability to rapidly increase specific immune cells in blood samples from PDAC mice (Figure 1). Numerous studies confirm the potent local and systemic antitumor response from IRE (22,29,40,43,44,65,66). This response involves disrupting the tumor environment, induction of tumor cell apoptosis, and activation of immune cells (29,64,65). A recent study suggests IRE boosts the efficiency of DC vaccination in PDAC (22). Additionally, it has been demonstrated that IRE reduces PDAC fibrosis, aiding immune cells in accessing and infiltrating the tumor (30,43,44). Given these insights, taken together, IRE can combat the immunosuppressive environment of PDAC. Researchers should focus on optimizing a combined IRE-DC vaccination strategy to enhance PDAC treatment outcomes.
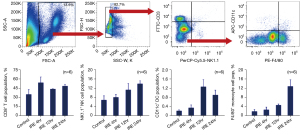
Translational potential of intraperitoneal delivery of DC vaccination
DC-vaccine delivery lacks a standardized method in research. Subcutaneous (s.c.) administration, commonly used in studies, allows only a limited amount of DC vaccines to reach lymph nodes (LNs) (67,68). Intratumoral injections expose vaccines to immunosuppressive tumor-released cytokines, diminishing their effect (69). Intranodal injections deliver DC vaccines directly to LNs but damage their structure, impacting DC-T cell interactions and reducing cytotoxic T lymphocyte (CTL) generation (70,71). However, intraperitoneal (i.p.) administration permits a larger dosage, directing DC vaccines to peritoneal LNs without harming LN integrity, and boosting the antitumor response (70,71). Studies have proven that the i.p. method surpassed the s.c. and i.t. approaches in therapeutic outcomes (72-74). Given these findings, establishing a single dose i.p. DC vaccine strategy combined with IRE ablation for PDAC for quicker clinical application should be the focus of today’s research.
Innovative AI-derived imaging biomarkers from conventional MRI data present new possibilities for understanding tumor pathophysiology and monitoring treatment response
A large volume of clinical imaging data is collected daily, but only a small fraction of this information is utilized to confirm or exclude medical conditions (75). Most information in clinical images remains unquantified due to the lack of reliable and efficient analysis methods (54). AI provides an effective means to automate image interpretation, transforming the clinical workflow of radiographic detection (76). Recent progress in radiomics and AI algorithms enables AI to identify complex patterns within imaging data, providing quantitative evaluations of radiographic features with minimal additional cost and high speed (54,76,77). The heterogeneity and changes in the TME that occur in response to IRE ablation or cancer immunotherapy will be detected using our AI-derived MRI biomarkers, which represent unique microstructural properties of tissues shortly after treatment (78,79). Researchers should employ AI-derived MRI biomarkers to evaluate TME in preclinical tumor models and validate the findings using histopathological data.
AI-enhanced MRI biomarkers: monitoring and optimizing PDAC treatment responses through IRE and DC vaccination
Recent advancements underscore the profound potential of utilizing AI-driven MRI biomarkers to monitor and assess treatment responses in PDAC. In the study conducted by Figini et al., rabbits with VX2 liver tumors underwent immediate MRI post-IRE ablation, which displayed regions of IRE and RE on TRIP-MRI (43). Classification models were developed and evaluated for their effectiveness, with RF classifiers achieving notable accuracies of up to 97% (Figure 2) (80). In another study using the KPC mouse model for PDAC, machine learning (ML) models, leveraging MRI data, predicted OS with remarkable precision (81). A synergistic approach, combining IRE ablation and DC vaccination in the KPC tumor-bearing mouse model, further demonstrated extended median survival, thereby illuminating a promising avenue for PDAC treatment that merits in-depth exploration in clinical scenarios (Figure 3) (22).
