
Comparing surgical techniques and results of secondary ischemic mitral regurgitation: a state-of-the-art literature review
Introduction
Following current guidelines, the surgical community largely endorses the use of mitral valve replacement (MVR) for secondary ischemic mitral regurgitation (SIMR), with most patients undergoing chord-sparing surgery to replace the ischemic mitral valve (1,2). The use of restrictive mitral annuloplasty (RMA) has progressively decreased over the past 8 years. RMA was introduced in the 1980s following the recommendations of the French correction (3). However, its use has declined because the randomized clinical trial (RCT) by the Cardiothoracic Surgical Trials Network (CTSNet) reported poor outcomes (4). However, the use of the MitraClip device to perform edge-to-edge transcatheter repair, which has expanded the range of mechanical interventions available, remains controversial due to conflicting evidence regarding the benefits in terms of efficacy and safety (5,6).
Surgeons may be hesitant to use two-stage mitral valve repair, combining subvalvular repair (SVR) with RMA, due to limited evidence of clinical benefits. This is based on observational studies (OS) (7-9) and only a few RCTs (10,11). Although two-stage mitral valve repair has been shown to be safe and effective in randomized trials, with low rates of recurrent mitral regurgitation (MR) and reoperation, this combined procedure based on papillary muscle (PM) surgery has been performed in trials that were individually underpowered to detect differences in the frequency of clinical events. It is currently unclear whether SVR can improve clinical outcomes over time. Additionally, there is a contradiction between the proven benefits of handling PMs and their limited use in clinical practice. I present this article in accordance with the Narrative Review reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-24-39/rc) (Figure 1).
Methods
Search strategy and selection criteria
This seminar presents a narrative review based on the author’s knowledge. The literature search was conducted using PubMed, Medline, and Embase with the following terms: ‘mitral valve disease’, ‘secondary mitral regurgitation’, ‘secondary ischemic mitral regurgitation’, ‘functional mitral regurgitation’, ‘restrictive mitral annuloplasty’, ‘subvalvular repair’, ‘Trans Catheter Edge to Edge Repair and echocardiography coupled with secondary mitral regurgitation’, ‘secondary ischemic mitral regurgitation’, and ‘functional mitral regurgitation’. To complete the task, the following terms were added: ‘subvalvular repair, papillary muscle approximation, papillary muscle relocation, and papillary muscle sling’. The literature reviewed comprised full articles of RCTs, propensity-matched observational series, meta-analyses, and unmatched OS published in the past 10 years up to the end of March 2024. However, some frequently referenced and widely cited earlier publications, including reviews, were not excluded. Key international guidelines and expert consensus reports were reviewed to categorize documents based on their relevance to clinical and echocardiographic diagnosis, classification and management, treatment strategies, long-term follow-up and risk factors. SIMR is a complex disorder with limited randomized studies available on optimal treatment. In some cases, urgent treatment may be necessary due to the rapid deterioration of ventricular function. This can limit the possibility of designing RCTs.
Patients must be at least 18 years of age. Patients must have undergone any surgical procedure, including MVR, mitral valve repair, or transcatheter mitral valve therapy. Exclusions apply to animal or pediatric studies, as well as non-primary studies like letters and editorials. Manuscripts were also excluded if a translation was unavailable or if they were only published as abstracts (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | January 1, 2024 to March 31, 2024 |
| Databases and other sources searched | PubMed, Medline and Embase |
| Search terms used | ‘mitral valve disease’, ‘secondary mitral regurgitation’, ‘secondary ischemic mitral regurgitation’, ‘functional mitral regurgitation’, ‘restrictive mitral annuloplasty’, ‘subvalvular repair’, ‘Trans Catheter Edge to Edge Repair and echocardiography coupled with secondary mitral regurgitation’, ‘secondary ischemic mitral regurgitation’, and ‘functional mitral regurgitation’ |
| Timeframe | Up to March 2024 |
| Inclusion and exclusion criteria | Inclusion criteria: |
| • Age 18 years or older | |
| • Chronic severe ischemic and non-ischemic mitral regurgitation (ERO ≥0.4 cm echocardiogram) with tethering as a major mechanism | |
| • Symptomatic secondary mitral regurgitation (3+ or 4+ by echocardiographic laboratory assessment) due to cardiomyopathy of either ischaemic or non-ischaemic etiology | |
| • The subject has been adequately managed according to applicable guidelines, including for coronary artery disease, LV dysfunction, mitral regurgitation and heart failure | |
| • English language | |
| Exclusion criteria: | |
| • Pediatric | |
| • Any echocardiographic evidence of structural (chordal or leaflet) mitral-valve disease | |
| • Papillary muscle rupture | |
| • Infective endocarditis | |
| Selection process | One author selected articles after screening for duplicates |
ERO, effective regurgitant orifice; LV, left ventricle.
Assessment of different procedures
The literature currently uses the terms ‘functional mitral regurgitation’ or ’secondary mitral regurgitation’. The condition may be caused by either ischemic or non-ischemic factors. In this seminar, the term used to describe the pathology involving the mitral valve is ’secondary ischemic mitral regurgitation’. However, some reports also describe patients affected by non-ischemic secondary MR, which were compared with those who had SIMR.
The presented evidence is from the most comprehensive reported data series on MVR and repair, which evaluates different mitral valve repair techniques, their reported outcomes, indications, procedural use in clinical practice, clinical proofs and limitations. The report also includes follow-up of patients with severe left ventricular ejection function (LVEF) dysfunction who were indicated to receive a left ventricular assist device (LVAD) (Tables 2,3).
Table 2
| Author, year | Treatment total number | Age (years), mean ± SD | Male | Diabetes | Previous MI | NYHA class I/II preop | NYHA class I/II postop | LVEF preop (mm), mean ± SD | LVEF postop (mm), mean ± SD | LV dimension preop, mean ± SD | LV dimension postop, mean ± SD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Iung (6), 2019 | OMT 152; OMT + Mitraclip 152 | 70.6±9.9; 70.6±9.9 | 107; 120 | 39; 50 | 52; 75 | 40; 35 | 84; 80 | 32.9±6.7; 33.3±6.5 | 34±6; 30±6 | LVEDV: 134.5±33.1 mL; LVEDV: 136.2±37.4 mL |
LVEDV: 141.5±42.5 mL; LVEDV: 134.2±37 mL |
| Stone (5), 2023 | OMT 312; OMT + Mitraclip 302 | 72.8±10.5; 72.8±10.5 | 192; 201 | 123; 106 | 160; 156 | 110; 130 | 115; 171 | 31.3±9.6; 31.3±9.1 | NR; NR | LVEDV: 191.0±72.9 mL; LVEDV: 194.4 ±69.2 mL |
LVEDV: 211.4 ±94.2 mL; LVEDV: 192.2±76.5 mL |
| Michler (14), 2016 | CABG 151; CABG + RMA 150 | 65.2±11.3; 65.2±11.3 | 99; 106 | 66; 76 | 97; 103 | 84; 95 | 98; 107 | 41.2±11.6; 39.3±10.9 | 46.1±10.5; 45.6±10.0 | LVESV: 54.8±24.9 mL; LVESV: 59.6±25.7 mL |
LVESV: 41.2±20.0 mL; LVESV: 43.2±20.6 mL |
| Bouchard (22), 2014 | CABG 16; CABG + RMA 15 | 65±12; 65±12 | 14; 12 | 8; 4 | 12; 9 | 8; 8 | 15; 14 | 41.5±17.4; 45.7±11.4 | 52±2; 48±2 | LVEDD: 59±8 mm; LVEDD: 54±7 mm |
LVEDD: 53±1 mm; LVEDD: 54±1 mm |
| Chan (23), 2012 | CABG 39; CABG + RMA 34 | 70.4±7.9; 70.4±7.9 | 29; 25 | 15; 12 | 28; 27 | 26; 23 | 30; 32 | 40.3±16.1; 40.0±17.3 | NR; NR | LVEDD: 56.5±12 mm; LVEDD: 56.5±12.6 mm |
LVEDD: NR; LVEDD: NR |
| Fattouch (24), 2022 | CABG 54; CABG + RMA 48 | 66±7; 66±7 | 35; 30 | 32; 28 | 54; 48 | 15; 13 | 27; 38 | 43±9; 42±10 | 45±7; 48±8 | LVEDD: 58±7 mm; LVEDD: 59±2 mm |
LVEDD: 56±8 mm; LVEDD: 52±7 mm |
| Mihaljevic (25), 2007 | CABG 54; CABG + RMA 54 | 66±9.2; 66±9.2 | 32; 37 | 54; 53 | 31; 34 | NR; NR | NR; NR | NR; NR | NR; NR | LVEDD: 56±6 mm; LVEDD: 58±7 mm |
LVEDD: NR; LVEDD: NR |
| Goldstein (4), 2016 | MVRpl 125; RMA 126 | 68±9; 68±9 | 78; 77 | 41; 48 | 88; 99 | 48; 54 | 93; 100 | 40.0±11.0; 42.4±12.0 | 37.6±11.8; 42.5±11.8 | LVESV: 65.7±27.4 mL; LVESV: 61.1±26.2 mL |
LVESV: 60.6±39.0 mL; LVESV: 52.6±27.7 mL |
| Lorusso (26), 2013 | MVRpl 244; RMA 244 | 66.1±8; 66.1±8 | 169; 178 | 86; 89 | 244; 244 | 48; 57 | 159; 159 | 34.9±2.9; 35±3.2 | 37.7±2.7; 41.2±2.9 | LVEDV: 108±18.7 mL; LVEDV: 108±16.6 mL |
LVEDV: NR; LVEDV: NR |
| Magne (27), 2009 | MVRpl 184; RMA 186 | 66±10; 66±10 | 110; 128 | 53; 61 | NR; NR | 44; 80 | NR; NR | 40±14; 45±15 | NR; NR | LVEDD: 56±6 mm; LVEDD: 58±7 mm |
LVEDD: NR; LVEDD: NR |
| Pausch (11), 2023 | RMA + S-repair 47; RMA 47 | 61.0±14.5; 61.0±14.5 | 30; 27 | 12; 8 | 29; 28 | 8; 12 | 35; 35 | 38.1±8.4; 38.4±9.8 | 33.4±11.4; 40.4±10.9 | LVEDD: 59.8±10.2 mm; LVEDD: 58.7±9.4 mm |
LVEDD: 57.3±5.3 mm; LVEDD: 57.3±5.3 mm |
| Nappi (10), 2016 | RMA + S-repair 48; RMA 48 | 62.9±7; 62.9±7 | 28; 30 | 18; 20 | 48; 48 | 0; 0 | 29; 26 | 35.0±5.3; 35.0±3.7 | 44.1±6; 39.9±3.9 | LVEDD: 62.7±3.4 mm; LVEDD: 61.4±3.7 mm |
LVEDD: 56.5±5.7 mm; LVEDD: 60.6±4.6 mm |
| Fattouch (28), 2012 | RMA + S-repair 55; RMA 55 | 62±12; 62±12 | 32; 34 | 15; 14 | 55; 55 | 38; 39 | 46; 43 | 42±8; 42±5 | 46±5; 45±4 | LVEDD: 58±8 mm; LVEDD: 58±2 mm |
LVEDD: 50±7 mm; LVEDD: 54±8 mm |
Adapted with permission from Nappi et al. (29). SD, standard deviation; MI, myocardial infarction; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; LV, left ventricle; OMT, optimal medial therapy; LVEDV, left ventricular end diastolic volume; NR, not report; CABG, coronary artery bypass grafting; RMA, restrictive mitral annuloplasty; LVESV, left ventricular end systolic volume; LVEDD, left ventricular end diastolic dimension; MVRpl, mitral valve replacement.
Table 3
| Procedure | Long-term mortality | Hospital mortality | Reoperation | Readmission | Composite endpoint | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |||||
| MitraClip (N=454) vs. optimal medical therapy (N=464)† | 0.77 (0.40–1.49) | 0.44 | 2.87 (0.97–8.51) | 0.06 | 0.38 (0.23–0.61) | <0.001 | 0.35 (0.04–3.06) | 0.34 | 0.39 (0.09–1.73) | 0.21 | ||||
| CABG alone (N=314) vs. CABG associated with MV procedure (N=301)‡ | 1.06 (0.65–1.71) | 0.82 | 0.83 (0.32–2.12) | 0.69 | 3.07 (0.68–13.89) | 0.14 | 0.53 (0.05–5.07) | 0.58 | 0.66 (0.22–2.01) | 0.47 | ||||
| MV replacement (N=553) vs. MV repair (N=556)§ | 1.12 (0.85–1.48) | 0.43 | 1.92 (1.18–3.12) | 0.009 | 0.59 (0.35–0.98) | 0.04 | 0.45 (0.31–0.65) | <0.001 | 0.95 (0.74–1.21) | 0.68 | ||||
| Restrictive annuloplasty alone (N=103) vs. restrictive annuloplasty with subvalvular repair (N=103)¶ | 0.78 (0.35–1.73) | 0.55 | 0.70 (0.21–2.28) | 0.55 | 0.39 (0.09–1.61) | 0.19 | 0.49 (0.24–1.01) | 0.05 | 0.30 (0.15–0.62) | 0.001 | ||||
Adapted with permission from Nappi et al. (29). †, an OR >1 favors optimal medical therapy, an OR <1 favors optimal medical therapy plus MitraClip; ‡, an OR >1 favors CABG alone, an OR <1 favors CABG associated with MV procedure; §, an OR >1 favors MV repair, an OR <1 favors MV replacement; ¶, an OR >1 favors restrictive annuloplasty alone, an OR <1 favors restrictive annuloplasty with subvalvular repair. CI, confidence interval; CABG, coronary artery bypass grafting; MV, mitral valve; OR, odds ratio.
