The proliferative potential of human cardiac stem cells was unaffected after a long-term cryopreservation of tissue blocks
Introduction
The initial attempt to treat heart failure patients with autologous c-kit-positive cardiac stem cells (CSCs) (1) was extremely successful with the absence of major adverse events (2,3). Accordingly, it would be plausible to treat repeatedly, whenever necessary, by administering his/her own CSCs at a certain interval. These therapies were initially contrived to target adult patients with ischemic disorders, but the potential applications of CSCs may not be limited to this area. For instance, the recent progress of surgical interventions on congenital heart diseases, which affect 0.8% of live births, rescued >85% of pediatric patients but at the same time necessitated the treatment of spontaneously developed heart failure later in adulthood (4).
Based on these potential clinical demands, this research aimed to characterize human CSCs prepared from cryopreserved tissue samples. Specifically, we have analyzed the proliferative potential of freshly prepared CSCs and compared it to that of CSCs derived from surgical specimens stored in liquid nitrogen for about 2 years. Also, CSCs prepared from the right atrium (RA), which were employed for the clinical trial mentioned above, were compared to those obtained from the left atrium (LA) or left ventricle (LV) in the present study.
We hope that in the near future a piece of resected tissue can be frozen during cardiac operation and preserved in a “bank” as a back-up resource for later therapeutic use, without initiating the CSC isolation procedure immediately after the surgery.
Methods
Ethical concern
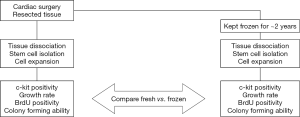
Every participant in this study provided a written consent. The entire research design, summarized in Figure 1, was viewed and approved by the Institutional Review Board of Sakakibara Heart Institute as well as that of Tokai University (#12I-18, #13I-27).
Tissue specimens
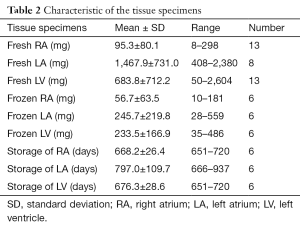
Tissue blocks of ~0.5 gram were obtained from RA and LV of patients (n=13 each) receiving left ventriculoplasty at Tokai University Hospital due to ischemic cardiomyopathy. The specimens of LA were harvested at the Sakakibara Heart Institute from patients (n=8) suffering mainly from valvular heart diseases coupled with refractory atrial fibrillation. The LA tissues were aseptically packaged upon surgery and transferred to Tokai University. Each cardiac tissue block was divided into a few pieces, and the largest piece was freshly used while the rest was stored for 1.8 to 2.5 years.
CSC isolation
CSCs were freshly isolated from the RA, LA, and LV as described previously (5). In brief, cardiac tissues were cut with scissors into pieces of ~1 mm in size. They were digested for 1 hour at 37 °C in 1–2 mg/mL of collagenase (Serva NB4) and further dissociated for 31 seconds in a gentleMACS C tube (Miltenyi Biotec) utilizing the preset program B_01. The resulting small cells were passed through a 40-µm filter and cultured in Ham’s F12 Nutrient Mixture (HyClone) containing 10% fetal bovine serum (HyClone), 0.2 mM glutathione (Tanabe, Japan), 10 µg/L trafermin (Kaken, Japan), 5 U/L epoetin alfa (Kyowa Hakko Kirin, Japan), and antimicrobial agents (“growth medium”). Subsequently, cells positive for c-kit were sorted using the MACS separation system and CD117 MicroBead (Miltenyi Biotec) (1), following the manufacturer’s instructions.
Cryopreservation of cardiac tissues
Upon cardiac operation at both hospitals, additional pieces of surgical specimens were immersed in the “freezing medium”, consisting of the growth medium supplemented with 10% dimethyl sulphoxide (DMSO, Sigma), in each cryogenic vial, respectively; they were housed in BICELL (Nihon Freezer Co., Ltd., Japan), a specialized container for cryopreservation of cells, and kept at −80 °C overnight. On the following day, the vials were transferred into boxes and kept in liquid nitrogen for ~2 years (range, 651 to 937 days). CSCs isolated from frozen RA, LA, and LV tissues (n=6 each), respectively, were analyzed and compared to the data obtained from the fresh samples of the same patients.