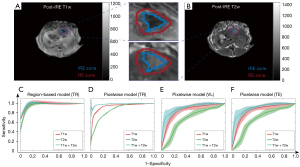
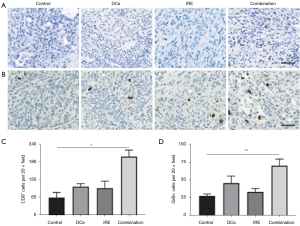
AI-derived MRI biomarkers, confirmed by gold-standard histological results, have not yet been employed to monitor responses to IRE ablation, immunotherapy, and IRE-immunotherapy
Locoregional-immunotherapy combinations are vital for treating cancers like PDAC (82,83). While animal and clinical studies show IRE ablation’s immunological effects, DC-based immunotherapy’s long-term outcomes for advanced PDAC differ. Boosting DC vaccination’s efficacy requires broadening immune response to counter tumor immunosuppression (53,84). IRE ablation, superior to other thermal techniques, can trigger antitumor immunity by altering the TME in PDAC mice, enhancing DC vaccination effectiveness (85). Further research is needed on the best combination of IRE immunotherapy. In the realm of research, scientists are delving into the potential of AI to develop new imaging biomarkers from mp-MRI data, hoping these will match histological tumor markers. There’s a growing consensus that these AI-powered MRI biomarkers could offer a deeper understanding of the TME, enhancing prognostic precision and predicting treatment responses. A pivotal step in this endeavor involves validating these AI-enhanced MRI markers against the gold-standard histological benchmarks, laying the groundwork for their prospective clinical applications.
IRE ablation destroys TME and triggers immune responses
Researchers have created pancreatic and liver tumor models to evaluate the effects of IRE, immunotherapy, and their combination. Preliminary findings indicate that IRE causes early changes in the TME, like cellular permeabilization, apoptosis, and reduction of tumor fibrosis in mouse PDAC models (43). Moreover, real-time imaging can fine-tune IRE procedures (44,86). Notably, IRE increases NK and CD8+ CTL accumulation in PDAC mouse tumors, hinting at enhanced antitumor immunity. IRE also results in more pronounced macrophage, NK, and CD8+ CTL infiltration compared to cryoablation in these models (30). Additionally, MRI’s potential for tracking early IRE responses non-invasively has been explored.
DC-based immunotherapy for PDAC
DCs have emerged as powerful agents in cancer immunotherapy. In the 1990s, DCs pulsed with protein antigens were shown to stimulate antigen-responsive T-cell proliferation in mice (87). Despite their ease of manufacture, peptides or proteins for DC pulsing are limited to tumors with identified tumor-associated antigens (TAAs). Recent advancements have seen DCs pulsed with DNA constructs, such as one where a recombinant adeno-associated virus carrying the AFP gene was used to induce a CTL response against HCC, showcasing the potential of combining multiple TAAs for enhanced efficacy (88). DCs pulsed with tumor lysates offer the promise of prolonged antigen presentation and the potential to initiate broad T-cell responses due to their encompassing multiple epitopes. Especially noteworthy is the development of a therapeutic cancer vaccine using tumor cell-derived autophagosomes, which has shown promise in mouse models (89). Another intriguing method involves the fusion of DCs and tumor cells, which presents a comprehensive array of TAAs, activating both CD4+ and CD8+ T cells (90). Though potent, its application has been hindered by challenges like low fusion efficiency and limited availability of viable autologous tumor cells.
In the continually advancing field of DC-based immunotherapy for PDAC in mouse models, several milestones stand out. Previous studies revealed the feasibility, safety, and immunogenicity of the allogeneic tumor lysate-based DC therapy, MesoPher, in patients with surgically resected PDAC who are free of local disease recurrence (91,92). Additionally, prophylactic DC vaccination through i.p. injection has been shown to effectively inhibit PDAC tumor growth and extend OS in PDAC mouse models (93). Furthermore, a recent pilot study revealed that i.p. injection of DC-vaccines results in better therapeutic outcomes in a genetically engineered mouse model of PDAC compared to i.t. and s.c. routes (72). More recently, it has been observed that IRE ablation augments the effectiveness of DC vaccination in mouse models of pancreatic cancer (22).
AI-derived tumor MRI biomarkers for evaluating treatment responses
In recent years, ML leveraging radiomic features has demonstrated robust efficacy in medical image analysis. Radiomics employs advanced pattern recognition techniques to extract a plethora of quantitative features from digital images. This extraction elucidates relationships between these features and the underlying pathophysiology, thereby enhancing diagnostic, prognostic, and predictive precision.
