Not all extracellular vesicles were created equal: clinical implications
Extracellular vesicles are lipid membrane-bound particles containing cytoplasmic and transmembrane proteins, DNA and RNA that are released from cells in a variety of circumstances and contribute to cell-to-cell communication and other processes. Recent research has emphasized the potential role of microvesicles in cancer cell signaling and tumor biology, from metastasis to cancer-associated thrombosis (1,2). In this regard, Muhsin-Sharafaldine et al. report in Oncogene on the contribution of diverse types of microvesicles (exosomes, microvesicles and apoptotic vesicles) to the procoagulant and immunogenic properties of melanoma (3).
Different types of extracellular vesicles
Mammalian and non-mammalian cells release different types of extracellular vesicles (4). Three main pathways for the generation of microvesicles are recognized (Figure 1A).
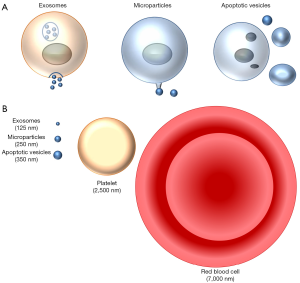
Exosomes are small particles (<150 nm) generated intracellularly inside multivesicular endosomes or multivesicular bodies (Figure 1B). Extracellular vesicles may be produced by budding from the extracellular membrane yielding particles from 100 to 1,000 nm known as microvesicles, ectosomes or microparticles. There are some specialized “microparticles”. Thus, platelets may be considered a very specialized form of extracellular vesicles released from megakaryocytes as the end products of membrane protrusions that extend into the sinusoidal vessels, where they are sheered off by blood flow in the process known as thrombopoiesis or platelet biogenesis (5). Finally, apoptotic vesicles are released during apoptotic cell death and are generally larger (100–5,000 nm in diameter). During apoptosis, proteases such as caspases are activated and breakdown intracellular proteins, resulting in nuclear condensation and fragmentation and cell fragmentation, with preservation of cell membrane integrity until very late stages of the process (6). Indeed, apoptotic cells are usually engulfed by adjacent cells before lysis, and apoptosis is usually considered a non-inflammatory form of cell death. Erythropoiesis shares some features with apoptosis, such as reduction in cell size, chromatin and nuclear condensation, and enucleation that require caspase-3 activity (7).
Extracellular vesicles in inflammation
Extracellular vesicles contribute to several pathophysiological responses. Thus, extracellular vesicles derived from epithelium activate wound repair circuits (8). However, they may also contribute to the pathogenesis of infection and sterile inflammatory conditions (9). Danger-associated molecular patterns (DAMPs), such as extracellular ATP, are released during tissue damage and activate the inflammasome in macrophages, amplifying inflammation. Inflammasome-induced activation of an intracellular caspase-1/calpain cysteine protease cascade facilitates the formation and release of phosphatidylserine-positive, highly procoagulant microparticles (10). Thus, galectin-3, a molecule contributing to atherothrombosis, is released from monocyte macrophages in exosomes (11). Parenchymal cells, such as kidney tubular cells, also release exosomes constitutively (12). Exosomes may be used as biomarkers (9). Thus, urinary exosomes from diabetic nephropathy patients differentially expressed a panel of 3 proteins (AMBP, MLL3, and VDAC1) when compared to non-diabetic controls (13), while osteoprotegerin was increased in urinary exosome-like vesicles from patients with autosomal dominant polycystic kidney disease (12). Furthermore, release of extracellular vesicles from non-mammalian cells may be involved in the pathogenesis of infectious disease. Thus, extracellular vesicles from Trypanosoma brucei mediate virulence factor transfer and erythrocyte remodeling, causing anemia (14).
Extracellular vesicles and cancer
Cancer cells also release several types of extracellular vesicles that are uptaken by other cells, potentially leading to transfer of functional mRNA and to altered cellular behavior (15). Indeed, extracellular vesicles released by malignant tumor cells are taken up by less malignant tumor cells located within the same and within distant tumors in vivo (15). Extracellular vesicles carry mRNAs involved in migration and metastasis and may indeed increase the migratory behavior and metastatic capacity of less malignant cells in vivo (15). However, the exact microvesicles that display this activity remain to be clearly identified (4).
Exosomes from lung-, liver- and brain-tropic tumor cells express specific integrins that allow preferential uptake by resident cells at their predicted destination, promoting the preparation of the pre-metastatic niche (16). Conversely, normal cell-derived exosomes mediate an intercellular transfer of miRNAs that specifically suppresses expression of tumor-related genes, as it is the case for astrocyte-derived exosomes and PTEN expression by brain metastasis (17). Melanoma-derived exosomes reprogrammed bone marrow progenitors toward a pro-vasculogenic phenotype (18). Reducing Met expression in exosomes diminished the pro-metastatic behavior of bone marrow cells. RNA interference for RAB27A, a regulator of membrane trafficking and exosome formation, decreased exosome production by melanoma cells, preventing bone marrow education and reducing tumor growth and metastasis. This information may be useful in designing novel therapeutic strategies. In addition, specific expression of certain proteins in tumor-derived exosomes may allow non-invasive, early diagnosis of tumors such as pancreatic cancer (19).
