
Association between sore throat and early immune responses against COVID-19 before and after the emergence of the Omicron variant
Highlight box
Key findings
• Coronavirus disease 2019 (COVID-19) caused by the Omicron variant is characterized by an increase in sore throat frequency and altered associations between sore throat and several immune indicators, including interferon (IFN)-α, interleukin-6, and IFN-λ1.
What is known and what is new?
• Omicron variant has a different symptom profile and is associated with a high prevalence of sore throat and low prevalence of smell or taste alteration compared to those observed in infections with its preceding variants.
• This study elucidated that in patients with COVID-19 having sore throat, early immune responses differ depending on the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant.
What is the implication, and what should change now?
• More studies that focusing on the association between a variety of clinical symptoms and immune responses against emerging SARS-CoV-2 variants are necessary to understand pathophysiology of each pandemic or endemic spread of COVID-19.
Introduction
The highly transmissible coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) became intermittently endemic worldwide after the emergence of the Omicron variant in late 2021 (1). Despite its higher transmissibility even in individuals who have received a vaccine against SARS-CoV-2 (2), the Omicron variant is less virulent than its preceding variants in terms of the rate of hospitalization, incidence of lower respiratory tract invasion, severe disease, and mortality (3,4). Recent large-scale epidemiological studies have reported that variants have different symptom profiles; for instance, a higher prevalence (60–70%) of sore throat (5-8) and a lower prevalence of smell or taste alteration are observed in Omicron infection (7-11). These differences are partly attributed to intrinsic viral factors, as suggested by the inefficient replication of Omicron in human alveolar organoids and ex vivo-infected lung tissues (12), as well as by its increased resistance to innate immune defenses, including type I interferons (IFNs) (13,14). However, the relationship between a variety of clinical symptoms and immune responses against emerging SARS-CoV-2 variants has not been fully elucidated.
Previously, we assessed the association between initial immune responses and development of pneumonia or hypoxic respiratory failure due to SARS-CoV-2 infection before and after the emergence of Omicron variants (15,16). We focused on the serum levels of the representative innate immune indexes as follows; IFN-α (type I IFNs), interleukin-6 (IL-6), C-X-C motif chemokine ligand 10 (CXCL10), vascular endothelial growth factor (VEGF), IFN-λ1, and IFN-λ3 (17-20). Moreover, we also assessed serum-neutralizing activity, which reflects humoral immune responses against SARS-CoV-2 infection and is used as an indicator of vaccine efficacy (21,22). We found that SARS-CoV-2 infection due to Omicron was associated with a decreased incidence of pneumonia and had a weaker correlation between serum IFN-α levels and extent of pulmonary lesions [computed tomography (CT) severity scores], compared to those due to the precedent variants (16). Using these data, we aimed to elucidate the association between sore throat, a prevalent COVID-19 symptom, and immune responses in the early phase of SARS-CoV-2 infection before and after the emergence of Omicron variants.
In this study, we aimed to quantify the associations between immune biomarkers and the presence or intensity of sore throat during the early phase of SARS-CoV-2 infection, and how these associations differ between the Omicron and precedent variants. We present this article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-24-36/rc).
Methods
Study design
This study was conducted as part of the Toyama University COVID-19 Cohort Study, an investigator-initiated prospective single-center observational study with non-probability sampling (15,16). The study period was between December 2020 to April 2022, which consisted of four major waves of the pandemic in Japan: the third (December 2020 to January 2021); fourth, mainly attributed to the Alpha variant (April–June 2021); fifth, mainly attributed to the Delta variant (July–October 2021); and sixth waves, mainly attributed to the Omicron BA.1 variant (January–April 2022). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), which was approved by the Ethical Review Board of the Toyama University Hospital (No. R2019167). Written informed consent was obtained from all the patients.
Diagnosis of COVID-19 in each participant was based on the findings of reverse transcription quantitative polymerase chain reaction (RT-qPCR) assays. Nasopharyngeal samples were collected for RT-qPCR, and chest CT was performed upon hospital admission. Serum samples were stored at −80 °C following each laboratory examination.
Participants and study protocol
Participants aged 18 years or older, who were admitted in our hospital during the study period and whose blood samples were collected within five days after symptom onset, were included in this study. Those who had received any treatment against SARS-CoV-2 before blood sampling or participated in another clinical trial were excluded.