The advancements in AI have painted a promising landscape for PDAC treatment. Notably demonstrating the potential of AI-derived MRI texture features to predict histopathological tumor markers and long-term outcomes in a mouse model of PDAC (2), developing and validating an MRI radiomics-based ML classifier for quantitatively evaluating the tumor-stroma ratio (TSR) in patients with PDAC (94), using ML model with radiomics features computed from diffusion weighted imaging (DWI) MRI data to predict OS in PDAC in clinical studies (95), and showing that AI-derived MRI imaging biomarkers accurately predict gene expression profiles and LN metastasis in patients with PDAC (96,97). Supporting the proposed research, preclinical studies have showcased strong correlations between AI-derived MRI and histological tumor biomarkers measured after immunotherapy. These AI biomarkers have been particularly insightful in distinguishing between IRE and RE zones post-IRE ablation. Emerging strategies focus on the longitudinal monitoring of IRE-DC vaccination to maximize the therapeutic response.
Future directions
Analysis of the multiparametric MRI texture using comprehensive feature models enables the computation of distinct characteristics of the tissues in which advanced statistical learning techniques are capable of distinguishing IRE ablation zones in alignment with histological tumor markers. Specifically, integrating traditional MRI with statistical analysis strategies may produce MRI indicators that discriminate between tumor RE and IRE zones post-IRE ablation of solid tumor models (80,98-101). These expert models heavily analyze the underlying characteristics of the TME following IRE ablation captured with multi-parametric MRI and associated with biological changes measured with histopathological analysis.
After IRE ablation, the cellular structures in the origin of the ablated region became nerotic due to delivered high-voltage pulses however, the cells in the perimeter of this region underwent recovery. The inner ablated region is anticipated to have higher apoptosis and lower fibrosis levels compared to the RE region which leads to characteristic changes that can be evaluated using different MRI sequences. Previous studies focused on the interpretation of conventional MRI data in the detection of these changes and suggested using more complex imaging techniques leading to repeatability issues (21,102). In a recent study, Eresen et al. (80) demonstrated that quantitative MRI biomarkers are capable of characterizing the IRE and RE ablation regions and differentiating them with high accuracy. However, further larger studies should be performed to validate the findings before translation to clinical trials.
Conclusions
DC vaccination, recognized for its feasibility, safety, and efficacy in PDAC treatment, has witnessed significant advancements with DC vaccines undergoing continuous optimization. However, the favorable outcomes observed in preclinical studies, notably improved OS, have not been consistently mirrored in clinical settings. IRE, a novel non-thermal ablation approach for tumors, offers in situ tumor vaccination by releasing critical damage-associated molecular patterns from ablated, non-extracted tumor tissues. As more studies explore IRE and as its technology advances, the combination of IRE with immunotherapy emerges as a compelling approach, particularly for patients with unresectable tumors. This evolving landscape highlights the imperative for interdisciplinary collaboration. The integration and advancement of AI-assisted systems, which can precisely discern the target population for immunotherapy, forecast efficacy, and improve prognostic accuracy, become paramount. Such synergistic endeavors not only optimize treatment outcomes but also solidify trust among both medical professionals and patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1882/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1882/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1882/coif). Zigeng Zhang reports a grant from Society of Interventional Radiology Foundation. A.E. reports a pilot grant from the University of California Irvine Anti-Cancer Challenge. V.Y. and Zhuoli Zhang report grants from the National Institute of Health. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yang J, Hu S, Shangguan J, et al. Dendritic cell immunotherapy induces anti-tumor effect in a transgenic mouse model of pancreatic ductal adenocarcinoma. Am J Cancer Res 2019;9:2456-68. [PubMed]
- Eresen A, Yang J, Shangguan J, et al. Prediction of therapeutic outcome and survival in a transgenic mouse model of pancreatic ductal adenocarcinoma treated with dendritic cell vaccination or CDK inhibitor using MRI texture: a feasibility study. Am J Transl Res 2020;12:2201-11. [PubMed]
- Pan L, Shang N, Shangguan J, et al. Magnetic resonance imaging monitoring therapeutic response to dendritic cell vaccine in murine orthotopic pancreatic cancer models. Am J Cancer Res 2019;9:562-73. [PubMed]
- Eresen A, Yang J, Shangguan J, et al. MRI radiomics for early prediction of response to vaccine therapy in a transgenic mouse model of pancreatic ductal adenocarcinoma. J Transl Med 2020;18:61. [Crossref] [PubMed]
- Christenson ES, Jaffee E, Azad NS. Current and emerging therapies for patients with advanced pancreatic ductal adenocarcinoma: a bright future. Lancet Oncol 2020;21:e135-45. [Crossref] [PubMed]
- Hosein AN, Dougan SK, Aguirre AJ, et al. Translational advances in pancreatic ductal adenocarcinoma therapy. Nat Cancer 2022;3:272-86. [Crossref] [PubMed]
- Grossberg AJ, Chu LC, Deig CR, et al. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J Clin 2020;70:375-403. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011;378:607-20. [Crossref] [PubMed]
- Jiang H, Hegde S, DeNardo DG. Tumor-associated fibrosis as a regulator of tumor immunity and response to immunotherapy. Cancer Immunol Immunother 2017;66:1037-48. [Crossref] [PubMed]
- Ware MB, El-Rayes BF, Lesinski GB. Mirage or long-awaited oasis: reinvigorating T-cell responses in pancreatic cancer. J Immunother Cancer 2020;8:e001100. [Crossref] [PubMed]
- O'Reilly EM. Refinement of adjuvant therapy for pancreatic cancer. JAMA 2010;304:1124-5. [Crossref] [PubMed]
- Farmer W, Hannon G, Ghosh S, et al. Thermal ablation in pancreatic cancer: A scoping review of clinical studies. Front Oncol 2022;12:1066990. [Crossref] [PubMed]
- Keane MG, Bramis K, Pereira SP, et al. Systematic review of novel ablative methods in locally advanced pancreatic cancer. World J Gastroenterol 2014;20:2267-78. [Crossref] [PubMed]
- Lambert A, Schwarz L, Borbath I, et al. An update on treatment options for pancreatic adenocarcinoma. Ther Adv Med Oncol 2019;11:1758835919875568. [Crossref] [PubMed]
- Rai ZL, Feakins R, Pallett LJ, et al. Irreversible Electroporation (IRE) in Locally Advanced Pancreatic Cancer: A Review of Current Clinical Outcomes, Mechanism of Action and Opportunities for Synergistic Therapy. J Clin Med 2021;10:1609. [Crossref] [PubMed]
- Eresen A, Yang J, Scotti A, et al. Combination of natural killer cell-based immunotherapy and irreversible electroporation for the treatment of hepatocellular carcinoma. Ann Transl Med 2021;9:1089. [Crossref] [PubMed]
- Sugimoto K, Abe M, Yoshimasu Y, et al. Irreversible electroporation of hepatocellular carcinoma: the role of ultrasonography. Ultrasonography 2020;39:229-37. [Crossref] [PubMed]
- Bäumler W, Sebald M, Einspieler I, et al. Incidence and evolution of venous thrombosis during the first 3 months after irreversible electroporation of malignant hepatic tumours. Sci Rep 2019;9:19876. [Crossref] [PubMed]