Extracellular vesicles and the immune response
Extracellular vesicles play a key role in immune regulation (20). During transplant rejection, exosome-like extracellular vesicles from donor dendritic cells interact with recipient dendritic cells, leading to their activation and triggering of alloreactive T cell activation (21). Dendritic cells are also thought to be important for immune cell-dependent tumor rejection and early clinical trials have demonstrated the feasibility and safety of the approach (22). Extracellular vesicles from cytotoxic T cells are loaded with cytokines promoting cell death. By contrast, extracellular vesicles also contribute to tumor-mediated immune suppression. Tumor-derived exosomes carry immunosuppressive molecules and factors that interfere with immune cell functions (23). Melanoma-derived extracellular vesicles disseminate via lymphatics and preferentially bind subcapsular sinus CD169+ macrophages in tumor-draining lymph nodes (24). CD169+ macrophage may act as tumor suppressors by containing the spread of vesicles. In this regard, they prevent the interaction of melanoma-derived vesicles with lymph node cortex B cells, an interaction that may foster tumor-promoting humoral immunity (24).
Properties of diverse melanoma-derived extracellular vesicles
As discussed above, extracellular vesicles derived from tumor cell may contribute to multiple tumor-related complications, from metastasis to suppression of the anti-tumor immune response to promotion of this immune response to the thrombotic complications of cancer. A correct understanding of the specific molecule and the specific vesicles involved in each of these complications may be used to design therapeutic approaches that differentially modulate the amount and properties of the different vesicles to optimize the therapeutic benefit, potentiating some functions of extracellular vesicles while dampening others. Muhsin-Sharafaldine et al. have now explored the specific molecular composition and properties of exosomes, microvesicles and apoptotic vesicles derived from melanoma cells (3). The pattern of surface and cytoplasmic molecule expression differed between the vesicle types and this was associated with different procoagulant and immunogenic functions.
Melanoma cells were either killed by adding doxorubicin to generate apoptotic vesicles or cultured for 48 hours to generate exosomes or microvesicles. As a result, there is no information of the relative number of the different vesicles released under the same culture conditions. An approach based on amount of protein suggested that the bigger the vesicles, the higher the amount of protein, but it did not provide information on number of vesicles.
The composition of the vesicles was assessed using proteomic approaches and the main findings are summarized in Table 1. Exosomes were enriched in histones and heat shock proteins, and the ten most abundant ion scores in exosomes included histones (H2A, H2B, H3.1 and H4), heat shock proteins (GRP78 and HSC71) and the tetraspanin CD81.
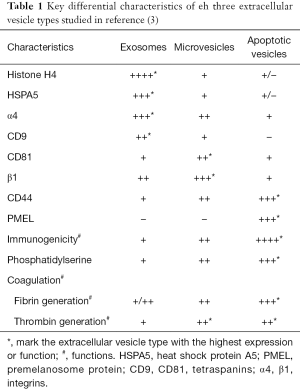
Full table
From a functional point of view, apoptotic vesicles had the highest functional impact, assessed either as immunogenicity or as a prothrombotic potential (Table 1). Apoptotic vesicles were more immunogenic, and mice immunized with antigen-pulsed apoptotic vesicles and challenged with melanoma cells were protected up to 60 days, while lower protection rates were afforded by microvesicles and exosomes. In this regard, only apoptotic vesicles showed enrichment for the melanoma associated antigen PMEL, thus potentially contributing to generate tumor-specific immunity. However, the immunogenicity of apoptotic vesicles is a surprising finding, since apoptosis is, in general, a non-inflammatory, non-immunogenic form of cell death. The observed immunogenicity of apoptotic vesicles may be due to the fact that under the culture conditions, these vesicles were not rapidly engulfed and cleared by adjacent cells, as would occur in vivo, in which different cell types, including macrophages are present. Rather, the cell culture conditions of a homogenous cell type exposed to a toxic that kills or injures all cells in the culture may impair engulfment of apoptotic vesicles. If this were the case, the immunogenicity observed under the experimental conditions may not occur in vivo due to rapid clearance of apoptotic vesicles. Another possibility is that doxorubicin also caused immunogenic forms of cell death, such as necroptosis. In this regard, lethal stimuli may cause both apoptosis and necroptosis within the same cell culture (25,26). While the generation of vesicles by necroptotic cells has not been described, it is conceivable the simultaneous occurrence of apoptosis and necroptosis may result in the generation immunogenic apoptosis vesicles. This may occur through adsorption of immunogenic components on the surface of apoptotic bodies. This hypothesis merits further exploration. The observed immunogenicity of apoptotic vesicles may be used to develop anti-tumor vaccines. In addition, it may identify a further mechanism by which chemotherapeutic agents may promote anti-tumor immunity in vivo, especially if these agents also induce necroptosis.