The first monovalent mRNA vaccine against SARS-CoV-2 wild-type (WT), which has less efficacy against the Omicron variant, became widely available during the fifth wave in Japan. Only few inpatients were vaccinated until the fifth wave, whereas numerous inpatients got vaccinated twice before admission during the sixth wave. Therefore, we included the following participants for further analysis (Figure 1): those who did not receive the vaccine during the third, fourth, and fifth waves and those who received the BNT162b2 or mRNA-1273 vaccine twice at least two weeks before admission during the sixth wave. We also analyzed the levels of immune indicators in unvaccinated participants infected with the Omicron variant; however, the results were not shown as the main findings of this study due to the small sample size.

Data on participant demographics, comorbidities, clinical presentation, laboratory findings, therapy regimen, and prognosis were collected from medical charts.
During the study period, participants were given a daily questionnaire on the presence of symptoms related to COVID-19, including fatigue, sore throat, headache, smell or taste alteration, joint/muscle pain, and diarrhea. Sore throat was assessed from admission until discharge using an analog scale (0= no pain, 1= throat irritation, 2= throat pain, 3= severe pain with difficulties in swallowing). The duration of sore throat was defined as the initiation of sore throat (at least from grade 1) to absolute resolution (grade 0). Clinical information was followed until discharge.
Two experienced pulmonary radiologists (K.N. and Y.Y.) reviewed the previous chest CT scans. When a newly developed inflammatory lesion was detected in the chest CT performed upon admission, COVID-19 pneumonia was confirmed, and further categorized according to the Fleischner Society Glossary of Terms for Thoracic Imaging (23-25). Hypoxemia requiring oxygen therapy was defined as a blood oxygen saturation (SpO2) level of ≤93% at rest/motion in room air, as defined previously (26). The degree of severity of COVID-19 was defined as follows: mild (symptomatic patients without pneumonia), moderate (patients having pneumonia but not requiring oxygen supplementation), severe (patients having pneumonia and requiring oxygen supplementation).
Cytokine and chemokine measurement
Serum cytokines and chemokines [IFN-α, CXCL10, IL-6, VEGF, IFN-λ1 (IL-29), and IFN-λ3 (IL-28B)] were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits, according to the manufacturers’ instructions (Table S1). Each sample was measured using a first saw. The analyte signal was set to zero, in case it fell below the background signal. If the signal was detectable but below the manufacturer’s lower limit of quantification, it was set to the lower limit of detection.
RT-qPCR
RT-qPCR was performed as previously described (27). Quantification quality was controlled using the AcroMetrix COVID-19 RNA Control (Thermo Fisher Scientific, Waltham, MA, USA). The detection limit was approximately 0.4 copies/µL. Viremia (RNAemia) was determined when SARS-CoV-2 was detected in the blood serum specimens.
The presence of N501Y/L452R mutation on SARS-CoV-2 was investigated with the screening PCR tests using Primer/Probe N501Y (Takara Bio Inc., Shiga, Japan) or Primer/Probe L452R Ver.2 (Takara Bio Inc.), which were conducted as administrative tests at Toyama Institution of Health (Toyama, Japan) during the fourth and fifth waves. Omicron BA.1 mutation was confirmed via multiplex real-time one-step RT-PCR assays using Primer/Probe L452R Ver.2 and Primer/Probe G339D (Takara Bio Inc.), which were conducted at Toyama University. In cases where the G339D mutation was detected but L452R was not, the variant was identified as Omicron BA.1, in agreement with the local epidemic situation analyzed at the Toyama Institute of Health (28).
Pseudovirus neutralization assay
As previously described (22,29), we measured the neutralizing activity of human serum against pseudoviruses using a high-throughput chemiluminescent reduction neutralizing test. The values for samples without and with the pseudovirus but without serum were defined as 0% and 100% infections (100% and 0% inhibition), respectively. Four pseudoviruses with expression plasmids for the truncated S protein of SARS-CoV-2 were used for measurement of the neutralizing activity against the infecting variant of each pandemic wave: pCAG-SARS-CoV-2 S (Wuhan; WT), pCAGG-pm3-SARS2-Shu-d19-B1.1.7 (alpha-derived variant), pCAGG-pm3-SARS2-Shu-d19-B1.617.2 (Delta-derived variant), and pCAGG-pm3-SARS2-Shu-d19-B1.1.529.1 (Omicron BA.1-derived variant) (22,29).
Statistical analysis
The participants’ medical and demographic characteristics was expressed using medians [interquartile ranges (IQR)] or numbers (percentages). To evaluate differences between the two groups, the Mann-Whitney U test or Fisher’s exact test were used to compare continuous and nominal variables, respectively.