Immunocytochemistry for c-kit
An aliquot of CSCs were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) and incubated overnight in a refrigerator with Rabbit Anti-Human CD117 (Dako) diluted 1:40 in PBS. After washing it with PBS, Rhodamine-conjugated Donkey Anti-Rabbit IgG (Jackson ImmunoResearch) at a concentration of 1:50 was applied, and the sample was incubated for an hour at 37 °C. Nuclei were stained using 4',6-diamidino-2-phenylindole (DAPI; Sigma), and Vectashield (Vector Labs) was mounted prior to the observation using a fluorescent microscope BZ-9000 (Keyence, Japan). At the same time, another group of cells was treated without the primary antibody and served as a negative control. The c-kit positivity was assessed utilizing the ImageJ software (available at: http://imagej.nih.gov/ij/).
Growth rate assay
Five thousand CSCs were seeded on a 35-mm dish equipped with a 2-mm grid. The cell number in each of the pre-determined nine squares was counted and summed up on 4 consecutive days. When the cell number increases from N1 to N2 in a given time interval (dT), N1 × 2^(dT/PDT) = N2, in which PDT stands for population doubling time. Therefore, dT/PDT = log2(N2/N1) = log2N2 − log2N1. By plotting the time on the X-axis versus the cell number in a base-2 logarithmic scale on the Y-axis, 1/PDT = dY/dX, i.e., the slope of the trend line. Finally, the growth rate is defined as the number of doublings that occur per unit of time, which is 1/PDT.
BrdU immunostaining
On a 35-mm dish, 50,000 CSCs were seeded one day prior to the 1-hour incubation with a medium supplemented with 1× BrdU (Roche). Subsequently, the cells were immersed in a fixing solution containing 15 mM glycine and 70% ethanol (pH 2.0) and incubated for 30 minutes with Anti-BrdU (Roche) diluted in the Incubation Buffer (Roche). After washing the specimen, Anti-mouse IgG-FITC (Roche) was applied and kept at 37 °C for half an hour, before adding DAPI. BrdU positivity was calculated as the ratio of BrdU-positive cells to the total number of cells.
Colony forming unit (CFU) assay
For this purpose, 1,000 CSCs were plated on a 100-mm dish so that each cell would be well isolated from the others. A week later, the number of newly formed colonies on the entire dish was counted and recorded as the CFU per 1,000 cells; a colony was defined as a group of 10 cells or more within a high-power field, which went through more than three rounds of division during the 7-day observation period. In most cases, a colony held ~30 cells reflecting ~5 divisions.
Statistical analysis
Results are reported as the mean ± standard deviation (SD). SPSS Statistics Version 24 (IBM) was used for the analysis. The normality of the measurements was evaluated by the Shapiro-Wilk test prior to the analyses. The comparison of the data among CSCs derived from the RA, LA, and LV tissues was performed using the Bonferroni method. For comparing the CSCs isolated from fresh and frozen tissue blocks of the same patient, the frozen-to-fresh ratio of the previously mentioned measurements was calculated; since the SDs of these values of freshly prepared CSCs were higher than 30% of the respective measurements, we considered any difference larger than 30% to be meaningful. P value of less than 0.05 was considered statistically significant.
Results
CSC preparation from fresh and frozen specimens
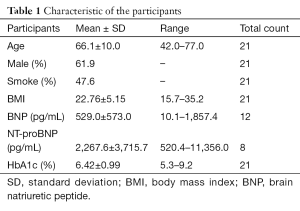
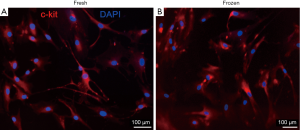
The clinical data of the participants and the nature of cardiac tissues are summarized in Tables 1,2, respectively. Following the primary cultivation of cardiac cells, CSCs were sorted utilizing magnetic microbeads conjugated with an anti-c-kit antibody. The yielded proportion of CSCs was between 2% and 8% of the parental population in the majority of cases. Most importantly, we were able to isolate and culture over 1 million CSCs from both fresh and frozen tissues of all patients; this quantity was used for the preceding clinical trial (2,3). As represented in Figure 2, after immunostaining every sample, the positivity of c-kit was found to fall mostly in the range of 80% to 95%; there was no clear difference between the fresh and frozen samples used to prepare the CSCs.