- Figini M, Zhou K, Pan L, et al. Transcatheter intra-arterial perfusion (TRIP)-MRI biomarkers help detect immediate response to irreversible electroporation of rabbit VX2 liver tumor. Magn Reson Med 2020;84:365-74. [Crossref] [PubMed]
- Buijs M, de Bruin DM, Wagstaff PG, et al. MRI and CT in the follow-up after irreversible electroporation of small renal masses. Diagn Interv Radiol 2021;27:654-63. [Crossref] [PubMed]
- Yang J, Eresen A, Shangguan J, et al. Irreversible electroporation ablation overcomes tumor-associated immunosuppression to improve the efficacy of DC vaccination in a mice model of pancreatic cancer. Oncoimmunology 2021;10:1875638. [Crossref] [PubMed]
- Vroomen LGPH, Scheffer HJ, Melenhorst MCAM, et al. MR and CT imaging characteristics and ablation zone volumetry of locally advanced pancreatic cancer treated with irreversible electroporation. Eur Radiol 2017;27:2521-31. [Crossref] [PubMed]
- Hidalgo M. Pancreatic cancer. N Engl J Med 2010;362:1605-17. [Crossref] [PubMed]
- He J, Page AJ, Weiss M, et al. Management of borderline and locally advanced pancreatic cancer: where do we stand? World J Gastroenterol 2014;20:2255-66. [Crossref] [PubMed]
- Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 2010;28:3617-22. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Lellouche L, Palmieri LJ, Dermine S, et al. Systemic therapy in metastatic pancreatic adenocarcinoma: current practice and perspectives. Ther Adv Med Oncol 2021;13:17588359211018539. [Crossref] [PubMed]
- Paiella S, De Pastena M, D'Onofrio M, et al. Palliative therapy in pancreatic cancer-interventional treatment with radiofrequency ablation/irreversible electroporation. Transl Gastroenterol Hepatol 2018;3:80. [Crossref] [PubMed]
- White SB, Zhang Z, Chen J, et al. Early Immunologic Response of Irreversible Electroporation versus Cryoablation in a Rodent Model of Pancreatic Cancer. J Vasc Interv Radiol 2018;29:1764-9. [Crossref] [PubMed]
- Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143-52. [Crossref] [PubMed]
- Lai YC, Chang WC, Chen CB, et al. Response evaluation for immunotherapy through semi-automatic software based on RECIST 1.1, irRC, and iRECIST criteria: comparison with subjective assessment. Acta Radiol 2020;61:983-91. [Crossref] [PubMed]
- Hodi FS, Ballinger M, Lyons B, et al. Immune-Modified Response Evaluation Criteria In Solid Tumors (imRECIST): Refining Guidelines to Assess the Clinical Benefit of Cancer Immunotherapy. J Clin Oncol 2018;36:850-8. [Crossref] [PubMed]
- Marciscano AE, Thorek DLJ. Role of noninvasive molecular imaging in determining response. Adv Radiat Oncol 2018;3:534-47. [Crossref] [PubMed]
- Gerwing M, Herrmann K, Helfen A, et al. The beginning of the end for conventional RECIST - novel therapies require novel imaging approaches. Nat Rev Clin Oncol 2019;16:442-58. [Crossref] [PubMed]
- José A, Sobrevals L, Ivorra A, et al. Irreversible electroporation shows efficacy against pancreatic carcinoma without systemic toxicity in mouse models. Cancer Lett 2012;317:16-23. [Crossref] [PubMed]
- He C, Huang X, Zhang Y, et al. T-cell activation and immune memory enhancement induced by irreversible electroporation in pancreatic cancer. Clin Transl Med 2020;10:e39. [Crossref] [PubMed]
- Martin RC 2nd, McFarland K, Ellis S, et al. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol 2013;20:S443-9. [Crossref] [PubMed]
- Kluger MD, Epelboym I, Schrope BA, et al. Single-Institution Experience with Irreversible Electroporation for T4 Pancreatic Cancer: First 50 Patients. Ann Surg Oncol 2016;23:1736-43. [Crossref] [PubMed]
- Zhao J, Wen X, Tian L, et al. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat Commun 2019;10:899. [Crossref] [PubMed]
- Brock RM, Beitel-White N, Coutermarsh-Ott S, et al. Patient Derived Xenografts Expand Human Primary Pancreatic Tumor Tissue Availability for ex vivo Irreversible Electroporation Testing. Front Oncol 2020;10:843. [Crossref] [PubMed]