MVR
General knowledge and indications
In recent years, mitral valve repair has become more popular than replacement. However, a recent randomized trial from CTSNet suggested that replacement is superior for patients with severe ischemic mitral regurgitation (IMR). The rate of recurrent moderate or severe MR over 2 years was higher in the repair group than in the replacement group, resulting in a higher incidence of heart failure (HF) and repeat hospital admissions. Valve replacement may be more beneficial than valve repair in cases of secondary MR, as repairs are less durable in these cases. It is important to note that this study highlights the difference between primary and secondary MR as two distinct diseases (4). Importantly, RMA was confidently performed using an approved complete rigid or semi-rigid annuloplasty ring that was downsized (1 or 2 size) to correct for annular dilatation. As pointed out by the investigators the recurrence of MV regurgitation in recipients of RMA requires further investigation to understand the underlying mechanism. MR may persist or recur after RMA due to augmented leaflet tethering caused by the anterior displacement of the posterior leaflet, as well as progressive adverse global and localized LV remodeling (4).
Chordal-sparing MVR may be a superior alternative to downsized annuloplasty repair for patients with coronary artery disease (CAD) and chronic severe secondary MR due to LV systolic dysfunction <50% (Stage D) who require mitral valve surgery because of severe symptoms [New York Heart Association (NYHA) class III or IV] that persist despite best medical therapy for HF (2,4). The American College of Cardiology/American Heart Association classifies the recommendation for surgical valve replacement as Class of Recommendation (COR) 2b and Level of Evidence (LOE) B–R (2). Nearly four decades ago, a seminal paper by David et al. (30) demonstrated that integrity with continuity between the mitral annulus, the LV wall through the leaflets, the chordae tendineae, and the PM is essential for LV function after mitral valve surgery.
Procedural use in clinical practice
The use of complete chordal preservation surgery, as opposed to posterior leaflet only, results in a superior long-term reduction in LV chamber dimensions and systolic afterload (31). Several individual studies have documented various drawbacks in patients who received partial chordal preservation compared to those who underwent mitral valve repair. These disadvantages included higher operative mortality, worse LV function, and poorer long-term survival (32-34). The unique morphological characteristics likely account for the superior effectiveness of complete chordal sparing preservation in improving wall motion in the apical and diaphragmatic regions of the left ventricle (35).
The Khonsari I procedure, as described by the Stanford group (36), involves dividing the anterior mitral leaflet into two or four segments. These segments are then repositioned using valve sutures to their normal anatomical position. In an RCT, Yun et al. (31) demonstrated that both complete and partial chordal sparing MVR led to a decrease in left ventricular end diastolic volume (LVEDV). The mean change from baseline was −58±12 (P<0.0001) for total replacement and −31±5 (P<0.0001) for partial replacement. Preserving the entire subvalvular apparatus may lead to a sustained and incremental decline LVEDV at postoperative discharge. After one year, the decrease was seen to be more significant compared to partial preservation (112±41 vs. 107±28; mean change from baseline −69±8 vs. 33±16; P=0.63). Furthermore, in comparison to partial preservation, patients who underwent complete chordal preservation had a further decrease in left ventricular end systolic volume (LVESV) over time (60±13 vs. 40±11 mL; mean change from baseline −20±2 mL; P=0.0001), whereas no significant differences were noted in patients who underwent partial chordal preservation (46±24 vs. 50±20 mL; mean change from baseline 4±4 mL; P=0.9) (Figure 2).

Clinical proofs
Only one RCT has been published to assess the outcomes of complete chordal preservation vs. mitral valve repair. The trial, known as the CTSNet RCT, enrolled 251 patients in 23 countries. LV reverse remodeling, defined as LVESV at one year after randomization, was the primary endpoint. At the 2-year interim analysis, there was no meaningful group difference in LV reverse remodeling (LVESV 60.6±39.0 mL with chordal-sparing MVR vs. 52.6±27.7 mL with MV repair; mean change from baseline, −6.5 and −9.0 mL, respectively) (4). The outcome of the CTSNet trial beyond 2 years will not be published due to the study design’s limited follow-up period, which did not exceed 2 years. Currently, there is a considerable body of comprehensive data available to advocate the use of chordal-preserving MVR surgery, as it has been shown to provide an additional benefit on MR recurrence over mitral valve repair (37,38). However, nearly a decade ago, a systematic review of matched cohorts of 600 patients who underwent MV repair (n=416) or MVR (n=184) showed that the long-term risk of death was 35% greater in the replacement arm vs. the repair arm [hazard ratio (HR) =1.352; 95% confidence interval (CI): 1.131–1.618] (39).
Two largely independent studies in the past 20 years have failed to provide definitive conclusions about the relative effects of the two surgical procedures on survival (27,40). One study, which included 370 patients, reported an overall 6-year survival rate that was not statistically different between MV repair and MVR (73%±4% vs. 67%±4%; P=0.17; HR =1.2; 95% CI: 0.7–1.9; P=0.52) (39). Another study involved 1,250 patients with a mean follow-up duration of over 5 years. The study reported a 10-year survival rate of 36% for patients who underwent MVR and 33% for those who received restrictive mitral valve repair. There was no significant difference between the two groups for patients over 60 years old (P=0.34). However, the survival benefit of chordal sparing operations was less evident in patients under 60 years of age. For those who received mitral repair, the survival rate was 81%, whereas it was 55% for those who received valve replacement (P=0.0001) (27).
Limitation
The main concern regarding the surgical procedure of MVR is the potential for increased risk of impaired postoperative ejection performance when a replacement is performed without sparing the valve apparatus at the time of IMR surgery. This pathophysiology is related to the sudden increase in systolic wall stress resulting in the loss of the low impedance pathway for ejection into the left atrium and ventricular dysfunction due to fracture of the mitral valve apparatus.
Rozich et al. (41) demonstrated that mitral chordal preservation at the time of replacement resulted in a substantial reduction in the incidence of LV end-diastolic and end-systolic volume reduction, and a marked decrease in LV end-diastolic wall stress in the chordal preservation group (P<0.05). In the chordal sparring group, end-systolic wall stress declined from 95±6 to 66±6 g/cm2 (P<0.05) and in the chordal transection group, it increased from 89±9 to 111±12 g/cm2 (P<0.05). The ejection fraction remained constant before and after mitral valve surgery (from 0.63±0.01 to 0.61±0.02 g/cm2) in patients who underwent chordal-sparing surgery, but was significantly reduced in patients who did not undergo chordal-sparing surgery (from 0.60±0.02 to 36±0.02 g/cm2). However, the occurrence of severe ventricular dysfunction can be considerably lowered by careful patient eligibility and by selection of the appropriate surgical technique for IMR repair. In young patients, it may be advisable to opt for RMA with SVR instead of total chordal sparing MVR if they exhibit preoperative echocardiographic asymmetric leaflet tethering, a posterior leaflet tethering angle of less than 45 degrees, tenting height (TH) of less than 11 mm, the absence of a basal aneurysm/dyskinesis, no greater degree of LV dilation [left ventricular end diastolic dimension (LVEDD) less than 60 mmHg], and an LV sphericity index (42). Michler also found that an MV tenting area of more than 3.1 cm2, but not an LVEDD of more than 64 mm or a left ventricular end systolic dimension (LVESD) of more than 54 mm, greatly decreased the risk of sustained or repeated MR when chordal-sparing MVR was performed (25).
Mitral valve repair procedures
RMA
General knowledge and indications
RMA was pioneered in mitral valve surgery in the 1980s (3) and has been extensively applied by the surgical world treating patients with moderate to severe ischemic and non-IMR. SIMR is typically associated with regional inferior wall motion abnormalities, resulting in posterior leaflet tethering and posteriorly directed MR (Carpentier type IIIb) (Figure 3).

The results of a large series of patients with end-stage cardiomyopathy undergoing mitral valve repair were pioneered by Bolling and Bach (43,44). The purpose of RMA is to decrease the anteroposterior diameter by tightening the leaflets, thereby minimising the tenting area and favouring the restoration of normal coaptation length. In addition to adequate annular correction in the setting of mitral annular undersizing (i.e., RMA), it may also be helpful in reducing LVESD or LVESV. Hence, removing MR and reducing the size of the left ventricle can lead to a decrease in stress on the LV wall, resulting in an enhancement of LV reverse remodeling (45). The use of RMA combined with coronary artery bypass grafting (CABG) in patients with moderate secondary IMR is still a topic of ongoing controversy.
Penicka et al. (12) showed that viable myocardium can be indicated by improvements in global and regional wall motion, as well as reverse LV remodeling after CABG alone. The researchers found that postoperative relief of MR was correlated with more viable segments and less LV dyssynchrony at baseline in their study of patients with moderate SIMR who received CABG alone. The results of the CTSNet study reinforce the importance of CABG and the presence of viable myocardium, as patients who did not receive CABG had no improvement in regional wall motion. Thus, the RMA proved to be ineffective, hindering the leaflets from achieving the necessary coaptation zone to reduce TH and area (3).
Procedural use in clinical practice
The commonly accepted practice in RMA is to downsize by two ring sizes, for example, using size 28 when measuring size 32. The choice between rigid or partial bands, or flexible complete rings, as well as overcorrection by undersizing, can impact MR recurrence rates. Although there is some evidence to support a higher incidence of recurrent MR in patients undergoing partial band or flexible full ring repair (46), recurrent MR rates remain high even in patients undergoing full rigid ring repair (13,39,47). Generally, RMA involves downsizing the ring by 2 sizes if LVEDD is less than 60 mm and the LVESD is less than 55 mm. In a small RCT, the use of a double row overlapping suture was described. This technique has been shown to be effective in preventing MR recurrence at the 2-year follow-up, compared to the single row technique (48). In this population of patients with IMR, all were completely revascularized.
The majority of surgeons have conventionally favoured tight overcorrection using RMA (3,10,13,33,42,47,48). However, recent research has shown that this approach may not be appropriate due to the impact of RMA on the spatial relationship between the left ventricle and the mitral annulus (4,49). The use of RMA can exacerbate the tethering of the posterior leaflet and further disrupt this ischaemia-induced abnormal geometric spatial relationship. The persistent displacement of the lateral and posterior PM relative to the mitral annulus supports this phenomenon. From an anatomical point of view, the anterior leaflet (AL) is connected to the fibrous trigones, so that the posterior leaflet is predominantly involved in RMA, with a reduction of the mitral annular area and a lowering of the anterior-posterior dimension. Overcorrection may exacerbate the geometric mismatch between the left ventricle and the mitral annulus in the setting of sustained posterior-inferior-lateral LV wall displacement. This may worsen posterior leaflet tethering. Based on these findings, the CTSNet investigators suggested that the ratio of LVESD to prosthetic annuloplasty ring size may be useful in assessing the risk of persistent or recurrent MR. It has been shown that a ratio of LV end-systolic dimension to ring size of 2 or greater is linked to an elevated risk of sustained or recurrent MR. Overcorrection of the annular dimension may result in increased tethering between the PM and the leaflet edge. The authors stated that although RMA is a mandatory procedure, it is a weak approach to the management of severe SIMR (4,49). Understanding the pathophysiology of SIMR allows surgeons to conduct a thorough preoperative evaluation and make informed decisions in the operating room (50-54).
Clinical proofs
According to the most comprehensive echocardiographic studies, recurrence of moderate or more MR in patients ranges from 32.6% at 6 months to 56.8% at 24 months (4) and 55.9% at 5 years (10,42). A critical factor in predicting MR recurrence is the severity of preoperative MR. It is commonly accepted that the recurrence of MR should be observed in patients with centrally directed or multiple jets, a higher degree of LV enlargement, symmetric AL tethering, the presence of a basal aneurysm/dyskinesis, a coaptation height of 11 mm or more, and a posterior leaflet angle greater than 45° (44,50-55).
Kwon et al. (47) found that patients with severe SIMR who had recurrent MR after undersized MV repair had worse LVESV at 2-year follow-up than those without recurrent MR (45±10 vs. 42±10; P=0.97; mean change from baseline, −16.1 vs. −19.1 mL, respectively). Similarly, Nappi et al. (10,42) definitively demonstrated that patients with moderate to severe MR recurrence exhibited a significant worsening of LVEDD at 5-year follow-up, with a mean change from baseline of −0.2 mm. The RA group demonstrated a significant reduction in LVEDD of −2.3±4.1 compared to the papillary muscle approximation (PMA) group (−5.8±4.1, P<0.001) (10).
RMA is the valve repair procedure with the most evidence from isolated trials. There have been a number of reports of comparisons between RMA and MVR. Trials with follow-up periods longer than 5 years have consistently reported a substantially higher rate of MR recurrence for undersized mitral valve repairs compared to MVRs. However, the survival rates were substantially equivalent at longer follow-up. Two studies, an RCT (4) and a propensity-matched study (26), suggest a tendency for an observed increase in the incidence of clinical adverse events in RMA patients.
According to the data from Bishawi et al. (56), the number of surgical patients undergoing restrictive mitral valve repair (n=416) vs. partial or total chordal sparing surgery (n=106) shifted in the later years (from 2000 to 2006) over a 20-year period (from 1986 to 2006). The choice between MV repair and replacement appears to be primarily determined by the operating surgeon’s judgment and preference. This is highlighted by the fact that Wald’s χ2 test showed that surgeon choice was by far the most important factor in this decision (χ2=58.9, P=0.0001). However, the severity of MR significantly predicted the decision to undergo MVR [Wald χ2=19.9, odds ratio (OR) =3.377, 95% CI: 1.977 to 5.766, P=0.0001], with a greater proportion of patients with moderate-to-severe MR undergoing MVR compared to RMA. In addition, patients who were screened for replacement were more likely to have a better ejection fraction (Wald χ2=11.4, OR =1.277, 95% CI: 1.108 to 1.472, P=0.0007) (33).