Full table
Full table
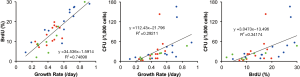
Growth potential of freshly isolated CSCs
Cultured CSCs were analyzed during passages 4 to 7, where the cells seeded immediately after tissue dissociations were designated as passage 0. In order to estimate their growth potential, we employed three independent parameters: growth rate (number of doublings per day), BrdU positivity, and CFU (per 1,000 cells). Figure 3 depicts the interrelationship of these values found in CSCs isolated from fresh tissues. The average values of all regions combined were 0.48±0.19 for the growth rate, 15.0%±7.6% for the BrdU positivity, and 32.2±39.5 for the CFU. While the values of CFU were not normally distributed based on the Shapiro-Wilk test, the growth rate and BrdU positivity showed a strong correlation (P<0.001, Pearson’s coefficient 0.866) among the comparisons of these indicators. Accordingly, we focused on the growth rate and BrdU positivity in the following analyses.
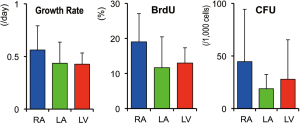
Growth potential of CSCs from different regions
The growth rate and BrdU positivity were calculated in CSCs obtained from each cardiac region (Figure 4). As shown, CSCs of all three areas showed a great proliferative ability. Among them, the values of the right atrial samples tended to be higher than the other two groups, without a statistical significance. Note that the right atrial CSCs and left ventricular CSCs were obtained from the same participants, while those from the LA were isolated from patients of different etiologies.
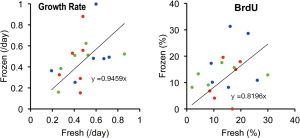
Difference in the growth capacity of CSCs from fresh and frozen tissues
We were able to successfully isolate and culture CSCs from all tissue specimens kept frozen for ~2 years. The subsequently measured growth rate and BrdU positivity were 0.47±0.22 and 13.7%±7.9%, respectively (Figure 5), which were similar to the values of fresh tissues described above. In order to strictly compare CSCs obtained from these materials, the frozen-to-fresh ratio of each individual was calculated; the 95% confident intervals of the ratio of the growth rate and BrdU positivity were (0.830, 1.287) and (0.744, 1.785), respectively. As we preset a difference of 30% to be meaningful (see methods), the growth potential of the CSCs derived from frozen tissues was not inferior to that of freshly prepared cells. In other words, the long-term cryopreservation of specimens did not significantly decline the proliferative activity of the derivative CSCs.
Discussion
In our attempt, CSCs were successfully obtained from every surgical specimen that was kept frozen for ~2 years.
A former study depicted the maintained phenotypes of human mesenchymal stromal cells (MSCs) after 1-week-long cryopreservation of the parental bone marrow mononuclear cell (BM-MNC) population (6). Similarly, the cryopreservation of human BM-MNCs, for a period of 1 to 2 months, did not spoil the cellular function of the subsequently isolated MSCs (7). More recently, Shen et al. described the effectiveness of human MSC isolation from bone marrow cells that were cryopreserved for 23–25 years, although they compared these cells with freshly isolated MSCs from different individuals. The adherence rate of the preserved cells decreased in the initial culture; then surprisingly at the third passage, the MSCs derived from both groups’ possessed similar features (8).
Subsequently, solid organs and connective tissues also became the focus of research; there are several former studies investigating the phenotypes of mesenchymal or stem/progenitor cells isolated from fresh and cryopreserved surgical specimens, respectively. The targeted tissues include deciduous teeth (9), dental follicles (10), striated muscles (11), adipose tissues (12), arterial walls (13), umbilical cords (14), and testicular tissues (15). All these studies demonstrated the feasibility of cell isolation from frozen materials as well as the qualitative equivalence of such prepared cells compared to those freshly obtained.
To the best of our knowledge, however, there is no preceding research on the characteristics of stem/progenitor cells derived from the human heart, before and after 1-year-long cryopreservation. Our current study suggested that the growth potential of CSCs did not significantly decline after the prolonged storage of the original tissue in liquid nitrogen, which is consistent with the previous studies on various organs as mentioned above.
Regarding the comparison among different cardiac regions, the distinct etiology may have influenced the properties of CSCs; especially, the left ventricular samples taken during the left ventriculoplasty, involving infarcted and scarred myocardium, may contain more fibrous materials than the other specimens. In addition, accompanying medication and other external factors might have affected the outcome. These elements compose the limitations of this study.