- Schaft N, Dörrie J, Müller I, et al. A new way to generate cytolytic tumor-specific T cells: electroporation of RNA coding for a T cell receptor into T lymphocytes. Cancer Immunol Immunother 2006;55:1132-41. [Crossref] [PubMed]
- Figini M, Wang X, Lyu T, et al. Diffusion MRI biomarkers predict the outcome of irreversible electroporation in a pancreatic tumor mouse model. Am J Cancer Res 2018;8:1615-23. [PubMed]
- Zhang Z, Li W, Procissi D, et al. Rapid dramatic alterations to the tumor microstructure in pancreatic cancer following irreversible electroporation ablation. Nanomedicine (Lond) 2014;9:1181-92. [Crossref] [PubMed]
- Wagstaff PG, Buijs M, van den Bos W, et al. Irreversible electroporation: state of the art. Onco Targets Ther 2016;9:2437-46. [Crossref] [PubMed]
- Verloh N, Jensch I, Lürken L, et al. Similar complication rates for irreversible electroporation and thermal ablation in patients with hepatocellular tumors. Radiol Oncol 2019;53:116-22. [Crossref] [PubMed]
- Neumann E, Rosenheck K. Permeability changes induced by electric impulses in vesicular membranes. J Membr Biol 1972;10:279-90. [Crossref] [PubMed]
- Paunesku T, Ke T, Dharmakumar R, et al. Gadolinium-conjugated TiO2-DNA oligonucleotide nanoconjugates show prolonged intracellular retention period and T1-weighted contrast enhancement in magnetic resonance images. Nanomedicine 2008;4:201-7. [Crossref] [PubMed]
- Leroy-Willig A, Bureau MF, Scherman D, et al. In vivo NMR imaging evaluation of efficiency and toxicity of gene electrotransfer in rat muscle. Gene Ther 2005;12:1434-43. [Crossref] [PubMed]
- Leroy-Willig A, Fromes Y, Paturneau-Jouas M, et al. Assessing gene and cell therapies applied in striated skeletal and cardiac muscle: is there a role for nuclear magnetic resonance? Neuromuscul Disord 2003;13:397-407. [Crossref] [PubMed]
- Okino M, Mohri H. Effects of a high-voltage electrical impulse and an anticancer drug on in vivo growing tumors. Jpn J Cancer Res 1987;78:1319-21. [PubMed]
- Heller R, Gilbert R, Jaroszeski MJ. Clinical applications of electrochemotherapy. Adv Drug Deliv Rev 1999;35:119-29. [Crossref] [PubMed]
- Zhang Y, Guo Y, Ragin AB, et al. MR imaging to assess immediate response to irreversible electroporation for targeted ablation of liver tissues: preclinical feasibility studies in a rodent model. Radiology 2010;256:424-32. [Crossref] [PubMed]
- Weiss J, Hoffmann U, Aerts HJWL. Artificial intelligence-derived imaging biomarkers to improve population health. Lancet Digit Health 2020;2:e154-5. [Crossref] [PubMed]
- Cardobi N, Dal Palù A, Pedrini F, et al. An Overview of Artificial Intelligence Applications in Liver and Pancreatic Imaging. Cancers (Basel) 2021;13:2162. [Crossref] [PubMed]
- Thust SC, Heiland S, Falini A, et al. Glioma imaging in Europe: A survey of 220 centres and recommendations for best clinical practice. Eur Radiol 2018;28:3306-17. [Crossref] [PubMed]
- Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 2015;16:e534-42. [Crossref] [PubMed]
- Girelli R, Frigerio I, Salvia R, et al. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg 2010;97:220-5. [Crossref] [PubMed]
- Li J, Chen X, Yang H, et al. Tumour cryoablation combined with palliative bypass surgery in the treatment of unresectable pancreatic cancer: a retrospective study of 142 patients. Postgrad Med J 2011;87:89-95. [Crossref] [PubMed]
- He C, Huang X, Zhang Y, et al. A Novel Prediction Tool Based on Large Cohorts to Determine the Cancer-Specific Survival Probability of Patients With Locally Advanced Pancreatic Cancer After Irreversible Electroporation Treatment. Front Oncol 2020;10:952. [Crossref] [PubMed]
- Martin RC 2nd. Use of irreversible electroporation in unresectable pancreatic cancer. Hepatobiliary Surg Nutr 2015;4:211-5. [PubMed]
- Timmer FEF, Geboers B, Ruarus AH, et al. Irreversible Electroporation for Locally Advanced Pancreatic Cancer. Tech Vasc Interv Radiol 2020;23:100675. [Crossref] [PubMed]