The association between each pair of biomarkers and sore throat grade was determined using Spearman’s rho correlation coefficient. Correlations between immune parameters are presented using a heatmap. Statistical significance was set at P<0.05. Statistical analyses were performed using the GraphPad Prism 9 software (San Diego, CA, USA).
Results
Clinical features of participants before and after the emergence of Omicron variant
The clinical features of the participants before and after emergence of the Omicron variant are summarized in Tables 1,2, respectively. A total of 136 participants infected with Delta and precedent variants (39, 50, and 47 in the third, fourth, and fifth waves, respectively), and 47 infected with Omicron after vaccination (sixth wave) were included for further analysis. The entire study population was included in our previous studies (15,16). The incidence of sore throat, SARS-CoV-2 pneumonia, and hypoxemic respiratory failure in entire cohort were found to be 42%, 52%, and 24%, respectively. There were two participants who had a previous history of malignancy or surgical treatment on head/neck that might have affected the sensation of the throat; two participants had undergone thyroidectomy for thyroid tumor. All participants survived COVID-19 for at least 30 days after symptom onset. Median period of hospital stay was 6 days (IQR: 4–9 days) in the entire study population. Owing to temporary exhaustion of in-hospital medical services in our region, the part of the cohort with mild COVID-19 (26% of participants with Delta and precedent variants and 36% of those with Omicron variant) was transferred to the shielding accommodation facilities within 4 days after admission to our hospital.
Table 1
| Features | Total (n=136) | Sore throat | |||
|---|---|---|---|---|---|
| G0 (n=79) | G1 (n=23) | G2 (n=24) | G3 (n=10) | ||
| Age (years) | 49 [32–54] | 49 [37–54] | 49 [31–53] | 42 [24–56] | 38 [27–51] |
| Sex, male | 78 [57] | 51 [65] | 7 [30] | 17 [71] | 3 [30] |
| Pandemic period | |||||
| Third wave | 39 [29] | 20 [25] | 9 [39] | 8 [33] | 2 [20] |
| Fourth wave | 50 [37] | 29 [37] | 6 [26] | 12 [50] | 3 [30] |
| Fifth wave | 47 [35] | 30 [38] | 8 [35] | 4 [17] | 5 [50] |
| Underlying disease | |||||
| None | 74 [54] | 42 [53] | 12 [52] | 14 [58] | 6 [60] |
| Hypertension | 24 [18] | 17 [22] | 1 [4] | 5 [21] | 1 [10] |
| Diabetes mellitus | 8 [6] | 6 [8] | 1 [4] | 1 [4] | 0 [0] |
| Previous history of malignancy/surgery on head/neck | 1 [0.7] | 0 [0] | 1 [4] | 0 [0] | 0 [0] |
| Body mass index (kg/m2) | 22.4 [21–25] | 23.3 [21–26] | 20.8 [20–23] | 22.5 [21–25] | 21.4 [20–26] |
| Presence of sore throat | 57 [42] | – | – | – | – |
| Duration of sore throat (days) | 4 [2–5] | – | 2 [2–5] | 4 [3–5] | 7 [4–8] |
| Nasal viral load (log) | 4.9 [3.8–5.6] | 4.5 [3.8–5.3] | 5.2 [4.4–5.8] | 5.3 [4.2–5.7] | 5.0 [3.3–5.5] |
| Viremia | 31 [23] | 22 [28] | 3 [13] | 5 [21] | 1 [10] |
| Severity | |||||
| Mild | 65 [48] | 31 [39] | 14 [61] | 14 [58] | 6 [60] |
| Moderate-to-severe (developed SARS-CoV-2 pneumonia) | 71 [52] | 48 [61] | 9 [39] | 10 [42] | 4 [40] |
| Development of respiratory failure | 32 [24] | 22 [28] | 2 [9] | 5 [21] | 3 [30] |
| Treatment | |||||
| Remdesivir | 28 [21] | 19 [24] | 2 [9] | 5 [21] | 2 [20] |
| Dexamethasone | 32 [24] | 19 [24] | 2 [9] | 8 [33] | 3 [30] |
| Heparin | 31 [23] | 19 [24] | 2 [9] | 8 [33] | 2 [20] |
| Antibody | 20 [15] | 13 [16] | 4 [17] | 2 [8] | 1 [10] |
Continuous variables are reported as median [interquartile range (IQR): 25–75]. Categorical variables are reported as numbers [percentages]. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 2
| Features | Total (n=47) | Sore throat | |||
