Finding ways to solve or prevent aminoglycoside-induced ototoxicity?
Aminoglycoside (AG), which was first discovered in 1944, is widely used in treating severe Gram-negative bacterial infections (1). Gentamicin, kanamycin, amikacin and neomycin are the major AGs used nowadays, and vancomycin is the drug of choice for methicillin-resistant Staphylococcus aureus (MRSA) infection. Although other antibiotics can also target Gram-negative bacteria such as penicillins and cephalosporins, AGs remain to be the popular choice because of their economical and clinical advantages including low cost, rapid bactericidal activity and low incidences of resistance (2). However, the adverse effects of reversible nephrotoxicity and irreversible ototoxicity limit their use to short term therapy (3,4). Precaution is needed for treating senior patients who are prone to have hearing, vestibular and renal dysfunctions. Identification of the predisposing factors of such toxicities will allow us to maximize the benefit of using AGs and greatly improve the therapeutic outcome.
The link of ototoxicity, which is manifested as tinnitus or severe permanent hearing loss, with AGs was first determined in 1984 from three prospective, randomized, double-blind clinical trials of gentamicin, tobramycin and amikacin (5). In the United States, 2% to 5% of patients receiving AGs develop hearing loss (6,7). The longer duration of treatment and the higher dose being used, the higher susceptibility of developing ototoxicity. AG can be rapidly taken up by cochlear hair cells following systemic administration via endocytosis(8) or mechanoelectrical transduction channels at their apical membranes (9). The accumulation of AG in cochlear hair cells leads to increased production of reactive oxygen species (ROS) and activation of stress kinases and caspases, resulting in apoptosis of these cells (10). Older age, renal dysfunction, mitochondrial dysfunction and genetic factors are the known predisposing factors of the ototoxicity; however, the related mechanisms remain unclear (7,11,12). In the early studies in 1984, the researchers had already found that patients with auditory toxicity underwent AG therapy for a longer period and were more likely to be bacteremic (7). Why does AG-induced ototoxicity depend more on the duration of treatment rather than the plasma concentration of AG? Is the ototoxicity related to the endotoxin level as AGs are mainly indicated for Gram-negative bacterial infections? A recent study conducted by Koo et al. has given us insight in answering these questions (13).
By tracing fluorescently-tagged gentamicin (GTTR) in the LPS-induced endotoxemia model, Koo et al. found that the augmented accumulation of AG in cochlear walls and different cell types in the ear was proportional to the dose of LPS administered into mice, indicating LPS can potentiate the uptake and trafficking of AG across the blood-labyrinth barrier (BLB). The auditory toxicity is associated with the concentration of AG in the inner ear (14). In this study, low-dose LPS injection was shown to enhance the accumulation of GTTR in cochlear but not in serum, which is congruent with the earlier finding that the ototoxicity was independent of plasma AG concentration (7). Administration of LPS acutely increased the expressions of pro-inflammatory molecules in both cochleae and circulation but the expressions of interleukin 1-beta (IL-1β) and interleulin-10 (IL-10) were induced in circulation only. The LPS-induced inflammation in cochleae sustained till 24 hours after the initial LPS injection when the plasma concentrations of inflammatory proteins had already subsided. LPS can induce pro-inflammatory responses through activating toll-like receptor (TLR) pathways, and the observed potentiating effect of LPS on AG-induced ototoxicity was TLR4 dependent. LPS failed to elicit inflammatory response in TLR4-hyporesponsive C3H/HeJ mice, and concurrently the accumulation and uptake of AG were also reduced. More importantly, Koo et al. discovered that repeated low-dose LPS administration exacerbated AG-induced auditory brainstem response (ABR) threshold shifts, and acute LPS-induced endotoxemia did not modulate auditory threshold, suggesting the integrity of BLB remains intact in LPS-aggravated ototoxicity of AG. Low-dose LPS was shown to dilate the basal strial capillaries and decrease vasoconstrictive serotonin in serum, without affecting the permeability of BLB. It is noteworthy to investigate whether restoration of serotonin level or local application of vasoconstrictor can alleviate the endotoxemia-aggravated ototoxicity of AG. In addition, as decreased cochlear expression of proinflammatory molecules together with attenuated capillary dilation and reduced AG accumulation were observed in hyporesponsive TLR4 mice injected with LPS, local antagonism of TLR4 signaling (e.g., eritoran), or removal of LPS (e.g., polymixin B) may serve as a therapeutic option to prevent or treat AG-induced auditory damage (Figure 1).