Recently, Bishawi et al. (56) performed a propensity-matched analysis of patients with SIMR vs. non-ischemic secondary mitral regurgitation (NI-SMR). Survival was worse with SIMR vs. NI-SMR, although similar repair durability was observed. In addition, the cumulative moderate regurgitation rate was 27% at 10 years with rare severe regurgitation or mitral reoperation. Mitral repair for SIMR or NI-SMR can improve symptoms with persistent mild regurgitation in most patients up to 10 years in selected patients with relatively preserved function. The excellent outcome described in the latest publication from the Duke group appears to be related to lower preoperative LVEDD (4.3±1.1 cm) and preserved LV ejection fraction (40%±13%). These findings are consistent with those reported in the CTSNet study for patients who received RMA with lower preoperative LVESV (3). At the 2-year follow-up, 74 patients who were enrolled in the CTSNet trial and had severe IMR but no MR recurrence had left ventricles that were significantly smaller (43±26 mL/m2) compared to those who had recurrent MR post-RMA alone (63±27 mL/m2). The LVESV measures remained lower than those who underwent surgical MVR (61±39 mL/m2) (4).
Limitation
One limitation of RMA is the significant geometric deformation of the left ventricle and the associated increased leaflet tethering with adverse mitral leaflet remodeling (Figure 4). Some studies have consistently demonstrated that RMA is inadvisable whether performed with 1 or 2 sizes (4,10,11,42). The Osaka group (13,57,58) made a significant contribution to understanding the limitations of RMA. RMA transiently alleviates MR when LVEDD is under 60 mm and LVESD is under 55 mm. At a median follow-up of 66 months, LV size was smaller in subjects with severe IMR without sustained or relapsing MR after RMA compared to subjects with relapsing MR after RMA alone. Furthermore, 33 patients had improved LV function with decreased anterior-posterior PM tethering distance, AL angle, and interpapillary muscle distance (IPMD) (58).

CABG and moderate SIMR
General knowledge and indications
At present, there is no widely accepted surgical standard of care for moderate IMR at the time of CABG, and there is no consensus on a single approach. Two contrasting views exist: some experts believe that revascularization alone can lead to lower rates of MR in patients with moderate IMR. This is based on the observation that regional and global LV function and geometry are significantly enhanced after CABG (24,59). The opponents of this approach argue that revascularization alone can reduce the rate of MR. In this way, additional adverse remodeling may be prevented and the subsequent HF is likely to be reduced (59) (Figure 5).

Procedural use in clinical practice
This section discusses OS examining the differences in MR recurrence between the 2 surgical strategies. The results of studies comparing CABG with or without RMA are often inconsistent and limited by methodological or sample size limitations. Although some studies suggest that RMA may improve event-free survival, the evidence is not conclusive. Retrospective cohort studies, even if improved by statistical analysis based on the propensity score, are limited by confounding factors that are often unmeasurable. The surgeon’s decision to include a safe reserve to allow for restoration of SIMR after myocardial revascularization that results in favorable LV remodeling is the most important (12,25,59).
Clinical proofs
CABG alone vs. CABG plus RMA in moderate IMR with well-defined MR measurement criteria has been compared in four recent RCTs (14,22-24). Bouchard et al. (22) found that adding a ring did not affect the clinical outcome after CABG operation. At 12 months, there were no significant changes in echocardiographic measures of residual MR (P=0.316), LVESV (P=0.427), or LV function (P=0.204). In addition, there was no significant difference in improvement between CABG alone and the combined strategy as evaluated by the Minnesota quality of life (QOL) score and brain natriuretic peptide (BNP) measurements.
A study sponsored by the National Heart, Lung, and Blood Institute’s CTSNet (14) compared CABG alone with CABG and RMA in 301 consecutive patients. The patients were observed for a maximum of 2 years. Patients receiving the combination procedure (RMA group) had substantially lower MR recurrence rates at 1 and 2 years compared to CABG alone, with no worsening to severe MR in the RMA group [31.0% (moderate, 25.9%; severe, 5.2%) vs. 11.2% (moderate, 10.4%; severe, 0.8%); P<0.001]. No substantial improvement in 2-year mortality was observed in patients who received the combination of CABG and RMA over those who received CABG alone (survival, 10% vs. 10.6%; HR in the combined group, 0.90; 95% CI: 0.45 to 1.83; P=0.78). NYHA functional class, LV ejection fraction, survival, and major adverse cardiac and cerebrovascular events at 2 years were also similar between the two groups. The researchers found that patients in the RMA group had a prolonged hospital stay after the operation, a high number of postoperative supraventricular arrhythmias during the first year (24 vs. 11 events, P=0.04), and an increased occurrence of postoperative neurological events (14 vs. 4 events, P=0.02), which comprised metabolic encephalopathy, convulsions, transient ischemic attacks, and stroke (14).
Long-term follow-up results from the randomised trials POINT and RIME have demonstrated the benefits of RMA at the time of CABG in preventing further progression of SIMR, mitral re-intervention, and LV remodelling. Untreated SIMR was associated with a significantly higher NYHA class and rehospitalization. However, both POINT and RIME investigators reported that the association of RMA with CABG did not result in superior rates of improved death (24,59).
The RIME trial (23) enrolled 73 patients with moderate IMR, of whom 39 were assigned to CABG alone and 34 to CABG plus RMA. Over a median follow-up of 12 months, subjects who underwent the combination procedure had a 28% decline in LVESV index (LVESVI) from baseline (78.4±26.5 vs. 56.2±14.9 mL; mean baseline −22.2±25.6 mL, and 71.8±16.1 vs. 67.4 ± 20.1 mL; mean baseline −4.4±17.4 mL).
In the POINT RCT (24), 102 patients randomized to CABG alone or CABG plus RMA were evaluated for the effect of surgery on long-term outcomes. At 15-year follow-up (mean follow-up 160.4±45.5 months), the 48 patients who underwent combined RMA plus CABG showed a significant reduction in LVEDD compared with those who underwent CABG alone (54.7±6.9 vs. 51.6±6 mm; P=0.03) for the treatment of moderate MR. At 15 years, the probability of survival was 72% in the CABG-alone group and 80% in the CABG plus RMA group (P=0.18). CABG plus RMA also resulted in greater freedom from at least moderate IMR or reintervention at final follow-up (P<0.001). CABG with RMA also resulted in a lower NYHA class (P<0.001) and a lower readmission rate (P=0.002) (24).
Two meta-analyses combined results from RCTs, and large OS have evaluated the use of RMA plus CABG vs. CABG alone (22,60). Significant advantages in the prevention of MR relapse with RMA were observed in all studies with a mean follow-up of more than one year. A meta-analysis pooled nine retrospective studies, involving a total of 2,479 patients with moderate IMR (grades 2.2 to 3.9) undergoing CABG (n=1,515) or CABG plus mitral valve surgery (n=964). The study found no significant difference in long-term survival rates between patients who underwent RMA and those who received CABG alone (risk ratio =1.02; 95% CI: 0.90 to 1.14; P=0.73). However, patients who underwent RMA had a greater reduction in MR grade than those who underwent CABG alone [standardized mean difference (SMD) =−0.9; 95% CI: −1.250 to −0.559; P<0.0001]. There was no statistically significant improvement in NYHA class (SMD =−0.26; 95% CI: −0.766 to −0.24; P=0.30) (60).
The unique pooled meta-analysis of four RCTs and 15 OS with clinical and echocardiographic endpoints evaluated patients with moderate-to-severe IMR who underwent CABG alone vs. CABG combined with mitral valve surgery [18 RMA and five mitral valve surgery (MVS)] (61). CABG/MVS was not associated with increased perioperative mortality [RCT, relative risk (RR) =0.89, 95% CI: 0.26–3.02; OS, RR =1.40, 95% CI: 0.88–2.23] but was associated with fewer cardiac events (MI, HF, ischemic stroke) in restrictive annuloplasty (RA) (RR =0.49; 95% CI: 0.28–0.87; P=0.014). Patients who underwent combined CABG and MV surgery had a significantly lower incidence of moderate-to-severe MR at follow-up (RCTs, RR =0.16, 95% CI: 0.04–0.75; OS, RR =0.20, 95% CI: 0.09–0.48). Late mortality was similar between surgical approaches in RCTs (HR =1.20; 95% CI: 0.57–2.53) and OS (RR =0.99; 95% CI: 0.81–1.21). Echocardiographic findings were not substantially affected (61).
Limitation
It is worth noting that while there is no conclusive evidence to suggest MV surgery alters the natural course of dilated ischemic cardiomyopathy or improves survival rates, the use of RMA during MV surgery may result in severe complications due to increased posterior leaflet tethering (34,62). Following evidence of increasingly severe leaflet tethering due to anterior displacement of the posterior leaflet and ongoing adverse global and localised LV remodelling, concerns have been raised about the risk of re-escalation of MR associated with overcorrected RMA repair (15,63-65). This result is most likely the anatomical basis for the proven limited benefit of long-term surgical management in patients with restrictive MV repair (10,15,29,42,49-54,63-68), although such a procedure in combination with CABG in a patient with moderate IMR is still widely used in the surgical community (22,59). The majority of scientific literature relies on pairwise meta-analysis (26,29,39,60), and the absence of network meta-analysis is the primary obstacle to definitively assessing differences between groups (Figure 5).
Subvalvular procedures
General knowledge and indications
In order to restore the function and configuration of the subvalvular apparatus, subvalvular procedures are often used in addition to annuloplasty (Figures 6,7). The primary impact of SVR is to relieve the tethering forces applied to both leaflets of the MV due to lateral and posterior displacement of the PMs. In contrast to RMA, which may have an indirect effect on restoring LV geometry through conducive reverse remodelling, SVR provides a direct effect on improving LV geometry. There are several surgical procedures for dealing with the subvalvular apparatus, based on specific principles. Each approach should be chosen according to the direction of MV tethering (apical, lateral or posterior). Three techniques with possible variants are reviewed here: the PM approximation, the PM relocation, and the ring + string procedure (7,8,69,70) (Figure 6B,6C).


Procedural use in clinical practice
The ability to restore the correct spatial configuration of the left ventricle, which has been impaired by the vectorial displacement of the PMs (Figure 6B,6C), is the principle that underpins the three main surgical approaches to PM management. In fact, three main dimensions, the anteroposterior diameter of the annulus, the tenting area and the IPMD, are affected by IMR as a disorder that causes changes in the spatial configuration of the MV. The aim of performing SVR in conjunction with RMA is not only to ensure direct improvement of the IPMD, but also to monitor for overcorrection of the annuloplasty to prevent the complication of mitral valve stenosis (16,71,72). The importance of the movement of the PMs in three directions (apical, lateral and posterior) has been widely recognised by the community of cardiologists and cardiac surgeons, with the result that it guides both the indication for surgery and the choice of treatment (16,73,74). As suggested by Bothe et al. (17), posterolateral PM displacement is the prevailing pathology leading to apical leaflet tethering in SIMR.
PM approximation
Procedural use in clinical practice
As originally reported by Hvass et al. (8), the rapprochement of the PM was carried out by means of a 4 mm Goretex prosthesis. The PMs were fully approximated by guiding the prosthesis through the ventricular trabeculae. The effect of the increased chamber size of the left ventricle on the overcorrection of the PMs and the different morphology of the PM, which can have a profound effect on the surgical procedure, are not considered in the authors’ description of the technique. Instead, two key aspects were highlighted: the anatomical relevance of the PMs and the degree of approximation based on the type of tethering and the extent of ventricular enlargement. Taking into account the different anatomy of the PM, Rama et al. (70) simplified the technique. They first applied a single suture using a pledgeted piece of autologous pericardium (Figure 7A) as an optimal alternative to the 4 mm Goretex prosthesis (Figure 7D).
Our group performed SVR using 4-0 Goretex suture with pledget in cases classified as PM I, II and III and 4-0 Goretex prosthesis for types IV and V (10,42). In patients with transmural anterior LV wall scar, Wakasa et al. reported complete side-by-side PMA through an anterior LV incision. SVR and reconstruction of the LV wall was accompanied by annuloplasty of the MV (75) (Figures 6B,6C,7A,7B,7D).
Clinical proofs
The PMA RCT included 96 patients with moderate to severe MR and evaluated the effect of PMA on long-term outcomes (10,42). The 48 patients treated with a combination of SVR and RMA did not have a markedly better 5-year survival rate than those (n=48) whose IMR was treated with RMA alone (22.9% vs. 29.2%; HR =0.76; 95% CI: 0.35 to 1.68, P=0.502). At 2 years (RMA 13.2% vs. PMA 15.0%), there was no statistically significant difference between the two groups in terms of moderate to severe MR recurrence. The data shows a slightly higher percentage of surviving patients with relapsed severe MR in the RMA group compared to the PMA group at the 5-year follow-up (23.5% vs. 10.8%, P=0.153). These results demonstrate the effectiveness of RMA plus PMA in improving patient outcomes and suggest that it may be a preferable treatment option. Additionally, patients who underwent RMA in addition to SVR had a lower rate of hospital readmission for HF compared to those who underwent RMA alone at the 5-year post-operative follow-up (23.8% vs. 38%, P=0.136). However, research is needed to confirm these findings. The effect of the combined procedure on the proportion of patients with a higher number of events for MACCE at both the valvular and subvalvular levels was surprising. Over 5 years, MACCE in the last year was considerably attenuated in the PMA group (HR =0.10; 95% CI: 0.02 to 0.49; P=0.004) (10,42).