|---|---|---|---|---|---|
| G0 (n=16) | G1 (n=13) | G2 (n=10) | G3 (n=8) | ||
| Age (years) | 62 [51–74] | 71 [63–75] | 59 [52–78] | 61 [47–69] | 49 [39–55] |
| Sex, male | 32 [68] | 12 [75] | 8 [62] | 8 [80] | 4 [50] |
| Underlying disease | |||||
| None | 15 [32] | 3 [19] | 3 [23] | 4 [40] | 5 [63] |
| Hypertension | 19 [40] | 9 [56] | 4 [31] | 4 [40] | 2 [25] |
| Diabetes mellitus | 7 [15] | 1 [6] | 2 [15] | 4 [40] | 0 [0] |
| Previous history of malignancy/surgery on head/neck | 1 [2] | 0 [0] | 1 [8] | 0 [0] | 0 [0] |
| Body mass index (kg/m2) | 25.0 [22–27] | 26.7 [24–29] | 24.9 [22–27] | 24.8 [23–27] | 23.1 [20–25] |
| Presence of sore throat | 31 [66] | – | – | – | – |
| Duration of sore throat (days) | 5 [4–7] | – | 4 [3–6] | 5 [4–6] | 7 [4–7] |
| Nasal viral load (log) | 4.4 [4.0–5.1] | 4.8 [4.2–5.2] | 4.3 [3.9–5.0] | 4.6 [4.4–4.7] | 4.1 [3.4–4.6] |
| Viremia | 4 [9] | 2 [13] | 0 [0] | 1 [10] | 1 [13] |
| Severity | |||||
| Mild | 32 [68] | 10 [62] | 9 [69] | 7 [70] | 6 [75] |
| Moderate-to-severe (developed SARS-CoV-2 pneumonia) | 15 [32] | 6 [38] | 4 [31] | 3 [30] | 2 [25] |
| Development of respiratory failure | 6 [13] | 5 [31] | 0 [0] | 1 [10] | 0 [0] |
| Treatment | |||||
| Remdesivir | 3 [6] | 3 [19] | 0 [0] | 0 [0] | 0 [0] |
| Dexamethasone | 3 [6] | 3 [19] | 0 [0] | 0 [0] | 0 [0] |
| Heparin | 3 [6] | 3 [19] | 0 [0] | 0 [0] | 0 [0] |
| Antibody | 14 [30] | 6 [38] | 3 [23] | 4 [40] | 1 [13] |
Continuous variables are reported as median [interquartile range (IQR): 25–75]. Categorical variables are reported as numbers [percentages]. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
All treatments were administered after the initial blood collection and included antiviral medication with remdesivir, corticosteroids, heparin, and SARS-CoV-2 antibodies. Only one participant infected with Delta received intermittent positive-pressure ventilation.
Among the participants infected with Omicron, the incidence of sore throat was 66%, which was significantly higher than that in participants infected with Delta and precedent variants (P<0.005). The incidence of pneumonia and hypoxemic respiratory failure in participants infected with Omicron was 32% and 13%, respectively, which were lower than those in participants infected with Delta and precedent variants (P<0.01 and P=0.12, respectively). During the study period, the participants who did not present with sore throat had a relatively higher incidence of pneumonia, respiratory failure or viremia than those presenting with sore throat. The following incidences were observed in participants with Delta and preceding variants (sore throat positive vs. negative): pneumonia: 47% vs. 61%, P=0.12; respiratory failure: 18% vs. 28%, P=0.16; and viremia: 16% vs. 28%, P=0.14. In vaccinated participants infected with Omicron variant, the incidence was as follows (sore throat positive vs. negative): pneumonia: 29% vs. 38%, P=0.74; respiratory failure: 3% vs. 31%, P<0.05; and viremia: 6% vs. 13%, P=0.60.
As the initial nasopharyngeal swab specimens were not available for validation of the viral load, the nasopharyngeal viral load could not be assessed in seven participants (5.1%) infected with the Delta and precedent variants and 14 participants (28.8%) infected with Omicron.
Serum immune biomarkers associated with COVID-19 sore throat
Among participants infected with Delta and precedent variants, a higher nasal viral load and lower IL-6 and LDH levels were observed in participants with sore throats compared to those without sore throats (P<0.05) (Figure 2). However, among participants infected with Omicron, no significant difference was observed between participants with and without sore throats.