- Narayanan G, Daye D, Wilson NM, et al. Ablation in Pancreatic Cancer: Past, Present and Future. Cancers (Basel) 2021;13:2511. [Crossref] [PubMed]
- Novickij V, Čėsna R, Perminaitė E, et al. Antitumor Response and Immunomodulatory Effects of Sub-Microsecond Irreversible Electroporation and Its Combination with Calcium Electroporation. Cancers (Basel) 2019;11:1763. [Crossref] [PubMed]
- Beitel-White N, Martin RCG, Li Y, et al. Real-time prediction of patient immune cell modulation during irreversible electroporation therapy. Sci Rep 2019;9:17739. [Crossref] [PubMed]
- Geboers B, Ruarus AH, Nieuwenhuizen S, et al. Needle-guided ablation of locally advanced pancreatic cancer: cytoreduction or immunomodulation by in vivo vaccination? Chin Clin Oncol 2019;8:61. [Crossref] [PubMed]
- Pizzurro GA, Barrio MM. Dendritic cell-based vaccine efficacy: aiming for hot spots. Front Immunol 2015;6:91. [Crossref] [PubMed]
- Curti A, Tosi P, Comoli P, et al. Phase I/II clinical trial of sequential subcutaneous and intravenous delivery of dendritic cell vaccination for refractory multiple myeloma using patient-specific tumour idiotype protein or idiotype (VDJ)-derived class I-restricted peptides. Br J Haematol 2007;139:415-24. [Crossref] [PubMed]
- Rodríguez-Ruiz ME, Perez-Gracia JL, Rodríguez I, et al. Combined immunotherapy encompassing intratumoral poly-ICLC, dendritic-cell vaccination and radiotherapy in advanced cancer patients. Ann Oncol 2018;29:1312-9. [Crossref] [PubMed]
- Shang N, Figini M, Shangguan J, et al. Dendritic cells based immunotherapy. Am J Cancer Res 2017;7:2091-102. [PubMed]
- Lesterhuis WJ, de Vries IJ, Schreibelt G, et al. Route of administration modulates the induction of dendritic cell vaccine-induced antigen-specific T cells in advanced melanoma patients. Clin Cancer Res 2011;17:5725-35. [Crossref] [PubMed]
- Yang J, Eresen A, Shangguan J, et al. Effect of route of administration on the efficacy of dendritic cell vaccine in PDAC mice. Am J Cancer Res 2020;10:3911-9. [PubMed]
- Eggert AA, Schreurs MW, Boerman OC, et al. Biodistribution and vaccine efficiency of murine dendritic cells are dependent on the route of administration. Cancer Res 1999;59:3340-5. [PubMed]
- Mullins DW, Sheasley SL, Ream RM, et al. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J Exp Med 2003;198:1023-34. [Crossref] [PubMed]
- Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016;278:563-77. [Crossref] [PubMed]
- Bi WL, Hosny A, Schabath MB, et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J Clin 2019;69:127-57. [Crossref] [PubMed]
- Hosny A, Parmar C, Quackenbush J, et al. Artificial intelligence in radiology. Nat Rev Cancer 2018;18:500-10. [Crossref] [PubMed]
- Larson DB, Magnus DC, Lungren MP, et al. Ethics of Using and Sharing Clinical Imaging Data for Artificial Intelligence: A Proposed Framework. Radiology 2020;295:675-82. [Crossref] [PubMed]
- Shiraishi J, Li Q, Appelbaum D, et al. Computer-aided diagnosis and artificial intelligence in clinical imaging. Semin Nucl Med 2011;41:449-62. [Crossref] [PubMed]
- Eresen A, Sun C, Zhou K, et al. Early Differentiation of Irreversible Electroporation Ablation Regions With Radiomics Features of Conventional MRI. Acad Radiol 2022;29:1378-86. [Crossref] [PubMed]
- Eresen A, Yang J, Shangguan J, et al. Detection of Immunotherapeutic Response in a Transgenic Mouse Model of Pancreatic Ductal Adenocarcinoma Using Multiparametric MRI Radiomics: A Preliminary Investigation. Acad Radiol 2021;28:e147-54. [Crossref] [PubMed]
- Chiaravalli M, Reni M, O'Reilly EM. Pancreatic ductal adenocarcinoma: State-of-the-art 2017 and new therapeutic strategies. Cancer Treat Rev 2017;60:32-43. [Crossref] [PubMed]