Ninety patients with SIMR were studied in another report by Wakasa et al. (75). Thirty patients underwent isolated RMA, while 60 patients underwent combined SVR without (n=26) or with (n=34) left ventriculoplasty. The linearised mortality rate including all-cause mortality and cardiac-related mortality was similar (P=0.61 and P=0.92) in patients with two-stage valvular and SVR vs. single stage valvular repair during a median follow-up of 3.4 years. In 26 patients, SVR without LV reconstruction was undertaken. This surgical approach appears to have a lower risk of grade ≥2 + MR relapse when compared to SVR combined with left ventriculoplasty or a single RMA procedure (P=0.09).
Roshanali et al. (18) compared two-stage MV repair with isolated RMA in 100 consecutive patients [74% ischaemic dilated cardiomyopathy (DCM) and 26 non-ischaemic DCM]. They were followed for a mean of 40.8 months. There appears to be a trend towards a lower relapse rate of 3+ to 4+ MR in patients who underwent two-stage MV repair with SVR/PMs compared to isolated RMA in both ischemic and non-ischemic dilated cardiomyopathy (PMA 3.4% vs. RMA ischaemic DMC 8% vs. RMA non-ischaemic DMC 11%, P=0.428). NYHA functional class at final follow-up was 1.57%±0.62% in the RMA group and 1.45%±0.57% in the combination group; there was no significant difference in NYHA functional class between baseline and final follow-up (P>0.05).
Pausch et al. (76) confirmed improved results with the use of SVR in patients with non-ischemic dilated cardiomyopathy. Relocating both PM in a standardized manner to correct SMR resulted in satisfactory in-hospital and 1-year outcomes for both SIMR-induced cardiomyopathy and NI-SMR. Patients with dilated cardiomyopathy showed a similar improvement in symptoms of HF and in LV remodelling compared to patients with SIMR.
A recent publication from Osaka Rosai Hospital (13) highlights the key relevance of IPMD (Figures 6B,6C,7A,7B,7D). The authors demonstrated the relationship between LV function, severity of MR and leaflet tethering measures after RMA. A study was conducted on 44 patients who received a stand-alone primary operation with the use of RMA between 2004 and 2015. LV function, anterior and posterior PM tethering distance, AL angle and IPMD were found to significantly decrease in 33 patients during a median follow-up of 66 months. The study found that a reduction in IPMD (from 31±6 to 25±5 mm) and posterior PM tethering (from 37±4 to 32±4 mm) was independently associated with a reduced risk of MR relapse (parameter estimate of 0.299 with a standard error of 0.110; P=0.013 and parameter estimate of −0.104 with a standard error of 0.045; P=0.035). Furthermore, the variation in IPMD was assessed independently of the difference in LV end-systolic dimension (parameter estimate of 0.299 with standard error of 0.110; P=0.013), leading to greater improvement in IPMD which is linked to more conducive reverse remodelling (13).
Limitation
The controversy surrounding the use of the two-stage MV repair, consisting of the PMA and RMA procedures, are warranted when the outcomes are examined in detail. Indeed, at 5-year follow-up, we observed a rate of rehospitalization for HF of 23.8% and a rate of moderate to severe MR of 27% [preoperative effective regurgitant orifice area (EROA) 41.0±5.3 mm2vs. a postoperative EROA 41.1±1.1 mm2]. Part of the explanation for this negative finding is that the clinical usefulness of adding PM approximation has multiple factors. Two-stage MV repair surgery, including SVR procedure, is recommended to address adverse LV remodelling in patients with a dilated left ventricle, extensive scar tissue formation, dyskinesia, or basal aneurysm. However, there are currently no prospective studies on the use of two-stage MV repair to demonstrate whether adding SVR results in improvements in LV remodelling. Postoperative tethering was not found in patients with LV lateral wall dysfunction, persistent LV dyskinesis, and severe alteration of LV sphericity, as well as predominant lateral displacement of both leaflets due to symmetric tethering (10,42,51,66).
Surgery may not directly improve prognosis in patients who have undergone a two-stage MV repair with RMA and SVR and have severe and proportionate MV regurgitation, characterized by a severely enlarged LV chamber and moderate MV regurgitation. Ischemic cardiomyopathy is the leading disease in 23.8% of patients, often irrespective of the severity of MR. At the 5-year follow-up, there was adverse global and localized LV reverse remodeling. The LVEDD was 62.7±3.4 mm compared to 63.5±2.4 mm at baseline, with a mean change of −6.4±0.49 mm. The EROA was 41.0±5.3 mm2 compared to 41.1±1.11 mm2 at baseline, with a mean change of −6.4±0.49 mm2. The results were consistent with adverse global and localized LV reverse remodeling (5,6,10,42,77). Lastly, 2 women (5%) who underwent combined surgery with two-stage MV repair required further surgical intervention between 30 days and 5 years, although there was some improvement in adverse LV reverse remodelling (mean compared with baseline −6.5±0.7 mm at 5 years). The mortality rate was higher for women with SIMR who underwent two-stage MV repair with combined SVR procedure compared to men, despite no significant differences in the degree of reverse LV remodeling between the sexes. This finding is consistent with the analysis of female participants in the CTSNet RCT (10,42,77).
PM relocation
Procedural use in clinical practice
In 2000, Liel-Cohen conducted an initial experiment (78). Kron et al. (7) were the first to describe the widespread use of posteromedial PM relocation in 18 patients with transmural infarction of the inferior wall and moderate to severe SIMR. Since then, the relocation of the PM has been used in clinical routine. The procedure requires suturing the fibrous portion of the posterior PM tip twice using 3-0 Prolene suture. To bring the posteromedial PM tip closer to the annulus, bring the suture down to the mitral annulus just posterior to the right fibrous trigone. Identify the final position of the tip of the posterior PM by locating the point at which the leaflets coaptation in the plane of the mitral annulus.
Pausch et al. (11) recently employed an innovative approach to posteromedial PM relocation consisting of the following key stages: (I) both PM are realigned apico-laterally in a standardized manner; (II) their sutures are fixed on the posterior side of the annuloplasty ring; and (III) the procedure is systematically applied in a three-dimensional endoscopic mini-thoracotomy setting (Figures 6A,7C).
Clinical proofs
The landmark trials showed no short-term mortality at 2 months and no need for reintervention with the subvalvular PM relocation procedure (11,28). In a study of 110 patients with over 48 months of follow-up, there was no difference in the overall 5-year freedom from cardiac-related death between the PM relocation arm and the isolated RMA arm (90.9%±1.8% and 89%±1.6% (P=0.82). Compared to RMA, SVR has a higher 5-year freedom from cardiac-related events rate (83%±2.1% vs. 65.4%±1.2%, P<0.001).
A total of 101 patients were studied in a single RCT. It compared two-stage MV repair using combined PM relocation and RMA with isolated RMA procedure. During a maximum follow-up period of one year, the 51 patients who underwent a two-stage mitral valve repair with PM relocation procedure achieved a significantly better 1-year survival rate than those whose IMR was treated with single-stage MV repair including isolated RMA (0% vs. 1.0%, P=0.025). Additionally, they showed a significant trend towards better event-free MR recurrence (98% vs. 86.7%, P=0.045) (11).
There are few candidates with a postero-basal myocardial infarction and asymmetric tethering who are unable to undergo a two-stage MV repair with relocation of the PM. The technique has a reduced incidence of severe complications and effectively preserves the normal three-dimensional relationship between the posterior medial PM tip, leaflets, and the annulus. To reduce mitral valve tenting, it is important to relocate both tips of the posterior PM, as suggested by Kron et al. and Fattouch et al. (7,28). Although it is possible to relocate one head of the anterolateral PM, it is important to consider the anatomical features of the posteromedial PM and the different anatomy related to types III, IV, and V. Therefore, it is crucial to relocate both heads of the papillary muscle position monitoring (PMPM). The chordae responsible for determining the seagull sign and the tenting are derived from the anterior head of the PM and are destined for the AL, whereas the chordae for the P2 and P3 scallops are derived from the posterior head of the PMPM, as shown in Figures 6A,7C. The results of the REFORM-MR (Reform-Mitral Regurgitation) registry are promising for establishing a standard approach to two-stage valve and subvalve repair. In a multicentre setting, this study defined the outcomes of treating SIMR with combined PM relocation and ring annuloplasty. It aims to enhance comprehension of the limitations of subannular repair procedures in treating patients with type III SIMR (79).
Limitation
There have been extensive studies of the biomechanical profile of SVR and the use of PM approximation or relocation has been shown to have no additional perioperative risk. However, PM relocation may have potential adverse biomechanical effects, unlike PM approximation. The previous statement does not address the fact that the PM can move in multiple directions, causing increased tension in the posterior tri-gone and PM (19,50-54,80-86). Watanabe et al. (81) demonstrated that relocating the PM may restrict the mitral valve if it is directed only towards the posterior leaflet, causing a tilting effect on the posterior annulus and increasing its posterior tethering. In the largest series by Fattouch et al. and Pausch et al., it was found that PM relocation was effective only when combined with a non-restrictive annuloplasty (11,28,76,79).
Ring plus string procedure
Procedural use in clinical practice
The ring and string procedure, which has been adopted in mitral valve surgery since the 2009 by Langer et al. (69), consists of the use of an RMA ring (RING) combined with papillary muscle repositioning (STRING). The concerns about the extent of occurrence of recurrent MR due to LV remodelling, for which TH is one of the more easily determined quantitative parameters, have been alleviated by the evidence of TH exceeding 10 mm. In these conditions, nearly all patients experience a recurrence of MR in the context of an unsuccessful reverse remodelling. It has been suggested that complementing RMA with PM repositioning may offer potential benefits for patients with secondary MR greater than grade 3 and TH greater than 10 mm (29,50,51,66,68).
An undersized ring, 1–2 sizes smaller than the intertrigonal distance, is used in RMA. The procedure begins with a horizontal aortotomy, followed by passing a double-armed Teflon pledgeted 4-0 expanded polytetrafluoroethylene (e-PTFE-Goretex) suture (STRING) through the head of the PM. Next, the suture is threaded from the LV cavity through the aorto-mitral continuity below the commissure between the noncoronary and left coronary aortic cusps and then exteriorised.
Clinical proofs
For patients with SIMR caused by uneven tethering and local LV remodeling (infero-basal scar tissue formation), a single string for the PMPM is sufficient. However, for patients with nonischemic cardiomyopathy or IMR characterized by global LV remodeling, it is more appropriate to use two strings, one for each PM (69).
Limitation
The main problem with these procedures is that the mitral subvalvular apparatus is not fully exposed with the transverse aortotomy, which can be a limiting factor. For type IV or V anatomy, the procedure aims to correct both tips of the posteromedial PM. It is recommended to add a second stitch in this case to prevent the occurrence of a Segul sign by fixing the PMPM (19,28). Although Langer et al. have published results indicating that this technique is useful and safe, it is important to note that this type of approach may not be representative for a large series of controlled patients with long-term follow-up. While Langer et al. have reported positive results regarding the efficacy and safety of this technique, it is important to note that these findings may not be representative of a larger, controlled group of patients followed over time (69).
Mitral valve leaflet repair procedures
Edge-to-edge repair
General knowledge and indications
Alfieri et al. outlined the rationale for the surgical procedure of edge-to-edge leaflet coaptation. They demonstrated that creating a double-orifice mitral valve immediately reduces the degree of MR (87). The advantage of the edge-to-edge procedure in combination with RMA is that it allows the MV to be targeted directly at the level of the jet of regurgitant flow. The durability of the mitral valve repair can be enhanced and recurrent MR can be avoided by suturing the edges of the mitral valve leaflets close together in the area of the regurgitant flow. The Alfieri procedure improves results by ensuring early closure of the MV. This is likely to be achieved by decreasing the closing forces to lower systolic tension (88). During systole, the subvalvular apparatus usually applies vertical tension to avoid leaflet prolapse, which is disturbed by the remodeling of the LV. Thus, the chordae tendineae, the PMs and the adjacent LV wall could be subjected to upward stress when the leaflets are anchored together. This action has the potential to reverse the course of adverse LV remodelling (19,82,83). For patients with MR caused by ventricular disease, whether non-ischemic or ischaemic remodelling, the edge-to-edge technique is appropriate. The procedure is valid for either disease, considering the degree of coaptation depth or TH, as there are pathophysiological analogies between the conformational spatial changes in the two phenotypes. The Alfieri procedure’s best results are achieved through careful patient choice. Therefore, when dealing with the mitral valve, the assessment of the gap between the annular plane of the MV and the point of leaflet coaptation needs to be taken into account. As a matter of fact, this parameter reflects the extent of mitral leaflet tethering, independent of LV function and tethering shape (15,64,65,89,90). For patients with TH >10 mm, some recommend using edge-to-edge surgery in addition to RMA (50,51,66,67).
Procedural use in clinical practice
Before undergoing the edge-to-edge operation, patients should have a transthoracic echocardiogram to determine the degree and mechanism of MR and to select the location of the approximating suture. Symmetric tethering is identified by a central jet located between the A2 and P2 scallops of the mitral valve, which can be treated with a central edge-to-edge procedure that results in a double-orifice MV shape. In cases of IMR where the regurgitant flow is positioned at the posterior commissure, a commissural edge-to-edge suture is used to produce a single hole MV with a proportionally lower surface area. The length of the suture is always limited to the shortest possible length to reduce the risk of postoperative MV stenosis: between a few millimetres and 1 cm in most patients. An implanted prosthetic ring, which is typically 1 or 2 sizes smaller than the AL surface (87,91-93), must be complete and either rigid or semi-rigid.