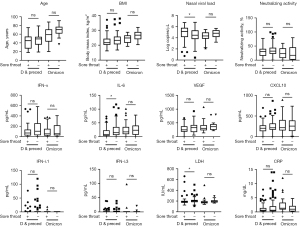
Correlation between serum immune biomarkers and the grade of COVID-19 sore throat
Among participants infected with Delta and precedent variants, serum IFN-α level was weakly correlated with sore throat grade (r=−0.176, P<0.05) (Figure 3). Among participants infected with Omicron, age, body mass index (BMI), and serum IFN-λ1 level were correlated with sore throat grade (r=−0.419, P<0.005; r=−0.376, P<0.005; r=0.315, P<0.05).

Association between serum immune biomarker levels and other COVID-19 symptoms
We also assessed the association between the biomarkers and representative COVID-19 symptoms other than sore throat: headache, smell or taste alteration, and joint or muscle pain.
The incidence of COVID-19 symptoms in the study cohort is summarized in Table S2. The incidence of joint or muscle pain was significantly higher in participants infected with Omicron compared to those infected with the Delta and precedent variants (32% vs. 18%, P<0.005). However, the incidence of smell and taste alteration was lower in participants infected with Omicron (17% vs. 32%, P=0.05).
Figures S1-S3 show the association of immune biomarker levels with other COVID-19 symptoms. Among participants infected with Delta and precedent variants, LDH and IFN-α levels were significantly higher (P<0.05) in participants with headache and joint/muscle pain, respectively. No significant associations between immune biomarker levels and smell/taste alterations were observed.
Serum immune biomarker levels and sore throat in unvaccinated participants infected with Omicron
The clinical features and serum immune biomarker levels in the unvaccinated participants infected with Omicron are summarized in Table S3 and Figure S4. As shown in Table S3, the incidence of sore throat and respiratory failure in unvaccinated participants infected with the Omicron was similar to those in vaccinated participants (unvaccinated 13% vs. vaccinated 13%, P>0.99), whereas the incidence of SARS-CoV-2 pneumonia was relatively higher (unvaccinated 50% vs. vaccinated 32%, P=0.20). In addition, except for VEGF, no apparent correlation was found between immune biomarker levels and sore throat (Figure S4).
Discussion
In this study, we observed a difference in the initial immune response related to sore throat among patients infected with Omicron and those with precedent variants. During the study period, hospital admission of COVID-19 patients for isolation was mandatory in Japan. Several patients with mild COVID-19 were admitted to our hospital and subsequently enrolled in the study (15,16). The incidence of sore throat in participants infected with Omicron was similar to that reported in previous studies (5-8), and was significantly higher than that in participants infected with Delta and precedent variants. The initial immune biomarker level analyses revealed that at the initial phase of COVID-19, nasopharyngeal viral load, IL-6, and IFN-α were associated with sore throat in participants infected with Delta and precedent variants, whereas, age, the BMI, and IFN-λ1 levels were associated with sore throat in participants infected with Omicron. To the best of our knowledge, this study is the first to describe the initial immune response related to sore throat in patients with COVID-19 caused by Omicron and precedent variants.
Furthermore, we found that nasopharyngeal viral load was proportional to sore throat in participants infected with Delta and precedent variants, consistent with previous studies conducted during the pre-Omicron era (30,31). Bae et al. (30) reported that a decline in viral load was correlated with a decrease in symptom count in 89 patients with COVID-19. Goldberg et al. (31) found that several symptoms including sore throat were associated with viral RNA load in 87 nonhospitalized participants with COVID-19. On the other hand, our results revealed that nasopharyngeal viral load was inversely proportional in participants infected with Omicron; to date, few descriptive studies have assessed this association. We also found that nasopharyngeal viral load was relatively high in Omicron patients without sore throat, which may partly reflect the effective suppression of Omicron by augmented local immunity due to throat pain. However, since nasopharyngeal viral load was assessed in only 30 participants infected with Omicron variant in our analysis, further investigation is necessary to clarify its association with COVID-19 sore throat.
Levels of immune biomarkers assessed in our study showed a decreased association with a various COVID-19 symptoms, although these were strongly associated with the presence of SARS-CoV-2 pneumonia, CT score, or respiratory failure, as described in our previous reports (15,16). This might be attributed to the nature of localized symptoms, which have little impact on systemic immune response and are reflected in attenuated associations with serum levels of cytokines, including IL-6 and CXCL10. However, we believe that several findings in this study could reveal the potential association between localized symptoms and the systemic immune response in COVID-19.