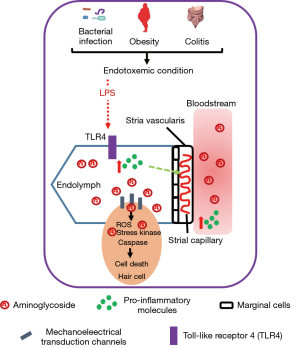
Majority of studies on AG-related ototoxicity often use healthy and uninfected animals, where the pathophysiological conditions are substantially different from patients with Gram-negative bacterial infection. In the infected patients, adaptive inflammatory response against bacterial infection is autonomous, and release of endotoxin due to the bacteriolytic effect of antibiotics contributes to endotoxemia. This study by Koo et al. highlights that this inevitable condition in patients can indeed aggravate AG-induced auditory toxicity. Their findings not only suggest the molecular mechanisms that provide insight in developing preventive or treatment options for ototoxicity, but also help to identify individuals that are susceptive to such adverse effect of AG. Patients with higher inflammatory status due to other conditions should avoid using AGs for Gram-negative infections. Metabolic endotoxemia, which is defined as a two- or three- fold increase of serum LPS level, is often observed in obese individuals (8). High-fat diet can increase intestinal permeability which allows the leaking of bacterial products such as LPS from the intestinal lumen into the bloodstream, consequently causing metabolic endotoxemia (8). Considering the growing prevalence of obesity, special attention is required for treating these patients. Furthermore, patients with autoimmune diseases such as colitis may also have weakened gut barrier and certain degree of endotoxemia (15). For all these conditions, clinicians may need to seek other options for treating Gram-negative bacterial infections.
AGs remain as the drug of choice for most of the Gram-negative bacterial infections because of their effectiveness; however, the concern of irreversible ototoxicity limits their use. In this study, Koo et al. emphasize the important effect of endotoxemia in developing of auditory toxicity, which provides an additional guideline to weighing the balance between risk and benefit of using AGs. It would make the best use of our existing effective antibiotics, and this is particularly important as very few new antibiotics have been identified in recent decades and resistance of antibiotics remains to be a major challenge for treating infectious diseases.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Begg EJ, Barclay ML. Aminoglycosides--50 years on. Br J Clin Pharmacol 1995;39:597-603. [PubMed]
- Avent ML, Rogers BA, Cheng AC, et al. Current use of aminoglycosides: indications, pharmacokinetics and monitoring for toxicity. Intern Med J 2011;41:441-9. [Crossref] [PubMed]
- Turnidge J. Pharmacodynamics and dosing of aminoglycosides. Infect Dis Clin North Am 2003;17:503-28. v. [Crossref] [PubMed]
- Lortholary O, Tod M, Cohen Y, et al. Aminoglycosides. Med Clin North Am 1995;79:761-87. [Crossref] [PubMed]
- HINSHAW HC, FELDMAN WH, et al. The clinical administration of dihydrostreptomycin in tuberculosis; a preliminary report. Am Rev Tuberc 1948;58:525-30. [PubMed]
- Rybak LP. Drug ototoxicity. Annu Rev Pharmacol Toxicol 1986;26:79-99. [Crossref] [PubMed]
- Moore RD, Smith CR, Lietman PS. Risk factors for the development of auditory toxicity in patients receiving aminoglycosides. J Infect Dis 1984;149:23-30. [Crossref] [PubMed]
- Hashino E, Shero M, Salvi RJ. Lysosomal targeting and accumulation of aminoglycoside antibiotics in sensory hair cells. Brain Res 1997;777:75-85. [Crossref] [PubMed]
- Li H, Steyger PS. Systemic aminoglycosides are trafficked via endolymph into cochlear hair cells. Sci Rep 2011;1:159. [Crossref] [PubMed]
- Huth ME, Ricci AJ, Cheng AG. Mechanisms of aminoglycoside ototoxicity and targets of hair cell protection. Int J Otolaryngol 2011;2011:937861.
- Henry KR, Chole RA, McGinn MD, et al. Increased ototoxicity in both young and old mice. Arch Otolaryngol 1981;107:92-5. [Crossref] [PubMed]
- Manian FA, Stone WJ, Alford RH. Adverse antibiotic effects associated with renal insufficiency. Rev Infect Dis 1990;12:236-49. [Crossref] [PubMed]
- Koo JW, Quintanilla-Dieck L, Jiang M, et al. Endotoxemia-mediated inflammation potentiates aminoglycoside-induced ototoxicity. Sci Transl Med 2015;7:298ra118. [Crossref] [PubMed]
- Beaubien AR, Ormsby E, Bayne A, et al. Evidence that amikacin ototoxicity is related to total perilymph area under the concentration-time curve regardless of concentration. Antimicrob Agents Chemother 1991;35:1070-4. [Crossref] [PubMed]
- Melgar S, Karlsson A, Michaëlsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol 2005;288:G1328-38. [Crossref] [PubMed]