Clinical proofs
Almost 15 years ago, a pioneering study from the San Raffaele University Hospital demonstrated the survival advantage of performing concomitant RMA with edge-to-edge suturing over isolated RMA (94). The better long-term freedom from MR relapse with the edge-to-edge suture is most likely due to its superiority. While some studies have reported poor outcomes with the use of the Alfieri technique, the focus of these reports has been on the edge-to-edge procedure in the absence of simultaneous RMA. The pioneer series assessed the impact of MV repair on 77 patients with moderate to severe functional MR, either idiopathic (n=26) or ischemic (n=51), with a follow-up duration of at least 18.4 months. In 54 patients (TH >10 mm), RMA was used in combination with the edge-to-edge procedure, while 23 patients (TH <10 mm) received isolated RMA. At 2.7 years, survival was markedly better (91.4%±4.1% vs. 89.2%±7.2%, P=0.9) in the 54 patients who underwent associated edge-to-edge conservative MR repair compared to those who underwent stand-alone RMA. For patients with MR relapse ≥3+/4+, the rate of patients receiving a simultaneous edge-to-edge repair was 3.7%, while the rate of patients undergoing an individual RMA was 21.7% (P=0.03). Freedom from repair failure was 95%±3.4% and 77%±12.1%, respectively (P=0.04). In the two groups, LV end-diastolic dimensions were reduced (67 to 58 mm after RMA and 68 to 62 mm after RMA with edge-to-edge procedure) and NYHA functional class was enhanced after repair. In patients receiving simultaneous RMA and edge-to-edge repair, the use of larger rings was noted in order to avoid stenosis at the time of edge-to-edge suturing (94).
Fifty-four patients with moderate to severe functional MR due to non-ischemic idiopathic dilated cardiomyopathy were evaluated in another study. The edge-to-edge technique was used, but despite favoring reverse LV remodeling, it was not associated with a reduced risk of death at a median follow-up of 4.2 years [77.7%±9.9% associated edge-to-edge technique to RMA and 87.7%±5.8% isolated RMA (P=0.5)]. The univariate HR was 2.3; 95% CI: 0.9 to 6.1; P=0.01; multivariate HR was 1.8; 95% CI: 0.6 to 4.8; P=0.2) (95).
Limitation
Follow-up studies using echocardiography have shown that the edge-to-edge procedure can result in a significant decrease in MV area, which can cause clinically important mitral valve stenosis. This approach has been applied in patients with a ring size >36 mm and a TH >10 mm. Anterior commissure measurements were taken using an 18 mm Hegar probe, while posterior measurements used a 17 mm probe (92,93). Careful selection of the annuloplasty ring size, downsizing by one measure, is imperative to avoid significant MV stenosis (87,93-95). In cases of severe dilation of the left ventricle (LVEDD >65 mm) characterised by highly progressive LV remodelling, we did not use the edge-to-edge approach (66).
AL enlargement and cutting secondary chordae procedure
General knowledge and indications
AL augmentation was first introduced in the French correction to alleviate restricted systolic and diastolic leaflet motion in patients with rheumatic mitral valve disease (Carpentier type IIIa) (3). Since then, its use has been extended to address the leaflet coaptation defect caused by the tethering of the posterior PM (96-98). To enlarge the AL, either an autologous or bovine pericardial patch can be used. The autologous pericardial patch is typically treated with glutaraldehyde fixation prior to implantation.
Procedural use in clinical practice
Autologous pericardial patches have been used to treat Carpentier type IIIa and IIIb mitral valve pathology. This treatment has been proven effective and safe, allowing patients to lead an excellent lifestyle without the need for long-term anticoagulant treatment (98-101). A total of 90 patients (70 females, 20 males) were recorded, in which the use of anterior mitral leaflet augmentation with autologous pericardium fixed with glutaraldehyde was adjusted according to the aetiology of the patients. In 71 cases (78.9%), the dysfunction was isolated MR, and in 19 cases (21.1%), the dysfunction was combined with stenosis. Our experience has shown that even with an undersized annuloplasty, without augmentation of the AL, MR may still persist or recur due to the degree of tissue retraction that impedes leaflet coaptation (98-101). The size of the AL was assessed with the ring sizer. If the degree of leaflet retraction did not warrant reduction of the annuloplasty ring by two sizes (i.e., risk of stenosis), the AL augmentation technique was selected (48). All sutures for prosthetic ring annuloplasty (using 2-0 Ethibond from Ethicon Inc., Piscataway, NJ, USA) are placed prior to AL augmentation to minimise the risk of injury to the pericardial patch. RMA was undertaken with a flexible ring in 65 cases, a rigid ring in 16 cases, and 9 patients were managed without annuloplasty (98-102).
AL augmentation is a reproducible procedure that can be performed on both the AL (Carpentier types IIIa and IIIb) and commissural areas (Carpentier type IIIa). In order to mitigate the impact of leaflet tethering resulting from displacement of the PMs, the procedure may be combined with cutting of the secondary chordae (96,98,99,102). AL augmentation is useful when TH does not rise above 8 mm and when the LV chambers are not very dilated (LVESD <50 mm and LVEDD <60 mm). In these cases, favourable ventricular remodelling has been achieved by optimal CABG surgery. If ongoing tethering is likely, the procedure should not be performed (48,51,66).
Chordal cutting is a surgical technique that has been advocated to reduce leaflet tethering and MR in patients with ischaemic involvement of the mitral valve (96-101). Studies have shown that chordal cutting, when combined with RMA, can be beneficial for patients with chronic secondary MR caused by apical displacement and resulting in increased leaflet tethering. The procedure, which may also be performed via aortotomy (102), was aimed at achieving central coaptation during systole (96,98,99,102) (Figure 6A).
Borger et al. (102) found an echocardiographic and clinical superiority in patients who underwent chordal-cutting mitral valve repair (n=43) compared to those who underwent the use of a standard restrictive mitral valve repair. The authors observed a higher rate of tent area reduction in patients who underwent the chordal cutting procedure than in those who were treated with isolated RMA (53%±3% vs. 41%±3%; P=0.01). There was certainly greater AL mobility in the chordal cutting group which resulted in a narrowing of the gap between the free edge of the anterior mitral leaflet and the posterior LV wall (24%±3% vs. 11.4%; P=0.01). During the 2-year follow-up period, RMA alone had a higher incidence of relapsed MR, as shown by both univariate (37% vs. 15%; P=0.03) and multivariate Cox regression analysis (P=0.03). Furthermore, the use of chordal cutting did not have a negative impact on postoperative left ventricular ejection fraction (10%±5% relative increase in left ventricular ejection fraction vs. 11%±6% in the control group; P=0.9). For patients who exhibit a highly enlarged LV cavity, chordal cutting combined with AL enlargement is recommended (96).
Transcatheter edge-to-edge mitral valve repair
Studies have proven the safety and efficacy of transcatheter edge-to-edge repair (TEER) in patients with symptomatic moderate-to-severe secondary MR (5,6). This type of device implantation is based on percutaneous access and the use of the edge-to-edge technique (87,91-95) with the application of 2/3 metal clips (i.e., MitraClip procedure, Abbott Vascular, Santa Clara, CA, USA) (5,6). Thus, TEER, used in combination with the MitraClip system, has become an accepted minimally invasive procedure and offers an additional option for MV repair in SMR. For patients with significant MR who are not eligible for standard surgical intervention, heart teams may consider TEER based on the results of two randomized controlled trials (COAPT and MITRA-FR) (5,6). These trials evaluated the safety and effectiveness of TEER in patients with symptomatic HF and severe persistent MR despite best medical treatment (BMT) alone. The COAPT RCT findings showed that the device was safe and effective in lowering secondary MR over a 5-year follow-up period (5). However, results from the MITRA-FR trial (6) indicated that using MitraClip did not have a beneficial effect on the primary endpoint of all-cause mortality or HF hospitalisation at 12 months and 2 years when compared to using guideline-directed medical therapy (GDMT) only. According to the COAPT study (5), the use of MitraClip significantly reduced hospitalizations in HF patients requiring rehospitalization. The study also showed effectiveness for several secondary endpoints, including 2-year mortality from all causes.
Conflicting results from two RCT have sparked a heated debate. The discrepancies may in part be due to different study designs, resulting in heterogeneous patient populations. In addition, researchers have identified the effect size of the trials, the echocardiographic assessment of the severity of MR, and the use of optimised medical therapy as important points of discussion. In COAPT, patients had more severe secondary MR EROA of 41±15 mm2 compared to 31±10 mm2) and less LV enlargement (mean indexed end-diastolic volume of LV 101±34 mL/m2 compared to 135±35 mL/m2) than patients enrolled in MITRA-FR. The disproportionate severity of SMR in relation to LV size in patients in the COAPT trial may explain the difference in results. Patients in this trial were more likely to benefit from TEER in terms of reduced death and hospitalisation for HF (103). Therefore, further analysis is needed to understand the differences observed. Therefore, caution should be exercised when applying TEER to patients classified as intermediate or low risk (1,2,104) due to the inconsistency of results.
LVAD: when and how to use it
Previous studies have demonstrated that secondary IMR is a severe condition. Patients with deteriorating LV function may not respond to medical or conventional mechanical interventions, resulting in a poor long-term outcome. Those with worse LV remodeling are at risk of developing further HF and have an unfavourable pathological profile that can lead to further progression of LV dysfunction. For these patients, using the TEER procedure may sometimes not be the best option, although it has the advantage of avoiding the perioperative risks of the standard procedure (5,6).
In patients with more severely dilated LV cavities, the efficacy of the MitraClip compared to GDMT has shown inconsistent results in both the MITRA-FR (6) and COAPT (5) studies. In these patients, the multidisciplinary heart team should consider LVAD implantation as an alternative to LVAD therapy. Patients with more severe LV dysfunction who fall within the pathophysiological condition of proportionate, the multidisciplinary Heart Team should consider LVAD implantation as a reasonable option. The use of LVAD has improved over the past decade, resulting in better survival rates for patients who undergo the implant procedure. Technological advances in the armamentarium platform that underpins the LVAD implant surgery have paralleled these improvements. The multidisciplinary approach of the Heart Team has also improved patient selection, perioperative management and outpatient care.
Patients with severe cardiac HF treated with ventricular assist devices had better outcomes than those receiving GDMT, as evidenced by significant improvements in life expectancy (105,106). After LVAD implantation, survival rates of approximately 75% at 1 year have been observed (107). In some cases, LVAD surgery combined with mitral valve repair is indicated (108). However, because MR is minimal during LVAD surgery due to continuous aspiration of the device, this concomitant procedure is not performed in most centres. During weaning from the device, mild to moderate/severe MR may occur. There is a risk of thromboembolic events related to anticoagulation, bleeding and infection in patients with secondary MR who have undergone LVAD implantation. It is recommended to consider LVAD implantation before the right ventricular function deteriorates (109,110).
Conclusions
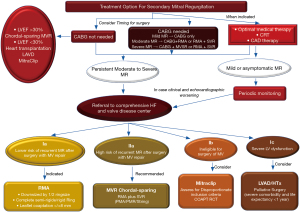
There is still no agreed strategy to determine how best to manage patients presenting with secondary IMR. Medical therapy and guideline-recommended combination resynchronisation therapy with device implementation remain the first-line treatment for these patients (1,2). Patients should be referred to HF referral centres if they are not benefiting from optimal medical therapy (1,2).
The care of patients with secondary MR must be managed by a multidisciplinary heart team consisting of experts in HF, interventional cardiology, arrhythmia cardiology, cardiac surgery and HF management. The team’s coordinated work aims to guide the patient through the best available therapeutic pathway (1,2) (Figure 8). For those with advanced disease and a life expectancy of <1 year, and for those with severe co-morbidities, referral to palliative care should be considered (1,2). The patient’s clinical course may be different and not restricted to functional mitral regurgitation (FMR) surgery. This may require additional interventions such as CABG, management of severe tricuspid regurgitation and direct mechanical intervention to correct arrhythmias.

Although largely driven by retrospective cohort studies, MV repair has long been accepted as the gold standard for clinical benefit. A veil of controversy surrounding the use of MV repair has been dispelled by the findings of the CTSNet RCTs (1,2,4,14), which support MVR in severe MR (4). Contrary to much of the published literature on this topic, the findings of this seminar assert that mitral-valve repair does not necessarily have several advantages over replacement. The literature reports lower operative mortality, improved LV function, and higher rates of long-term survival for mitral-valve repair (32-34,40,56). However, patients undergoing mitral-valve replacement tend to be older and have more coexisting illnesses than those undergoing repair. Therefore, adjustment for baseline differences has been critical in nonrandomized studies. However, it is important to note that even with the most advanced methods, there may still be unknown or unmeasured risk factors. It is worth mentioning that some studies have found no significant difference in short-term or long-term survival rates between repair and replacement groups (27), but the majority of studies have favored repair. Valve replacement with chordal sparing improves results compared to previous studies (32-34,40,56). Retaining the internal architectural support of the left ventricle preserves contractile efficiency and reduces LV dilatation and dysfunction (1,31,36). Goldstein et al. (4) found that moderate MR recurrence remained a progressive and excessive hazard for patients who underwent mitral valve repair. During the 2-year follow-up period, a staggering 58.8% of patients in the repair group experienced moderate or severe regurgitation, in stark contrast to the mere 3.8% in the replacement group. These findings demonstrate the clear superiority of MVR over repair in mitigating the risks associated with MR. The lack of durability in correcting MR is a significant concern due to the increased risk of HF, atrial fibrillation, and the need for repeat interventions and hospitalizations (2). Goldstein’s findings (4) indicate that patients in the repair group experienced more serious adverse events related to HF and were readmitted to the hospital for cardiovascular causes. The results of the Minnesota Living with Heart Failure Questionnaire were consistent with clinical events, and they were not inconclusive. The replacement group showed an average improvement of 7.9 points from baseline, which exceeded the 5-point threshold for clinically significant improvement, as reported in the study by Rector et al. (111). However, it was not statistically significant (P=0.07).