IFN-α and IFN-β are crucial innate immune factors in COVID-19 (32,33), which act as inhibitors of viral replication in infected cells and play a defensive role in uninfected cells. Correlation between serum IFN-α and sore throat in participants with Delta and precedent variants might reflect the augmented mucosal innate immune response; upregulation of IFN-α decreased the intensity of sore throat via limiting viral replication and localized inflammation. Several studies suggest that the weak association between IFN-α and sore throat by Omicron is due to an increasing IFN resistance of the variant (34,35). The attenuated association between IFN-α and SARS-CoV-2 pneumonia was also observed in participants with Omicron variant, which were described in our previous study (16). In addition, association between IFN-α and joint/muscle pain in participants infected with Delta and precedent variant suggests that IFN-α also affect these systemic COVID-19 symptoms during pre-Omicron era. Taken together, we found associations between IFN-α and several symptoms with COVID-19 due to the precedent variants, but not with Omicron variant.
IL-6 is widely recognized as a pivotal cytokine in the immune dysregulation of COVID-19 and is strongly associated with a systemic cytokine storm (17) or the extent of pulmonary lesions in SARS-CoV-2 pneumonia (16,36,37). Among participants infected with Delta and precedent variants, IL-6 levels were lower in participants with sore throats than in those without sore throats. These findings suggest that an attenuated mucosal innate immune response in the nasopharynx might induce the subsequent progression of SARS-CoV-2 infection with a lack of sore throat, which affects the systemic immune response with elevated serum IL-6 levels. The weak association between IL-6 and sore throat in patients infected with Omicron might be due to the attenuated virulence of this variant, which has little impact on the systemic immune response. The mucosal innate immune response against Omicron at the nasopharynx might be altered, which more relate with age, BMI and IFN-λ1 rather than IFN-α or IL-6. IFN-λ1 is the predominant antiviral cytokine present at the mucosal barriers in the upper respiratory tract of SARS-CoV-2-infected patients (38). Compared to IFN-I, IFN-λ1 acts as an effective local immune factor against SARS-CoV-2 (39). The low incidence of viremia in participants infected with Omicron supports this hypothesis. Although we could not clarify the detailed mechanisms, we consider that the weak association between IL-6 and sore throat caused by Omicron might be partly due to the attenuated virulence of the variant and augmented local immunity.
We also assessed the effect of vaccination on the immune response related to sore throat in patients infected with Omicron. The incidence of sore throats between vaccinated and unvaccinated patients was not significantly different. However, lower VEGF levels were observed in unvaccinated patients with sore throats than in those without sore throats. Moreover, VEGF levels were significantly correlated with sore throat grade, but not with age, BMI, and IFN-λ1. These findings suggest that vaccination might affect the initial mucosal immune response against Omicron, in addition to humoral immune responses against SARS-CoV-2 infection. As described in our previous report (16), VEGF was significantly correlated with IL-6 in participants infected with Omicron, regardless of vaccination status. However, the correlation of VEGF in COVID-19 symptoms was not explored due to the limited sample size. Further studies are necessary to clarify the effects of vaccines on innate immune responses, including VEGF, in patients with COVID-19.
This study has several limitations. First, the single-center observational study design, along with a modest sample size in the cohorts with Omicron variant, may have resulted in a selection bias. Second, the causative variant in the third pandemic wave remained unidentified, because genetic identification was not routinely available at that time. Third, we could not assess the nasopharyngeal SARS-CoV-2 viral load in a relatively large proportion of participants infected with Omicron. Fourth, this study could not sufficiently examine the time-dependent changes with sore throat and immune indicators due to the relatively short observational period. However, we believe that this study could elucidate a partial and important association between early immune responses and the SARS-CoV-2 variant in patients with COVID-19 having sore throat, which seemed not to be largely affected by these limitations.
Conclusions
We demonstrated that the early immune responses in COVID-19 patients with sore throats differ among SARS-CoV-2 variants. Participants infected with Omicron had an increased incidence of sore throat, and our results revealed altered association between sore throat and several immune biomarkers including IFN-α, IL-6, and IFN-λ1. Considering that new SARS-CoV-2 variants are emerging, more studies are necessary to elucidate the association between a variety of clinical symptoms and immune responses against various variants to understand pathophysiology of each pandemic or endemic spread of COVID-19.