- Segelov E, Lordick F, Goldstein D, et al. Current challenges in optimizing systemic therapy for patients with pancreatic cancer: expert perspectives from the Australasian Gastrointestinal Trials Group (AGITG) with invited international faculty. Expert Rev Anticancer Ther 2017;17:951-64. [Crossref] [PubMed]
- Garg AD, Vara Perez M, Schaaf M, et al. Trial watch: Dendritic cell-based anticancer immunotherapy. Oncoimmunology 2017;6:e1328341. [Crossref] [PubMed]
- Saxena M, Bhardwaj N. Turbocharging vaccines: emerging adjuvants for dendritic cell based therapeutic cancer vaccines. Curr Opin Immunol 2017;47:35-43. [Crossref] [PubMed]
- Wang ZJ, Behr S, Consunji MV, et al. Early Response Assessment in Pancreatic Ductal Adenocarcinoma Through Integrated PET/MRI. AJR Am J Roentgenol 2018;211:1010-9. [Crossref] [PubMed]
- Inaba K, Metlay JP, Crowley MT, et al. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med 1990;172:631-40. [Crossref] [PubMed]
- Yang JY, Cao DY, Xue Y, et al. Improvement of dendritic-based vaccine efficacy against hepatitis B virus-related hepatocellular carcinoma by two tumor-associated antigen gene-infected dendritic cells. Hum Immunol 2010;71:255-62. [Crossref] [PubMed]
- Chiang CL, Coukos G, Kandalaft LE. Whole Tumor Antigen Vaccines: Where Are We? Vaccines (Basel) 2015;3:344-72. [Crossref] [PubMed]
- Koido S. Dendritic-Tumor Fusion Cell-Based Cancer Vaccines. Int J Mol Sci 2016;17:828. [Crossref] [PubMed]
- Lau SP, Klaase L, Vink M, et al. Autologous dendritic cells pulsed with allogeneic tumour cell lysate induce tumour-reactive T-cell responses in patients with pancreatic cancer: A phase I study. Eur J Cancer 2022;169:20-31. [Crossref] [PubMed]
- Zhang Z, Li W, Procissi D, et al. Antigen-loaded dendritic cell migration: MR imaging in a pancreatic carcinoma model. Radiology 2015;274:192-200. [Crossref] [PubMed]
- Shangguan A, Shang N, Figini M, et al. Prophylactic dendritic cell vaccination controls pancreatic cancer growth in a mouse model. Cytotherapy 2020;22:6-15. [Crossref] [PubMed]
- Meng Y, Zhang H, Li Q, et al. Magnetic Resonance Radiomics and Machine-learning Models: An Approach for Evaluating Tumor-stroma Ratio in Patients with Pancreatic Ductal Adenocarcinoma. Acad Radiol 2022;29:523-35. [Crossref] [PubMed]
- Kaissis G, Ziegelmayer S, Lohöfer F, et al. A machine learning model for the prediction of survival and tumor subtype in pancreatic ductal adenocarcinoma from preoperative diffusion-weighted imaging. Eur Radiol Exp 2019;3:41. [Crossref] [PubMed]
- Li K, Yao Q, Xiao J, et al. Contrast-enhanced CT radiomics for predicting lymph node metastasis in pancreatic ductal adenocarcinoma: a pilot study. Cancer Imaging 2020;20:12. [Crossref] [PubMed]
- Li K, Xiao J, Yang J, et al. Association of radiomic imaging features and gene expression profile as prognostic factors in pancreatic ductal adenocarcinoma. Am J Transl Res 2019;11:4491-9. [PubMed]
- Yu Z, Zhang Z, Tan J, et al. Quantitative MRI Texture Analysis for Evaluating Treatment Response Following Irreversible Electroporation Ablation in Hepatocellular Carcinoma. J Vasc Interv Radiol 2023;34:S82. [Crossref]
- Liu K, Zhang Z, Hou Q, et al. Differentiation of Irreversible Electroporation Regions in Normal and Tumor Tissues Using MRI Textures. J Vasc Interv Radiol 2023;34:S25. [Crossref]
- Eresen A, Zhou K, Sun C, et al. Early assessment of irreversible electroporation ablation outcomes by analyzing MRI texture: preclinical study in an animal model of liver tumor. Am J Transl Res 2022;14:5541-51. [PubMed]
- Eresen A, Sun C, Zhou K, Shangguan J, Wang B, Pan L, et al. Correlation of histological tumor biomarkers with multivariable MRI texture model. J Vasc Interv Radiol 2022;33:S155-6. [Crossref]
- Shangguan AJ, Zhou K, Yang J, et al. Intraprocedural Transcatheter Intraarterial Perfusion (TRIP)-MRI for Evaluation of Irreversible Electroporation Therapy Response in a Rabbit Liver Tumor Model. Clin Exp Gastroenterol 2020;13:543-53. [Crossref] [PubMed]


