Intra-abdominal manifestations of pleural mesothelioma
Introduction
The majority of patients with treated or untreated pleural mesothelioma die as a result of disease persistence and progression within the thoracic cavity. Causes of death are most commonly an increasing extent of disease that causes progressive respiratory failure, pneumonia, or heart failure often with arrhythmias. Secondly, the disease progression within the mediastinum and chest wall produces an unrelenting pain syndrome which leads to cachexia. Finally, poor nutrition from dysphagia caused by tumor progression of the esophagus is common (1). However, in approximately one-third of patients, the disease penetrates the hemidiaphragm directly and may disrupt bowel function. Alternatively, in surgically-treated patients the disease may be disseminated into the abdomen at the time of diaphragm resection. These extrathoracic manifestations of the disease very often occur late in the course of the disease process. Approximately two-thirds of the patients will, at the time of death, have distant metastases with the most frequently involved organs being liver, adrenal gland, kidney, contralateral lung. When distant metastases do occur, they are predominantly of the sarcomatous histological type.
Intra-abdominal disease as a result of direct extension through the hemidiaphragm
Although unusual, direct extension of pleural mesothelioma through the hemidiaphragm to seed the peritoneal space can occur at the time of initial diagnosis of pleural disease. More frequently, direct extension occurs in patients with non-surgical management of the pleural mesothelioma allowing invasion of the chest wall and diaphragm with the resulting dissemination within the peritoneal space. A second mechanism for disease extension into the peritoneal space comes about as a result of diaphragm resection and the peritoneal contamination from dislodged mesothelioma cells or emboli into the peritoneal space. Whatever the cause of the dissemination process, patients with peritoneal involvement should be evaluated for possible favorable outcome from additional intraperitoneal surgical or regional chemotherapy interventions. In patients where combined surgical and regional chemotherapy treatments have achieved local control within the chest cavity, intra-abdominal surgery and regional chemotherapy treatment options should be considered (2,3). In patients who do not have local control within the pleural space, the treatment options are limited to systemic chemotherapy or best supportive care.
Patients with control of disease in the initially involved hemithorax, no extension to the opposite thoracic cavity, and no known metastatic disease other than in the peritoneal space should be considered for cytoreductive surgery and hyperthermic perioperative chemotherapy for disease control within the peritoneal space. Bidirectional adjuvant normothermic chemotherapy (BANC) within the peritoneal space may also be considered (4).
Table 1 presents the clinical features that suggest a favorable versus a low benefit outcome when cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) are used to treat peritoneal metastases. With some modifications, these same criteria for selection of patients for treatment by CRS and HIPEC apply not only to gastrointestinal and gynecologic cancer patients but also those who have peritoneal extension of pleural mesothelioma. Patients having an aggressive thoracic surgery, hyperthermic combined intraperitoneal and intrathoracic perioperative chemotherapy and then systemic chemotherapy may have a reduced performance status suggesting that they are at high risk for abdominal surgery. Also, this additional abdominal surgery may not be compatible with a complete recovery back to a reasonable quality of life.

Full table
The CT scan of abdomen and pelvis contrast-enhanced with both oral and intravenous contrast can be of great help in patient selection. The CT should show relative sparing of the small bowel and colon, an absence of disease outside of the abdomen and pelvis, an absence of liver metastases unless they are compatible with a wedge resection, and an absence of large volume disease within the porta hepatis. With pleural mesothelioma, a high grade malignancy, the CT peritoneal cancer index (CT-PCI) should be in the low range, certainly less than 20 (5-7).
Clinical features that suggest cytoreductive surgery and HIPEC should not be recommended include a poor performance status, rapid progression of the disease from chest into the peritoneal space with a short free interval, one or more of the concerning radiologic features, and question of control of the primary disease in the hemithorax. If the clinical features suggest a favorable outcome and no contraindications to an aggressive approach, the cytoreduction with HIPEC should be presented to the patient as a treatment option.
Cytoreductive surgery
The surgical technology required for a favorable outcome with pleural mesothelioma extending into the peritoneal space do not differ significantly from the surgical technology required to treat peritoneal mesothelioma. As shown in Table 2, this involves a series of five peritonectomy procedures and several visceral resections. All visible evidence of disease is to be removed surgically if prolonged disease control within the peritoneal space is to be achieved.
Full table
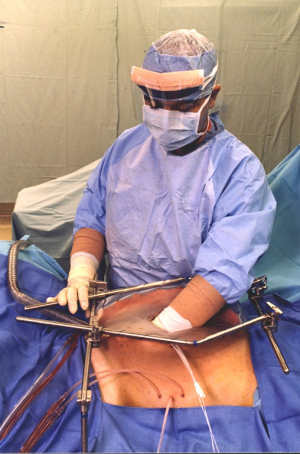
Perioperative chemotherapy as a planned part of the surgical procedure, a warm chemotherapy solution is used to wash the peritoneal surfaces following the peritonectomy procedures and visceral resections. Optimally, an open hyperthermic chemotherapy treatment prior to the completion of intestinal anastomoses, repair of seromuscular tears, and prior to closure of the abdomen will occur (Figure 1). This minimizes the possibility for tumor entrapment within suture lines or the abdominal closure (8).
Perioperative chemotherapy
There are two different HIPEC regimens that have been used in peritoneal mesothelioma patients and are appropriate for these patients with pleural mesothelioma metastatic to the peritoneal space. Selection of one treatment over the other may be influenced by molecular tumor profiling.
In general, those patients who seem to have had a response to cisplatin are treated with the chemotherapy regimen presented in the top portion of Figure 2. This is a 90-minute hyperthermic intraperitoneal treatment with cisplatin, intraperitoneal doxorubicin, and systemic ifosfamide plus mesna. In those patients who are thought to be resistant to cisplatin, hyperthermic intraperitoneal gemcitabine is recommended. This is a one-hour heated chemotherapy treatment with a single chemotherapy agent, gemcitabine. Alternatively, in patients who seem to have drug resistance to both cisplatin and gemcitabine, intraperitoneal melphalan has been shown to be of benefit.