Acknowledgments
Funding: This study was partly supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-24-36/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-24-36/dss
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-24-36/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-24-36/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Review Board of the Toyama University Hospital (No. R2019167) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jung C, Kmiec D, Koepke L, et al. Omicron: What Makes the Latest SARS-CoV-2 Variant of Concern So Concerning? J Virol 2022;96:e0207721. [Crossref] [PubMed]
- Basso P, Negro C, Cegolon L, et al. Risk of Vaccine Breakthrough SARS-CoV-2 Infection and Associated Factors in Healthcare Workers of Trieste Teaching Hospitals (North-Eastern Italy). Viruses 2022;14:336. [Crossref] [PubMed]
- Ulloa AC, Buchan SA, Daneman N, et al. Estimates of SARS-CoV-2 Omicron Variant Severity in Ontario, Canada. JAMA 2022;327:1286-8. [Crossref] [PubMed]
- Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet 2022;399:437-46. [Crossref] [PubMed]
- Menni C, Valdes AM, Polidori L, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet 2022;399:1618-24. [Crossref] [PubMed]
- Nakakubo S, Kishida N, Okuda K, et al. Associations of COVID-19 symptoms with omicron subvariants BA.2 and BA.5, host status, and clinical outcomes in Japan: a registry-based observational study. Lancet Infect Dis 2023;23:1244-56. [Crossref] [PubMed]
- Wang RC, Gottlieb M, Montoy JCC, et al. Association Between SARS-CoV-2 Variants and Frequency of Acute Symptoms: Analysis of a Multi-institutional Prospective Cohort Study-December 20, 2020-June 20, 2022. Open Forum Infect Dis 2023;10:ofad275. [Crossref] [PubMed]
- Vihta KD, Pouwels KB, Peto TE, et al. Omicron-associated changes in SARS-CoV-2 symptoms in the United Kingdom. Clin Infect Dis 2022;76:e133-41. [PubMed]
- Peña Rodríguez M, Hernández Bello J, Vega Magaña N, et al. Prevalence of symptoms, comorbidities, and reinfections in individuals infected with Wild-Type SARS-CoV-2, Delta, or Omicron variants: a comparative study in western Mexico. Front Public Health 2023;11:1149795. [Crossref] [PubMed]
- Hojo-Souza NS, Freitas VLS, Guidoni DL, et al. Clinical symptom profile of hospitalized COVID-19 Brazilian patients according to SARS-CoV-2 variants. Epidemiol Health 2023;45:e2023079. [Crossref] [PubMed]
- Song J, Jing Q, Zhu E, et al. Alterations in smell or taste in individuals infected with SARS-CoV-2 during periods of Omicron variant dominance. Int J Infect Dis 2023;128:278-84. [Crossref] [PubMed]
- Hui KPY, Ho JCW, Cheung MC, et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature 2022;603:715-20. [Crossref] [PubMed]
- Guo K, Barrett BS, Morrison JH, et al. Interferon resistance of emerging SARS-CoV-2 variants. Proc Natl Acad Sci U S A 2022;119:e2203760119. [Crossref] [PubMed]
- Alfi O, Hamdan M, Wald O, et al. SARS-CoV-2 Omicron Induces Enhanced Mucosal Interferon Response Compared to other Variants of Concern, Associated with Restricted Replication in Human Lung Tissues. Viruses 2022;14:1583. [Crossref] [PubMed]
- Nagaoka K, Kawasuji H, Takegoshi Y, et al. Predictive values of immune indicators on respiratory failure in the early phase of COVID-19 due to Delta and precedent variants. Front Immunol 2023;14:1197436. [Crossref] [PubMed]
- Nagaoka K, Kawasuji H, Takegoshi Y, et al. Dominant CT Patterns and Immune Responses during the Early Infection Phases of Different SARS-CoV-2 Variants. Viruses 2023;15:1304. [Crossref] [PubMed]
- Coperchini F, Chiovato L, Rotondi M. Interleukin-6, CXCL10 and Infiltrating Macrophages in COVID-19-Related Cytokine Storm: Not One for All But All for One! Front Immunol 2021;12:668507. [Crossref] [PubMed]
- Fabris M, Del Ben F, Sozio E, et al. Cytokines from Bench to Bedside: A Retrospective Study Identifies a Definite Panel of Biomarkers to Early Assess the Risk of Negative Outcome in COVID-19 Patients. Int J Mol Sci 2022;23:4830. [Crossref] [PubMed]
- Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020;370:eabd4585. [Crossref] [PubMed]
- Guerra-López JA, Amezcua-Castillo LM, González-Pacheco H, et al. Levels of Vascular Endothelial Growth Factor and Its Association with Pulmonary Embolism in COVID-19. J Interferon Cytokine Res 2022;42:444-8. [Crossref] [PubMed]
- Pradenas E, Ubals M, Urrea V, et al. Virological and Clinical Determinants of the Magnitude of Humoral Responses to SARS-CoV-2 in Mild-Symptomatic Individuals. Front Immunol 2022;13:860215. [Crossref] [PubMed]
- Kawasuji H, Morinaga Y, Tani H, et al. Neutralizing Antibody Response of the Wild-Type/Omicron BA.1 Bivalent Vaccine as the Second Booster Dose against Omicron BA.2 and BA.5. Microbiol Spectr 2023;11:e0513122. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Prokop M, van Everdingen W, van Rees Vellinga T, et al. CO-RADS: A Categorical CT Assessment Scheme for Patients Suspected of Having COVID-19-Definition and Evaluation. Radiology 2020;296:E97-E104. [Crossref] [PubMed]
- Machnicki S, Patel D, Singh A, et al. The Usefulness of Chest CT Imaging in Patients With Suspected or Diagnosed COVID-19: A Review of Literature. Chest 2021;160:652-70. [Crossref] [PubMed]
- Wang YC, Lu MC, Yang SF, et al. Respiratory care for the critical patients with 2019 novel coronavirus. Respir Med 2021;186:106516. [Crossref] [PubMed]
- Kawasuji H, Morinaga Y, Tani H, et al. SARS-CoV-2 RNAemia with a higher nasopharyngeal viral load is strongly associated with disease severity and mortality in patients with COVID-19. J Med Virol 2022;94:147-53. [Crossref] [PubMed]
- Itamochi M, Yazawa S, Inasaki N, et al. Neutralization of Omicron subvariants BA.1 and BA.5 by a booster dose of COVID-19 mRNA vaccine in a Japanese nursing home cohort. Vaccine 2023;41:2234-42. [Crossref] [PubMed]
- Morinaga Y, Tani H, Terasaki Y, et al. Correlation of the Commercial Anti-SARS-CoV-2 Receptor Binding Domain Antibody Test with the Chemiluminescent Reduction Neutralizing Test and Possible Detection of Antibodies to Emerging Variants. Microbiol Spectr 2021;9:e0056021. [Crossref] [PubMed]
- Bae S, Kim JY, Lim SY, et al. Dynamics of Viral Shedding and Symptoms in Patients with Asymptomatic or Mild COVID-19. Viruses 2021;13:2133. [Crossref] [PubMed]
- Goldberg SA, Lu S, Garcia-Knight M, et al. Viral Determinants of Acute COVID-19 Symptoms in a Nonhospitalized Adult Population in the Pre-Omicron Era. Open Forum Infect Dis 2023;10:ofad396. [Crossref] [PubMed]
- Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020;369:718-24. [Crossref] [PubMed]
- Acharya D, Liu G, Gack MU. Dysregulation of type I interferon responses in COVID-19. Nat Rev Immunol 2020;20:397-8. [Crossref] [PubMed]
- Nchioua R, Schundner A, Klute S, et al. Reduced replication but increased interferon resistance of SARS-CoV-2 Omicron BA.1. Life Sci Alliance 2023;6:e202201745. [Crossref] [PubMed]
- Shalamova L, Felgenhauer U, Wilhelm J, et al. Omicron variant of SARS-CoV-2 exhibits an increased resilience to the antiviral type I interferon response. PNAS Nexus 2022;1:pgac067. [Crossref] [PubMed]
- Chen LD, Zhang ZY, Wei XJ, et al. Association between cytokine profiles and lung injury in COVID-19 pneumonia. Respir Res 2020;21:201. [Crossref] [PubMed]
- Rutkowska E, Kwiecień I, Żabicka M, et al. Cytokines and Leukocytes Subpopulations Profile in SARS-CoV-2 Patients Depending on the CT Score Severity. Viruses 2021;13:880. [Crossref] [PubMed]
- Sposito B, Broggi A, Pandolfi L, et al. The interferon landscape along the respiratory tract impacts the severity of COVID-19. Cell 2021;184:4953-4968.e16. [Crossref] [PubMed]
- Gilbert C, Lefeuvre C, Preisser L, et al. Age-Related Expression of IFN-λ1 Versus IFN-I and Beta-Defensins in the Nasopharynx of SARS-CoV-2-Infected Individuals. Front Immunol 2021;12:750279. [Crossref] [PubMed]