Likewise, a meta-analysis compared the use of subvalvular procedures plus RMA to RMA alone and found that adding the double-stage level repair, which includes treating the PMS, resulted in a significantly lower frequency of MR ≥2 (OR =0.27; 95% CI: 0.19 to 0.38; P=0.0001) (60). Two recent pairwise meta-analyses have focused on either mortality rates or a range of clinical outcomes following surgical intervention for SIMR (29,39). Our meta-analysis indicates that MVR is linked to higher early mortality rates but lower reoperation and readmission rates compared to RMA for moderate-to-severe SIMR. Although isolated outcomes comparing valvular and adjunct subvalvular procedures do not show significant advantages, the inclusion of subvalvular procedures reduces the likelihood of significant postoperative adverse events. According to Vassileva et al. (39), patients who received MVR had a significantly higher likelihood of short-term mortality (summary OR =2.667; 95% CI: 1.859–3.817) and long-term mortality (summary HR =1.352; 95% CI: 1.131–1.618) compared to those treated with RMA.
For the subset of patients at high risk of adverse LV remodelling and/or relapse of MR, MV repair is not warranted. For patients with minimal LV geometric deformation (LVEDD <60 mm; LVESD <55 mm; TH between 5 and 8 mm), RMA is advised either with or without AL augmentation, provided that full revascularisation of the ischaemic myocardium is also achieved in case of non-extensive post-infarct scarring. The spread of the infarct area into the lateral territories supplied by the circumflex artery usually results in more extensive scarring, which is responsible for increased apical tethering. In such cases, the TH will exceed 8 mm, limiting the potential for a successful RMA. A composite procedure with two-stage level repair of the valvular (i.e., RMA) and subvalvular (i.e., SVR) components of the MV is indicated. It is important to note that in addition to the RMA, other surgical techniques that may be considered in this setting include the edge-to-edge technique and the RING + STRING technique. However, these techniques have not been widely adopted, resulting in a paucity of long-term evidence, although they may potentially improve long-term prognosis in terms of freedom from secondary ischaemic MR recurrence and reversal of LV remodelling (51,66).
In patients with moderate SIMR, RMA should be used in combination with CABG surgery if there is extensive post-infarct scarring, especially if SVR is required due to accentuated geometric ventricular deformation such as apical leaflet tethering (10,25,42,110). For patients with ischemic SIMR and a positive LV remodelling, CABG surgery alone is advised (14,22,23). Grayburn et al. (103,104) and Bartko et al. (20,21) identified and discussed a population of patients with proportionate MR who present with a highly dilated left ventricle, worse LV function and remodelling, and are therefore more challenging to manage. For patients in this group who have mainly NI-SMR due to underlying cardiomyopathy, it appears that neither the use of transcatheter mitral valve therapy nor the use of standard surgical mitral valve therapy leads to good outcomes due to a higher tendency for cardiomyopathy to progress (5,6,20,21,103,104).
The novel conceptualization of SMR pathology proposed by Grayburn et al. (103,104) has renewed attention on the ‘MV-LV unit’ and their functional relationships. It is important to consider these aspects. They introduced the concept of proportionate or disproportionate MR in relation to LV size to explain the prognosis of SMR and the differing results of the MITRA-FR (6) and COAPT (5) studies. In cases of significantly enlarged left ventricle, EROA values of approximately 0.2 can be accepted as proportionate and consistent with the degree of LV dilatation. This means that the severity of MR is a direct result of adverse remodeling and dilation of the left ventricle, and such EROA values are expected in these cases. In patients with MR severity proportional to the degree of LV enlargement, interventions targeting the MV are unlikely to be successful as they do not address the primary pathology (Figure 9, Tables 4,5). However, strategies to improve LV remodeling through medical therapy may be more promising, as demonstrated by the MITRA-FR trial. On the other hand, a disproportionately high degree of MR compared to the degree of LV enlargement suggests a repairable deficiency of the MV and may lead to better outcomes with primary valve intervention (103,104).

Table 4
| LVEDV (mL) | EROA (mm2) | EROA/LVEDV | |||
|---|---|---|---|---|---|
| Lower limit | Upper limit | Lower limit (mean: 1.136) |
Upper limit (mean: 1.603) |
||
| 100 | 115 | 161 | 1.15 | 1.61 | |
| 200 | 223 | 315 | 1.115 | 1.575 | |
| 250 | 284 | 400 | 1.136 | 1.6 | |
| 350 | 400 | 570 | 1.143 | 1.628 | |
†, data are extracted from Grayburn et al. (104). EROA, effective regurgitant orifice area; LVEDV; left ventricular end diastolic volume.
Table 5
| EROA/LVEDV ratio | MR classification | Clinical implication |
|---|---|---|
| >1.6 | Severe, disproportionate | Intervention on the mitral valve is beneficial |
| 1.1–1.6 | Severe, proportionate | Intervention on the mitral valve is not beneficial |
| < 1.1 | Non-severe | Intervention on the mitral valve is not indicated |
†, data are extracted from Grayburn et al. (104). EROA, effective regurgitant orifice area; LVEDV, left ventricular end diastolic volume; MR, mitral regurgitation.
The surgical literature on IMR has extensively investigated these concepts. Many of the proposed clinical reflections are supported by basic studies investigating surgical intervention outcomes. Across the literature, several geometrical laws have been applied to explain the success or failure of RMA (7,11,19,54,58). There has been increased interest in subvalvular apparatus surgery, such as PMA (11,50) and PM sling (8), as a result of the need for a more holistic approach to treating MR that includes both the MV and LV with subvalvular apparatus. These procedures aim to relieve the geometric stress on the LV and the valve tethering caused by an asymmetrically dilated LV.
Considerable effort has been dedicated to determining the optimal level of annular restriction required for mitral annuloplasty, taking into account the potential risks of stenosis, systolic anterior motion (SAM), and recurrent MR associated with overcorrection or excessive undersizing (10,57). Biomechanically, undersizing the annulus would result in forces on the heart’s fibrous skeleton that oppose the expected displacement vectors relative to LV dilation in these patients. The imbalance of internal forces within the ventricle can lead to a recurrence of MR. This is due to a shift towards an unstable mechanical equilibrium (54,74,112).
The stress on the mitral valve after surgery can be calculated as the difference between the pre-operative and post-operative mitral annulus diameter, divided by the left ventricular end-diastolic diameter. This calculation results in the mitral annulus stress index (MASI) (19,74,82,85) which is an easily measurable clinical indicator of the geometrical changes to the MV after annuloplasty (19). MASI has been shown to predict the recurrence of MR after repair (Figure 10). MASI simplifies the contrast between the conflicting geometrical vectors of the undersized annulus and the progressively dilating LV. This concept prompted the surgical community to address not only the valve but also the subvalvular apparatus by reducing the interpapillary distance. This was necessary to attenuate the detrimental effect of LV dilation and avoid repair failure (7,8,10,19,113).

The MASI index closely resembles and complements the concept enunciated by Grayburn et al. (103,104). A highly dilated LV indicates lack of coaptation. Since the pathology relies on the LV itself, any action on the MV will not influence the poor long-term outcomes. Restricting the mitral annulus or clipping the leaflets alone cannot restore proper valve function without additional intervention to reverse LV remodeling. Additionally, the more the mitral annulus is restricted in the context of a dilating left ventricle (i.e., high MASI index) (19), the greater the mechanical stress on the mitral valve-left ventricle unit, which can have negative long-term outcomes (51,53,80,82,85).
Braun et al. (114) reported that restrictive annuloplasty alone was insufficient to induce LV reverse remodeling for LVEDD >65 mm and LVESD >55 mm, necessitating subvalvular or ventricular adjunct procedures. These cutoff values were also predictors for late mortality, with a 5-year survival rate of 80% for LVEDD <65 mm compared to 49% for LVEDD >65 mm. A randomized study compared PMA to RMA alone for IMR and found a significant reduction in LVDD in the PMA group (LVEDD was 56.5±5.7 mm with PMA vs. 60.6±4.6 mm with RMA; P<0.001) after 5 years. This indicates long-term beneficial LV remodeling when subvalvular techniques are used. Kim et al. (115) showed that IPMD affects leaflet tethering, mitral tenting volume, and IMR severity in patients with LV dysfunction. Roshanali et al. (116) found that a preoperative interpapillary distance of ≥20 mm reliably predicts late MR after annuloplasty.
Kalra et al. (73) investigated the changes in interpapillary muscle dynamics in IMR. Regional myocardial ischemia causes the loss of lateral shortening in IPMD, leading to tethering of the leaflet edges and impaired systolic closure, resulting in MR. Even in small ventricles (103,104), there was evidence of disproportionate IMR, as Grayburn et al. have advocated. Shudo et al. (113) conducted a study comparing PM sling with RMA alone, and found that the latter was unable to significantly reduce IPMD postoperatively, resulting in increased TH. A significant correlation was found between the postoperative decrease in TH and the postoperative improvement in IPMD as a result of PM sling (113).
It appears that the most important predictor of prognosis in SIMR is the ability or the residual capacity of the LV to positively remodel and IPMD could be considered in this context as its surrogate, indicating the entity of such remodeling. If SIMR long-term treatment relies on LV adaptive abilities, selecting interventions able to modify the ventricular environment predisposing and assisting positively the LV towards reverse remodeling is paramount.
This is possible by a careful selection of the patients as elegantly demonstrated by Grayburn et al. (103,104) and with a “proportionated” action on the valve and/or the subvalvular apparatus, considering the MV-LV in its entirety. Unfortunately, in the context of percutaneous mitral intervention is difficult to establish the degree of annular restriction exerted by the mitral clip and its impact on LV geometry. However, the lesson learned from interventional cardiology adds a clinical reflection on what was simply considered as product of geometrical calculations by surgeons and can better elucidate the indications and the selection of patients and type of intervention to be performed. In this context, the use of the MitraClip procedure is recommended in patients with disproportionate secondary MR, in which severe MR is associated with non-severely dilated LV chambers (103,104). However, this proposal does not have unanimous consensus and requires further investigation, including transcatheter heart valve therapy (117-127).
Treating patients with advanced HF with a continuous-flow LVAD significantly improves the odds of stroke and device failure-free survival at 2 years compared with a pulsatile device. Additionally, both devices significantly improve quality of life and functional capacity (105). Mitral valve repair improves survival and reduces the incidence of HF events in patients undergoing continuous-flow LVAD (109). Pawale et al. (128) observed that concurrent mitral valve repair during LVAD implantation can be safely performed without an increase in perioperative adverse events. This repair is associated with a better reduction in the severity of MR and has the potential benefit of reducing readmissions for HF. Although concomitant MV surgery during LVAD implantation does not seem to have a significant impact on postoperative outcomes, it is important to consider patient-specific factors and characteristics when making decisions regarding MV surgery. Further research is necessary, particularly with prospective studies focusing on specific patient populations and newer LVAD devices, to provide more robust evidence and guide clinical practice in the management of valvular lesions in LVAD recipients. While there may be some debate on the impact of concomitant mitral valve surgery during LVAD implantation, it appears that postoperative outcomes, including early (OR =1.17; 95% CI: 0.63 to 2.17; P=0.63) and late survival (OR =0.83; 95% CI: 0.53 to 1.29; P=0.40) are not significantly affected, it is important to consider individual patient factors and characteristics when making the decision to perform MV surgery. Further research is necessary, particularly with prospective studies focusing on specific patient populations and newer LVAD devices, to provide more robust evidence and guide clinical practice in the management of valvular lesions in LVAD recipients (129).