Results of treatment
The number of patients treated to date is too few to make judgments regarding the efficacy of this approach and the prolonged benefit that may result. There is no doubt that patients who have peritoneal mesothelioma with direct extension into the pleural space may have long-term survival (9). This is conditional upon an aggressive approach to treatment of the direct extension of peritoneal mesothelioma into the pleural space. Of course, peritoneal mesothelioma is, for the most part, a much less aggressive disease process than pleural mesothelioma.
Rare manifestations of hematogenous metastases from pleural mesothelioma within the abdomen
In almost all patients who have peritoneal manifestations of pleural mesothelioma, this extension comes about through a direct and full-thickness invasion of the hemidiaphragm. Then cancer seeding occurs and is distributed by the normal peritoneal fluid around the abdomen and pelvis in a characteristic fashion. However, there are case reports of hematogenous metastases from pleural mesothelioma to abdominal and pelvic sites. The clinician should be aware that hematogenous metastasis, especially from biphasic or sarcomatoid pleural mesothelioma, is a real possibility. In Table 3, the clinical manifestations of hematogenous metastases from pleural mesothelioma are listed (10-15). For the most part, these metastases are to the highly vascularized portions of the gastrointestinal tract, the small bowel, stomach, and a single patient with colonic metastases. Also reported is a single patient with isolated pancreatic metastases from malignant pleural mesothelioma. The clinician taking care of pleural mesothelioma patients must be aware that metastatic disease within the abdominal viscera may lead to a perforation or bleeding of the gastrointestinal tract which requires emergency surgical intervention.

Full table
In summary, patients with direct extension of pleural mesothelioma into the peritoneal space may be considered for CRS and HIPEC. Disease control within the thorax, an absence of systemic metastases, and a mesothelioma distribution within the abdomen and pelvis compatible with a complete cytoreduction are the selection factors to be applied. Standard of care would indicate HIPEC to be a part of this intervention.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Pass HI, Carbone M, Krug LM, et al. Benign and malignant mesothelioma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA. editors. Cancer: Principles & Practice of Oncology, 10th Edition. Philadelphia: Wolten Kluwers Health, 2015:1738-60.
- Deraco M, Elias DM, Glehen O, et al. Peritoneal metastases and peritoneal mesothelioma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA. editors. Cancer: Principles & Practice of Oncology, 10th Edition. Philadelphia: Wolten Kluwers Health, 2015:1761-9.
- Alexander HR Jr, Baratti D, Chua TC, et al. Peritoneal mesothelioma. In: Kerr DJ, Haller DG, van de Velde CJ, et al. editors. Oxford Textbook of Oncology, Third Edition. Oxford: Oxford University Press, 2016:533-45.
- Sugarbaker PH, Turaga KK, Alexander HR Jr, et al. Management of Malignant Peritoneal Mesothelioma Using Cytoreductive Surgery and Perioperative Chemotherapy. J Oncol Pract 2016;12:928-35. [Crossref] [PubMed]
- Yan TD, Haveric N, Carmignani CP, et al. Computed tomographic characterization of malignant peritoneal mesothelioma. Tumori 2005;91:394-400. [PubMed]
- Yan TD, Brun EA, Cerruto CA, et al. Prognostic indicators for patients undergoing cytoreductive surgery and perioperative intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma. Ann Surg Oncol 2007;14:41-9. [Crossref] [PubMed]
- Yan TD, Haveric N, Carmignani CP, et al. Abdominal computed tomography scans in the selection of patients with malignant peritoneal mesothelioma for comprehensive treatment with cytoreductive surgery and perioperative intraperitoneal chemotherapy. Cancer 2005;103:839-49. [Crossref] [PubMed]
- Sugarbaker PH, Yan TD, Stuart OA, et al. Comprehensive management of diffuse malignant peritoneal mesothelioma. Eur J Surg Oncol 2006;32:686-91. [Crossref] [PubMed]
- Sugarbaker PH, Welch LS, Mohamed F, et al. A review of peritoneal mesothelioma at the Washington Cancer Institute. Surg Oncol Clin N Am 2003;12:605-21. xi. [Crossref] [PubMed]
- Chen HC, Tsai KB, Wang CS, et al. Duodenal metastasis of malignant pleural mesothelioma. J Formos Med Assoc 2008;107:961-4. [Crossref] [PubMed]
- Lin YT, Wu BS, Yang SF, et al. Isolated pancreatic metastasis of a malignant pleural mesothelioma. Kaohsiung J Med Sci 2009;25:395-400. [Crossref] [PubMed]
- Gocho K, Isobe K, Kaburaki K, et al. Malignant pleural mesothelioma presenting as an acute surgical abdomen due to metastatic jejunal perforation. Intern Med 2010;49:597-601. [Crossref] [PubMed]
- Sibio S, Sammartino P, Accarpio F, et al. Metastasis of pleural mesothelioma presenting as bleeding colonic polyp. Ann Thorac Surg 2011;92:1898-901. [Crossref] [PubMed]
- Falkenstern-Ge RF, Kimmich M, Bode-Erdmann S, et al. Pleural mesothelioma presenting as periumbilical metastasis: the first clinical documentation. Case Rep Oncol Med 2013;2013:198729. [Crossref] [PubMed]
- Navarro García MI, Sánchez Pérez A, Vázquez Rojas JL. Jejunal Perforation by Metastasis of Malignant Pleural Mesothelioma. Arch Bronconeumol 2015;51:366-7. [PubMed]