Microvesicles and apoptotic vesicles, especially the latter in some assays, were more procoagulant than exosomes and functional studies disclosed that tissue factor and phosphatidylserine were critical for procoagulant activity. In this regard, this may be an untoward effect of chemotherapeutic agents. A better understanding of the mechanisms involved may result in the development of novel therapeutic approaches that target specifically this well-known complication of cancer and its therapy.
Acknowledgements
Funding: Grant support—ISCIII and FEDER funds PI016/02057, Sociedad Española de Nefrologia, ISCIII-RETIC REDinREN/RD016/0009. Salary support—Programa Intensificación Actividad Investigadora (ISCIII/Agencia Laín-Entralgo/CM) to A Ortiz.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Kalluri R. The biology and function of exosomes in cancer. J Clin Invest 2016;126:1208-15. [Crossref] [PubMed]
- Kosaka N, Yoshioka Y, Fujita Y, et al. Versatile roles of extracellular vesicles in cancer. J Clin Invest 2016;126:1163-72. [Crossref] [PubMed]
- Muhsin-Sharafaldine MR, Saunderson SC, Dunn AC, et al. Procoagulant and immunogenic properties of melanoma exosomes, microvesicles and apoptotic vesicles. Oncotarget 2016;7:56279-94. [PubMed]
- Tkach M, Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016;164:1226-32. [Crossref] [PubMed]
- Eto K, Kunishima S. Linkage between the mechanisms of thrombocytopenia and thrombopoiesis. Blood 2016;127:1234-41. [Crossref] [PubMed]
- Sanz AB, Santamaría B, Ruiz-Ortega M, et al. Mechanisms of renal apoptosis in health and disease. J Am Soc Nephrol 2008;19:1634-42. [Crossref] [PubMed]
- Zhao B, Mei Y, Schipma MJ, et al. Nuclear Condensation during Mouse Erythropoiesis Requires Caspase-3-Mediated Nuclear Opening. Dev Cell 2016;36:498-510. [Crossref] [PubMed]
- Leoni G, Neumann PA, Kamaly N, et al. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest 2015;125:1215-27. [Crossref] [PubMed]
- Zhang W, Zhou X, Zhang H, et al. Extracellular vesicles in diagnosis and therapy of kidney diseases. Am J Physiol Renal Physiol 2016;311:F844-51. [Crossref] [PubMed]
- Rothmeier AS, Marchese P, Petrich BG, et al. Caspase-1-mediated pathway promotes generation of thromboinflammatory microparticles. J Clin Invest 2015;125:1471-84. [Crossref] [PubMed]
- Madrigal-Matute J, Lindholt JS, Fernandez-Garcia CE, et al. Galectin-3, a biomarker linking oxidative stress and inflammation with the clinical outcomes of patients with atherothrombosis. J Am Heart Assoc 2014;3:e000785. [Crossref] [PubMed]
- Benito-Martin A, Ucero AC, Zubiri I, et al. Osteoprotegerin in exosome-like vesicles from human cultured tubular cells and urine. PLoS One 2013;8:e72387. [Crossref] [PubMed]
- Zubiri I, Posada-Ayala M, Sanz-Maroto A, et al. Diabetic nephropathy induces changes in the proteome of human urinary exosomes as revealed by label-free comparative analysis. J Proteomics 2014;96:92-102. [Crossref] [PubMed]
- Szempruch AJ, Sykes SE, Kieft R, et al. Extracellular Vesicles from Trypanosoma brucei Mediate Virulence Factor Transfer and Cause Host Anemia. Cell 2016;164:246-57. [Crossref] [PubMed]
- Zomer A, Maynard C, Verweij FJ, et al. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 2015;161:1046-57. [Crossref] [PubMed]
- Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015;527:329-35. [Crossref] [PubMed]
- Zhang L, Zhang S, Yao J, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 2015;527:100-4. [Crossref] [PubMed]
- Peinado H, Alečković M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012;18:883-91. [Crossref] [PubMed]
- Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015;523:177-82. [Crossref] [PubMed]
- Robbins PD, Dorronsoro A, Booker CN. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J Clin Invest 2016;126:1173-80. [Crossref] [PubMed]
- Liu Q, Rojas-Canales DM, Divito SJ, et al. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J Clin Invest 2016;126:2805-20. [Crossref] [PubMed]
- Pitt JM, André F, Amigorena S, et al. Dendritic cell-derived exosomes for cancer therapy. J Clin Invest 2016;126:1224-32. [Crossref] [PubMed]
- Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest 2016;126:1216-23. [Crossref] [PubMed]
- Pucci F, Garris C, Lai CP, et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science 2016;352:242-6. [Crossref] [PubMed]
- Martin-Sanchez D, Ruiz-Andres O, Poveda J, et al. Ferroptosis, but Not Necroptosis, Is Important in Nephrotoxic Folic Acid-Induced AKI. J Am Soc Nephrol 2017;28:218-29. [Crossref] [PubMed]
- Linkermann A, Bräsen JH, Darding M, et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A 2013;110:12024-9. [Crossref] [PubMed]