Based on our observations, a resected specimen of any region of the heart could be kept frozen in the CSC freezing medium in an operation room to be used for therapeutic purposes in the future; specimens in the storage can be transferred to a tissue-processing facility for CSC isolation and preparation whenever necessary. The regenerative potential of CSCs isolated from frozen tissues may need to be examined in vivo by a future study, in order to complement and strengthen our findings.
Conclusions
Here we demonstrated that human CSCs in various cardiac regions preserved their proliferative potential following an extended storage of the tissues of origin.
Acknowledgements
The authors would like to acknowledge Dr. Emiko Hayashi and Ms. Yukiko Kirimura for their technical assistance in acquiring the data. Also, the authors express their deep appreciation towards Dr. Tomohiro Sawa for his helpful advice on the statistical analysis, Dr. Jun Umemura for strictly controlling the coding document to secure the patients’ identity and privacy, and Ms. Midori Hosoda for her critical reading of the manuscript.
Funding: This work was supported in part by the Research and Study Project of the Tokai University Educational System General Research Organization (PJ2013-03) as well as the Vehicle Racing Commemorative Foundation (#27-595).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of Sakakibara Heart Institute as well as that of Tokai University (#12I-18, #13I-27) and written informed consent was obtained from all patients.
References
- Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A 2007;104:14068-73. [Crossref] [PubMed]
- Bolli R, Chugh AR, D'Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 2011;378:1847-57. [Crossref] [PubMed]
- Chugh AR, Beache GM, Loughran JH, et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation 2012;126:S54-64. [Crossref] [PubMed]
- Gurvitz M, Burns KM, Brindis R, et al. Emerging Research Directions in Adult Congenital Heart Disease: A Report From an NHLBI/ACHA Working Group. J Am Coll Cardiol 2016;67:1956-64. [Crossref] [PubMed]
- Hayashi E, Cho Y, Inoue M, et al. The characterization of cardiac stem cells obtained from patients who have received left ventriculoplasty. Stem Cell & Translational Investigation 2015;2:e537.
- Haack-Sorensen M, Bindslev L, Mortensen S, et al. The influence of freezing and storage on the characteristics and functions of human mesenchymal stromal cells isolated for clinical use. Cytotherapy 2007;9:328-37. [Crossref] [PubMed]
- Casado-Díaz A, Santiago-Mora R, Jiménez R, et al. Cryopreserved human bone marrow mononuclear cells as a source of mesenchymal stromal cells: application in osteoporosis research. Cytotherapy 2008;10:460-8. [Crossref] [PubMed]
- Shen JL, Huang YZ, Xu SX, et al. Effectiveness of human mesenchymal stem cells derived from bone marrow cryopreserved for 23-25 years. Cryobiology 2012;64:167-75. [Crossref] [PubMed]
- Lee HS, Jeon M, Kim SO, et al. Characteristics of stem cells from human exfoliated deciduous teeth (SHED) from intact cryopreserved deciduous teeth. Cryobiology 2015;71:374-83. [Crossref] [PubMed]
- Park BW, Jang SJ, Byun JH, et al. Cryopreservation of human dental follicle tissue for use as a resource of autologous mesenchymal stem cells. J Tissue Eng Regen Med 2017;11:489-500. [Crossref] [PubMed]
- Sumino Y, Hirata Y, Hanada M, et al. Long-term cryopreservation of pyramidalis muscle specimens as a source of striated muscle stem cells for treatment of post-prostatectomy stress urinary incontinence. Prostate 2011;71:1225-30. [Crossref] [PubMed]
- Choudhery MS, Badowski M, Muise A, et al. Cryopreservation of whole adipose tissue for future use in regenerative medicine. J Surg Res 2014;187:24-35. [Crossref] [PubMed]
- Valente S, Alviano F, Ciavarella C, et al. Human cadaver multipotent stromal/stem cells isolated from arteries stored in liquid nitrogen for 5 years. Stem Cell Res Ther 2014;5:8. [Crossref] [PubMed]
- Choudhery MS, Badowski M, Muise A, et al. Utility of cryopreserved umbilical cord tissue for regenerative medicine. Curr Stem Cell Res Ther 2013;8:370-80. [Crossref] [PubMed]
- Baert Y, Braye A, Struijk RB, et al. Cryopreservation of testicular tissue before long-term testicular cell culture does not alter in vitro cell dynamics. Fertil Steril 2015;104:1244-52.e1-4.