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-24-39/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-24-39/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-24-39/coif). F.N. serves as an unpaid editorial board member of Annals of Translational Medicine from March 2023 to February 2025. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Writing Committee Members. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2021;77:e25-197. Erratum in: J Am Coll Cardiol 2021;77:509; J Am Coll Cardiol 2021;77:1275; J Am Coll Cardiol 2023;82:969. [Crossref] [PubMed]
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561-632. [Crossref] [PubMed]
- Braunberger E, Deloche A, Berrebi A, et al. Very long-term results (more than 20 years) of valve repair with carpentier's techniques in nonrheumatic mitral valve insufficiency. Circulation 2001;104:I8-11. [Crossref] [PubMed]
- Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N Engl J Med 2016;374:344-53. [Crossref] [PubMed]
- Stone GW, Abraham WT, Lindenfeld J, et al. Five-Year Follow-up after Transcatheter Repair of Secondary Mitral Regurgitation. N Engl J Med 2023;388:2037-48. [Crossref] [PubMed]
- Iung B, Armoiry X, Vahanian A, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation: outcomes at 2 years. Eur J Heart Fail 2019;21:1619-27. [Crossref] [PubMed]
- Kron IL, Green GR, Cope JT. Surgical relocation of the posterior papillary muscle in chronic ischemic mitral regurgitation. Ann Thorac Surg 2002;74:600-1. [Crossref] [PubMed]
- Hvass U, Joudinaud T. The papillary muscle sling for ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2010;139:418-23. [Crossref] [PubMed]
- Furukawa K, Yano M, Ishii H, et al. Clinical Outcomes of a Customized Mitral Valve Plasty for Functional Mitral Regurgitation with a Low Ejection Fraction and Implications for Preoperative Right Ventricular Function. Ann Thorac Cardiovasc Surg 2021;27:32-40. [Crossref] [PubMed]
- Nappi F, Lusini M, Spadaccio C, et al. Papillary Muscle Approximation Versus Restrictive Annuloplasty Alone for Severe Ischemic Mitral Regurgitation. J Am Coll Cardiol 2016;67:2334-46. [Crossref] [PubMed]
- Pausch J, Harmel E, Reichenspurner H, et al. Subannular repair in secondary mitral regurgitation with restricted leaflet motion during systole. Heart 2023;109:1394-400. [Crossref] [PubMed]
- Penicka M, Linkova H, Lang O, et al. Predictors of improvement of unrepaired moderate ischemic mitral regurgitation in patients undergoing elective isolated coronary artery bypass graft surgery. Circulation 2009;120:1474-81. [Crossref] [PubMed]
- Kainuma S, Funatsu T, Kondoh H, et al. Beneficial effects of restrictive annuloplasty on subvalvular geometry in patients with functional mitral regurgitation and advanced cardiomyopathy. J Thorac Cardiovasc Surg 2018;156:630-638.e1. [Crossref] [PubMed]
- Michler RE, Smith PK, Parides MK, et al. Two-Year Outcomes of Surgical Treatment of Moderate Ischemic Mitral Regurgitation. N Engl J Med 2016;374:1932-41. [Crossref] [PubMed]
- Zhu F, Otsuji Y, Yotsumoto G, et al. Mechanism of persistent ischemic mitral regurgitation after annuloplasty: importance of augmented posterior mitral leaflet tethering. Circulation 2005;112:I396-401. [Crossref] [PubMed]
- Topilsky Y, Vaturi O, Watanabe N, et al. Real-time 3-dimensional dynamics of functional mitral regurgitation: a prospective quantitative and mechanistic study. J Am Heart Assoc 2013;2:e000039. [Crossref] [PubMed]
- Bothe W, Timek TA, Tibayan FA, et al. Characterization of 3-dimensional papillary muscle displacement in in vivo ovine models of ischemic/functional mitral regurgitation. J Thorac Cardiovasc Surg 2019;157:1444-9. [Crossref] [PubMed]
- Roshanali F, Vedadian A, Shoar S, et al. Efficacy of papillary muscle approximation in preventing functional mitral regurgitation recurrence in high-risk patients with ischaemic cardiomyopathy and mitral regurgitation. Acta Cardiol 2013;68:271-8. [Crossref] [PubMed]
- Nappi F, Nenna A, Spadaccio C, et al. Predictive factors of long-term results following valve repair in ischemic mitral valve prolapse. Int J Cardiol 2016;204:218-28. [Crossref] [PubMed]
- Bartko PE, Arfsten H, Heitzinger G, et al. A Unifying Concept for the Quantitative Assessment of Secondary Mitral Regurgitation. J Am Coll Cardiol 2019;73:2506-17. [Crossref] [PubMed]
- Bartko PE, Heitzinger G, Arfsten H, et al. Disproportionate Functional Mitral Regurgitation: Advancing a Conceptual Framework to Clinical Practice. JACC Cardiovasc Imaging 2019;12:2088-90. [Crossref] [PubMed]
- Bouchard D, Jensen H, Carrier M, et al. Effect of systematic downsizing rigid ring annuloplasty in patients with moderate ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2014;147:1471-7. [Crossref] [PubMed]
- Chan KM, Punjabi PP, Flather M, et al. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation: final results of the Randomized Ischemic Mitral Evaluation (RIME) trial. Circulation 2012;126:2502-10. [Crossref] [PubMed]
- Fattouch K, Dioguardi P, Guccione F, et al. 10-Year Results of Mitral Repair and Coronary Bypass for Ischemic Regurgitation: A Randomized Trial. Ann Thorac Surg 2022;113:816-22. [Crossref] [PubMed]
- Mihaljevic T, Lam BK, Rajeswaran J, et al. Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. J Am Coll Cardiol 2007;49:2191-201. [Crossref] [PubMed]
- Lorusso R, Gelsomino S, Vizzardi E, et al. Mitral valve repair or replacement for ischemic mitral regurgitation? The Italian Study on the Treatment of Ischemic Mitral Regurgitation (ISTIMIR). J Thorac Cardiovasc Surg 2013;145:128-39; discussion 137-8. [Crossref] [PubMed]
- Magne J, Girerd N, Sénéchal M, et al. Mitral repair versus replacement for ischemic mitral regurgitation: comparison of short-term and long-term survival. Circulation 2009;120:S104-11. [Crossref] [PubMed]
- Fattouch K, Lancellotti P, Castrovinci S, et al. Papillary muscle relocation in conjunction with valve annuloplasty improve repair results in severe ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2012;143:1352-5. [Crossref] [PubMed]
- Nappi F, Antoniou GA, Nenna A, et al. Treatment options for ischemic mitral regurgitation: A meta-analysis. J Thorac Cardiovasc Surg 2022;163:607-622.e14. [Crossref] [PubMed]
- David TE, Uden DE, Strauss HD. The importance of the mitral apparatus in left ventricular function after correction of mitral regurgitation. Circulation 1983;68:II76-82. [PubMed]
- Yun KL, Sintek CF, Miller DC, et al. Randomized trial comparing partial versus complete chordal-sparing mitral valve replacement: effects on left ventricular volume and function. J Thorac Cardiovasc Surg 2002;123:707-14. [Crossref] [PubMed]
- Micovic S, Milacic P, Otasevic P, et al. Comparison of valve annuloplasty and replacement for ischemic mitral valve incompetence. Heart Surg Forum 2008;11:E340-5. [Crossref] [PubMed]
- Milano CA, Daneshmand MA, Rankin JS, et al. Survival prognosis and surgical management of ischemic mitral regurgitation. Ann Thorac Surg 2008;86:735-44. [Crossref] [PubMed]
- Silberman S, Oren A, Klutstein MW, et al. Does mitral valve intervention have an impact on late survival in ischemic cardiomyopathy? Isr Med Assoc J 2006;8:17-20. [PubMed]
- Natsuaki M, Itoh T, Tomita S, et al. Importance of preserving the mitral subvalvular apparatus in mitral valve replacement. Ann Thorac Surg 1996;61:585-90. [Crossref] [PubMed]
- Sintek CF, Pfeffer TA, Kochamba GS, et al. Mitral valve replacement: technique to preserve the subvalvular apparatus. Ann Thorac Surg 1995;59:1027-9. [Crossref] [PubMed]
- Gelsomino S, Lorusso R, De Cicco G, et al. Five-year echocardiographic results of combined undersized mitral ring annuloplasty and coronary artery bypass grafting for chronic ischaemic mitral regurgitation. Eur Heart J 2008;29:231-40. [Crossref] [PubMed]
- McGee EC, Gillinov AM, Blackstone EH, et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2004;128:916-24. [Crossref] [PubMed]
- Vassileva CM, Boley T, Markwell S, et al. Meta-analysis of short-term and long-term survival following repair versus replacement for ischemic mitral regurgitation. Eur J Cardiothorac Surg 2011;39:295-303. [Crossref] [PubMed]
- Thourani VH, Weintraub WS, Guyton RA, et al. Outcomes and long-term survival for patients undergoing mitral valve repair versus replacement: effect of age and concomitant coronary artery bypass grafting. Circulation 2003;108:298-304. [Crossref] [PubMed]
- Rozich JD, Carabello BA, Usher BW, et al. Mitral valve replacement with and without chordal preservation in patients with chronic mitral regurgitation. Mechanisms for differences in postoperative ejection performance. Circulation 1992;86:1718-26. [Crossref] [PubMed]
- Nappi F, Spadaccio C, Nenna A, et al. Is subvalvular repair worthwhile in severe ischemic mitral regurgitation? Subanalysis of the Papillary Muscle Approximation trial. J Thorac Cardiovasc Surg 2017;153:286-295.e2. [Crossref] [PubMed]
- Bolling SF, Deeb GM, Brunsting LA, et al. Early outcome of mitral valve reconstruction in patients with end-stage cardiomyopathy. J Thorac Cardiovasc Surg 1995;109:676-82; discussion 682-3. [Crossref] [PubMed]
- Bach DS, Bolling SF. Early improvement in congestive heart failure after correction of secondary mitral regurgitation in end-stage cardiomyopathy. Am Heart J 1995;129:1165-70. [Crossref] [PubMed]
- Tibayan FA, Rodriguez F, Langer F, et al. Undersized mitral annuloplasty alters left ventricular shape during acute ischemic mitral regurgitation. Circulation 2004;110:II98-102. [Crossref] [PubMed]
- Hu X, Zhao Q. Systematic evaluation of the flexible and rigid annuloplasty ring after mitral valve repair for mitral regurgitation. Eur J Cardiothorac Surg 2011;40:480-7. [Crossref] [PubMed]
- Kwon MH, Lee LS, Cevasco M, et al. Recurrence of mitral regurgitation after partial versus complete mitral valve ring annuloplasty for functional mitral regurgitation. J Thorac Cardiovasc Surg 2013;146:616-22. [Crossref] [PubMed]
- Nappi F, Spadaccio C, Chello M, et al. Double row of overlapping sutures for downsizing annuloplasty decreases the risk of residual regurgitation in ischaemic mitral valve repair. Eur J Cardiothorac Surg 2016;49:1182-7. [Crossref] [PubMed]
- Capoulade R, Zeng X, Overbey JR, et al. Impact of Left Ventricular to Mitral Valve Ring Mismatch on Recurrent Ischemic Mitral Regurgitation After Ring Annuloplasty. Circulation 2016;134:1247-56. [Crossref] [PubMed]
- Nappi F, Avatar Singh SS, Santana O, et al. Functional mitral regurgitation: an overview for surgical management framework. J Thorac Dis 2018;10:4540-55. [Crossref] [PubMed]
- Nappi F, Lusini M, Avtaar Singh SS, et al. Risk of Ischemic Mitral Regurgitation Recurrence After Combined Valvular and Subvalvular Repair. Ann Thorac Surg 2019;108:536-43. [Crossref] [PubMed]
- Nappi F. A Geometric Approach to Ischemic Mitral Regurgitation: Evaluating the Evidence of Valve Distortion. Ann Thorac Surg 2020;109:982. [Crossref] [PubMed]
- Nappi F, Spadaccio C, Mihos CG, et al. Biomechanics raises solution to avoid geometric mitral valve configuration abnormalities in ischemic mitral regurgitation. J Thorac Dis 2017;9:S624-8. [Crossref] [PubMed]
- Fraldi M, Spadaccio C, Mihos CG, et al. Analysing the reasons of failure of surgical mitral repair approaches-do we need to better think in biomechanics? J Thorac Dis 2017;9:S661-4. [Crossref] [PubMed]
- Lancellotti P, Moura L, Pierard LA, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010;11:307-32. [Crossref] [PubMed]
- Bishawi M, Milano C, Gaca J, et al. Late Durability of Mitral Repair for Ischemic Versus Nonischemic Functional Mitral Regurgitation. Ann Thorac Surg 2022;114:1358-65. [Crossref] [PubMed]
- Kainuma S, Taniguchi K, Daimon T, et al. Does stringent restrictive annuloplasty for functional mitral regurgitation cause functional mitral stenosis and pulmonary hypertension? Circulation 2011;124:S97-106. [Crossref] [PubMed]
- Yuh DD. What lies beneath (the mitral annulus): The geometric implications of restrictive annuloplasty. J Thorac Cardiovasc Surg 2018;156:639-40. [Crossref] [PubMed]
- Roshanali F, Mandegar MH, Yousefnia MA, et al. Low-dose dobutamine stress echocardiography to predict reversibility of mitral regurgitation with CABG. Echocardiography 2006;23:31-7. [Crossref] [PubMed]
- Harmel EK, Reichenspurner H, Girdauskas E. Subannular reconstruction in secondary mitral regurgitation: a meta-analysis. Heart 2018;104:1783-90. [Crossref] [PubMed]
- Virk SA, Tian DH, Sriravindrarajah A, et al. Mitral valve surgery and coronary artery bypass grafting for moderate-to-severe ischemic mitral regurgitation: Meta-analysis of clinical and echocardiographic outcomes. J Thorac Cardiovasc Surg 2017;154:127-36. [Crossref] [PubMed]
- Wu AH, Aaronson KD, Bolling SF, et al. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol 2005;45:381-7. [Crossref] [PubMed]
- Gelsomino S, van Garsse L, Lucà F, et al. Impact of preoperative anterior leaflet tethering on the recurrence of ischemic mitral regurgitation and the lack of left ventricular reverse remodeling after restrictive annuloplasty. J Am Soc Echocardiogr 2011;24:1365-75. [Crossref] [PubMed]
- Silbiger JJ. Mechanistic insights into ischemic mitral regurgitation: echocardiographic and surgical implications. J Am Soc Echocardiogr 2011;24:707-19. [Crossref] [PubMed]
- Kron IL, Hung J, Overbey JR, et al. Predicting recurrent mitral regurgitation after mitral valve repair for severe ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2015;149:752-61.e1. [Crossref] [PubMed]
- Nappi F, Avtaar Singh SS, Padala M, et al. The Choice of Treatment in Ischemic Mitral Regurgitation With Reduced Left Ventricular Function. Ann Thorac Surg 2019;108:1901-12. [Crossref] [PubMed]
- Nappi F. Left Ventricular Reconstruction With Mitral Surgery: Do Not Delay and Continue to Improve Repair. Ann Thorac Surg 2020;109:1951. [Crossref] [PubMed]
- Nappi F, Nenna A, Mihos C, et al. Ischemic functional mitral regurgitation: from pathophysiological concepts to current treatment options. A systemic review for optimal strategy. Gen Thorac Cardiovasc Surg 2021;69:213-29. [Crossref] [PubMed]
- Langer F, Kunihara T, Hell K, et al. RING+STRING: Successful repair technique for ischemic mitral regurgitation with severe leaflet tethering. Circulation 2009;120:S85-91. [Crossref] [PubMed]
- Rama A, Nappi F, Praschker BG, et al. Papillary muscle approximation for ischemic mitral valve regurgitation. J Card Surg 2008;23:733-5. [Crossref] [PubMed]
- Nappi F, Carotenuto AR, Avtaar Singh SS, et al. Euler's Elastica-Based Biomechanics of the Papillary Muscle Approximation in Ischemic Mitral Valve Regurgitation: A Simple 2D Analytical Model. Materials (Basel) 2019;12:1518. [Crossref] [PubMed]
- Xu D, McBride E, Kalra K, et al. Undersizing mitral annuloplasty alters left ventricular mechanics in a swine model of ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2022;164:850-61.e8. [Crossref] [PubMed]
- Kalra K, Wang Q, McIver BV, et al. Temporal changes in interpapillary muscle dynamics as an active indicator of mitral valve and left ventricular interaction in ischemic mitral regurgitation. J Am Coll Cardiol 2014;64:1867-79. [Crossref] [PubMed]
- Nappi F, Spadaccio C, Fraldi M. Reply: Papillary Muscle Approximation Is an Anatomically Correct Repair for Ischemic Mitral Regurgitation. J Am Coll Cardiol 2016;68:1147-8. [Crossref] [PubMed]
- Wakasa S, Kubota S, Shingu Y, et al. The extent of papillary muscle approximation affects mortality and durability of mitral valve repair for ischemic mitral regurgitation. J Cardiothorac Surg 2014;9:98. [Crossref] [PubMed]
- Pausch J, Sequeira Gross T, Müller L, et al. Subannular repair for functional mitral regurgitation type IIIb in patients with ischaemic versus dilated cardiomyopathy. Eur J Cardiothorac Surg 2021;60:122-30. [Crossref] [PubMed]
- Salsano A, Nenna A, Molinari N, et al. Impact of Mitral Regurgitation Recurrence on Mitral Valve Repair for Secondary Ischemic Mitral Regurgitation. J Cardiovasc Dev Dis 2023;10:124. [Crossref] [PubMed]
- Liel-Cohen N, Guerrero JL, Otsuji Y, et al. Design of a new surgical approach for ventricular remodeling to relieve ischemic mitral regurgitation: insights from 3-dimensional echocardiography. Circulation 2000;101:2756-63. [Crossref] [PubMed]
- Girdauskas E, Pausch J, Reichenspurner H, et al. Subannular repair for functional mitral regurgitation with reduced systolic ventricle function: rationale and design of REFORM-MR registry. J Cardiothorac Surg 2022;17:343. [Crossref] [PubMed]
- Nappi F, Spadaccio C. Obstructive Cardiomyopathy and Tethering in Ischemic Mitral Regurgitation: Two Sides of the Coin. Ann Thorac Surg 2019;107:1911-2. [Crossref] [PubMed]
- Watanabe T, Arai H, Nagaoka E, et al. Influence of procedural differences on mitral valve configuration after surgical repair for functional mitral regurgitation: in which direction should the papillary muscle be relocated? J Cardiothorac Surg 2014;9:185. [Crossref] [PubMed]
- Nappi F, Spadaccio C. Biomechanics of failed ischemic mitral valve repair: Discovering new frontiers. J Thorac Cardiovasc Surg 2017;154:832-3. [Crossref] [PubMed]
- Mihos CG, Capoulade R, Yucel E, et al. Mitral Valve and Subvalvular Repair for Secondary Mitral Regurgitation: Rationale and Clinical Outcomes of the Papillary Muscle Sling. Cardiol Rev 2018;26:22-8. [Crossref] [PubMed]
- Nappi F, Spadaccio C, Chello M, et al. Papillary muscle approximation in mitral valve repair for secondary MR. J Thorac Dis 2017;9:S635-9. [Crossref] [PubMed]
- Nappi F, Santana O, Mihos CG. Geometric distortion of the mitral valve apparatus in ischemic mitral regurgitation: Should we really forfeit the opportunity for a complete repair? J Thorac Cardiovasc Surg 2019;158:e91-2. [Crossref] [PubMed]
- Nappi F, Gambardella I. Combined Replacement and Subvalvular Repair for Functional Mitral Regurgitation: The New Frontier? Ann Thorac Surg 2020;109:303-4. [Crossref] [PubMed]
- Alfieri O, Maisano F, De Bonis M, et al. The double-orifice technique in mitral valve repair: a simple solution for complex problems. J Thorac Cardiovasc Surg 2001;122:674-81. [Crossref] [PubMed]
- Kinnaird TD, Munt BI, Ignaszewski AP, et al. Edge-to-edge repair for functional mitral regurgitation: an echocardiographic study of the hemodynamic consequences. J Heart Valve Dis 2003;12:280-6. [PubMed]
- Magne J, Sénéchal M, Dumesnil JG, et al. Ischemic mitral regurgitation: a complex multifaceted disease. Cardiology 2009;112:244-59. [Crossref] [PubMed]
- Lancellotti P, Marwick T, Pierard LA. How to manage ischaemic mitral regurgitation. Heart 2008;94:1497-502. [Crossref] [PubMed]
- Nenna A, Nappi F, Spadaccio C, et al. Systolic Anterior Motion (SAM) Complicating Mitral Valve Repair: Current Concepts of Intraoperative and Postoperative Management. Surg Technol Int 2020;37:225-32. [PubMed]
- Mihos CG, Xydas S, Nappi F, et al. Transaortic Alfieri Repair for Secondary Mitral Regurgitation: Effective and Underused. Ann Thorac Surg 2018;106:1264. [Crossref] [PubMed]
- Nappi F, Avataar Singh SS. Alfieri Edge-to-Edge Mitral Valve Repair for All Seasons? Ann Thorac Surg 2018;106:1258. [Crossref] [PubMed]
- De Bonis M, Lapenna E, La Canna G, et al. Mitral valve repair for functional mitral regurgitation in end-stage dilated cardiomyopathy: role of the "edge-to-edge" technique. Circulation 2005;112:I402-8. [Crossref] [PubMed]
- De Bonis M, Ferrara D, Taramasso M, et al. Mitral replacement or repair for functional mitral regurgitation in dilated and ischemic cardiomyopathy: is it really the same? Ann Thorac Surg 2012;94:44-51. [Crossref] [PubMed]
- Kincaid EH, Riley RD, Hines MH, et al. Anterior leaflet augmentation for ischemic mitral regurgitation. Ann Thorac Surg 2004;78:564-8; discussion 568. [Crossref] [PubMed]
- Wan S, Lee AP, Jin CN, et al. The choice of mitral annuloplastic ring-beyond "surgeon's preference". Ann Cardiothorac Surg 2015;4:261-5. [PubMed]
- Nappi F, Acar C. The Use of Anterior Mitral Leaflet Augmentation With Autologous Pericardium: Why Not? Ann Thorac Surg 2021;112:688-9. [Crossref] [PubMed]
- Olivito S, Lalande S, Nappi F, et al. Structural deterioration of the cryopreserved mitral homograft valve. J Thorac Cardiovasc Surg 2012;144:313-20, 320.e1.
- Acar C, de Ibarra JS, Lansac E. Anterior leaflet augmentation with autologous pericardium for mitral repair in rheumatic valve insufficiency. J Heart Valve Dis 2004;13:741-6. [PubMed]
- Aubert S, Flecher E, Rubin S, et al. Anterior mitral leaflet augmentation with autologous pericardium. Ann Thorac Surg 2007;83:1560-1. [Crossref] [PubMed]
- Borger MA, Murphy PM, Alam A, et al. Initial results of the chordal-cutting operation for ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2007;133:1483-92. [Crossref] [PubMed]
- Packer M, Grayburn PA. New Evidence Supporting a Novel Conceptual Framework for Distinguishing Proportionate and Disproportionate Functional Mitral Regurgitation. JAMA Cardiol 2020;5:469-75. [Crossref] [PubMed]
- Grayburn PA, Sannino A, Packer M. Proportionate and Disproportionate Functional Mitral Regurgitation: A New Conceptual Framework That Reconciles the Results of the MITRA-FR and COAPT Trials. JACC Cardiovasc Imaging 2019;12:353-62. [Crossref] [PubMed]
- Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. [Crossref] [PubMed]
- de By TMMH, Mohacsi P, Gahl B, et al. The European Registry for Patients with Mechanical Circulatory Support (EUROMACS) of the European Association for Cardio-Thoracic Surgery (EACTS): second report. Eur J Cardiothorac Surg 2018;53:309-16. [Crossref] [PubMed]
- Fang JC. Rise of the machines--left ventricular assist devices as permanent therapy for advanced heart failure. N Engl J Med 2009;361:2282-5. [Crossref] [PubMed]
- Petrus AHJ, Dekkers OM, Tops LF, et al. Impact of recurrent mitral regurgitation after mitral valve repair for functional mitral regurgitation: long-term analysis of competing outcomes. Eur Heart J 2019;40:2206-14. [Crossref] [PubMed]
- Sandoval E, Singh SK, Carillo JA, et al. Impact of concomitant mitral valve repair for severe mitral regurgitation at the time of continuous-flow left ventricular assist device insertion. Interact Cardiovasc Thorac Surg 2017;25:620-3. [Crossref] [PubMed]
- Michler RE. Surgical management of moderate ischemic mitral regurgitation at the time of coronary artery bypass grafting remains controversial. J Thorac Cardiovasc Surg 2018;156:1498-500. [Crossref] [PubMed]
- Rector TS, Tschumperlin LK, Kubo SH, et al. Use of the Living With Heart Failure questionnaire to ascertain patients' perspectives on improvement in quality of life versus risk of drug-induced death. J Card Fail 1995;1:201-6. [Crossref] [PubMed]
- Padala M. Papillary Muscle Approximation Is an Anatomically Correct Repair for Ischemic Mitral Regurgitation. J Am Coll Cardiol 2016;68:1146-7. [Crossref] [PubMed]
- Shudo Y, Matsumiya G, Sakaguchi T, et al. Assessment of changes in mitral valve configuration with multidetector computed tomography: impact of papillary muscle imbrication and ring annuloplasty. Circulation 2010;122:S29-36. [Crossref] [PubMed]
- Braun J, van de Veire NR, Klautz RJ, et al. Restrictive mitral annuloplasty cures ischemic mitral regurgitation and heart failure. Ann Thorac Surg 2008;85:430-6; discussion 436-7. [Crossref] [PubMed]
- Kim K, Kaji S, An Y, et al. Interpapillary muscle distance independently affects severity of functional mitral regurgitation in patients with systolic left ventricular dysfunction. J Thorac Cardiovasc Surg 2014;148:434-40.e1. [Crossref] [PubMed]
- Roshanali F, Mandegar MH, Yousefnia MA, et al. A prospective study of predicting factors in ischemic mitral regurgitation recurrence after ring annuloplasty. Ann Thorac Surg 2007;84:745-9. [Crossref] [PubMed]
- Sanz J, Weinsaft JW. Ischemic mitral regurgitation: is mitral valve physiology moving from global to local? J Am Coll Cardiol 2014;64:1880-2. [Crossref] [PubMed]
- Nappi F, Mazzocchi L, Avtaar Singh SS, et al. Complementary Role of the Computed Biomodelling through Finite Element Analysis and Computed Tomography for Diagnosis of Transcatheter Heart Valve Thrombosis. Biomed Res Int 2018;2018:1346308. [Crossref] [PubMed]
- Nappi F, Attias D, Avtaar Singh SS, et al. Finite element analysis applied to the transcatheter mitral valve therapy: Studying the present, imagining the future. J Thorac Cardiovasc Surg 2019;157:e149-51. [Crossref] [PubMed]
- Nappi F, Mazzocchi L, Timofeva I, et al. A Finite Element Analysis Study from 3D CT to Predict Transcatheter Heart Valve Thrombosis. Diagnostics (Basel) 2020;10:183. [Crossref] [PubMed]
- Spadaccio C, Fraldi M, Sablayrolles JL, et al. TAVI in Lower Risk Patients: Revolution or Nonsense? Keep Calm and Select Patients. J Am Coll Cardiol 2016;67:1380-1. [Crossref] [PubMed]
- Nappi F, Spadaccio C, Sablayrolles JL. Pushing the Limits in Transcatheter Aortic Valve Replacement: High-Volume Center's Effect, Overconfidence, or Something Else? JACC Cardiovasc Interv 2016;9:2186-8. [Crossref] [PubMed]
- Nappi F, Spadaccio C, Sablayrolles JL. Delayed prosthesis malposition after transcatheter aortic valve implantation causing coronaries obstruction. Eur J Cardiothorac Surg 2017;52:1227-8. [Crossref] [PubMed]
- Nappi F, Mazzocchi L, Spadaccio C, et al. CoreValve vs. Sapien 3 Transcatheter Aortic Valve Replacement: A Finite Element Analysis Study. Bioengineering (Basel) 2021;8:52. [Crossref] [PubMed]
- Nappi F, Nenna A, Sing SSA, et al. Mitral regurgitation: lessons learned from COAPT and MITRA-Fr. J Thorac Dis 2020;12:2936-44. [Crossref] [PubMed]
- Nappi F, Nenna A, Timofeeva I, et al. Mitral regurgitation after transcatheter aortic valve replacement. J Thorac Dis 2020;12:2926-35. [Crossref] [PubMed]
- Lugo D, Pulido Ramirez AL, Lo Presti S, et al. Structural heart disease: the year in valvular and complex coronary intervention trials. J Thorac Dis 2020;12:2910-8. [Crossref] [PubMed]
- Pawale A, Itagaki S, Parikh A, et al. Mitral valve repair for severe mitral valve regurgitation during left ventricular assist device implantation. J Thorac Cardiovasc Surg 2019;157:1841-1848.e1. [Crossref] [PubMed]
- Arjomandi Rad A, Fleet B, Zubarevich A, et al. Left ventricular assist device implantation and concomitant mitral valve surgery: A systematic review and meta-analysis. Artif Organs 2024;48:16-27. [Crossref] [PubMed]


