Ultrasound to assess diaphragmatic function in the critically ill—a critical perspective
Introduction
Ultrasonography (US) is an increasingly popular diagnostic tool in the intensive care unit (ICU), due to its easy applicability, low cost, non-invasiveness, fast learning curve and the range of information it can offer about different organs and tissues. As the enthusiasm around this topic spreads, so do the fields of application and the research around it. Over the past years an exponentially growing number of articles have been published on assessing the diaphragm through US (November 2016: >1,800 papers in English). It has been demonstrated that parameters such as diaphragm motion, thickness and thickening fraction (TF) can be used for the quantification of diaphragm dysfunction, atrophy and respiratory effort in mechanically ventilated patients. This may be important from a clinical perspective to, for instance, predict successful extubation in mechanically ventilated patients or to evaluate the effect of inspiratory muscle training.
Recently, a systematic review on the role of US to assess diaphragm function in critically ill patients has been published by Zambon and colleagues (1). In their study, they included 20 studies totalling a sum of 875 patients. The authors concluded that ultrasound could be a useful tool to assess diaphragm dysfunction in critically ill patients. While we largely share the enthusiasm around this topic, at this point we wish to warn that there are some aspects that need to be taken into consideration when using this technique in routine clinical care. In this paper we will outline the reasons behind our appeal to caution and take a brief look into the future of diaphragm US.
Accuracy
The diaphragm is a rather thin muscle with a mean thickness of 1.7–2 millimetre (mm) (95% CI: 1.7–2.0 mm) as evaluated in 109 healthy subjects when measured in the zone of apposition (2). If one measures muscle thickness (tdi) at end inspiration (tdi-end inspiration) and end expiration (tdi-end expiration) the diaphragm TF can be calculated [(tdi end-inspiration − tdi end-expiration)/tdi-end expiration ×100)]. Cut-off values for predicting successful extubation presented in earlier studies ranged from 30–36% (3-5). For healthy individuals thickening has an extremely large range with values ranging from 24.5% to 53.2% during normal breathing (6), up to 131% during forceful inspiration (7). As one might have already guessed, this means that changes considerably less than 0.5 millimetre have to be accurately assessed, while some probes have a smallest measurable distance of 0.1 mm (given that the speed of sound waves averages 1,540 m/s in human tissue, the range of high frequency probes lies around 3–15 MHz and that λ= v/f = 1,540/15×106 =0.1×10−3 m =0.1 mm) which equals 5–7% of the total thickness.
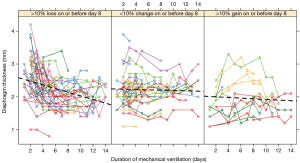
While on average data on diaphragm thickness seem to be accurate, a more careful evaluation of the individual data may be reason for concern. For instance, it can be derived from the individual patient data from the study by Goligher and colleagues (8) that differences as large as 0.5 mm occur within a set measurements and that thickness increases and decreases within two days (Figure 1, AJRCCM Figure E2). This is unlikely the result of muscle atrophy or hypertrophy. Another article by the same author already suggested, that for this reason thickness and thickening aren’t feasible for interpatient comparison (9).
Breathing effort
In healthy subjects, when the diaphragm contracts, it moves caudally and decreases pleural pressure resulting in generation of inspiratory flow. In mechanically ventilated patients, caudal movement of the diaphragm may also result from the positive pressure applied by the ventilator during inspiration. Obviously, under these conditions movement of the diaphragm cannot be used to assess diaphragm function. Equally, the same counts for changes in muscle thickness, because active contractile thickening of the diaphragm and thickening due to passive displacement can’t be distinguished. This makes it hard to assess diaphragm breathing effort.
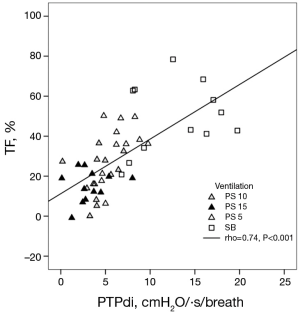
Vivier and colleagues (10) investigated if changes in diaphragm thickness do correlate with the work the diaphragm delivers during breathing. They conducted their study on 12 patients, measured diaphragm TF and calculated the transdiaphragmatic pressure-time product per breath (PTPdi) (PTPdi per breath = average inspiratory pressure × time/number of breaths) as an indicator of muscle effort. Even though they demonstrated a significant correlation between the two parameters, the data shows that a given TF can be associated with a wide range of PTPdi values (0 to approximately 8 cmH2O/·s/breath; Figure 2). These data are largely in line with data collected by Umbrello and colleagues (n=25 patients) (11), who evaluated several indices to assess muscle effort and their correlation with diaphragmatic parameters, such as thickening and motion. Just like Vivier and colleagues they found a significant correlation with thickening. Finally, Goligher (n=5 patients) (9) evaluated the correlation between diaphragm muscle effort expressed as transdiaphragmatic pressure and thickening of the muscle in patients after major surgery. They concluded that the degree of thickening varies strongly between patients at a given level of diaphragm effort and that inspiratory volumes and pressure generation are dissociated as well. This means that the amount of diaphragmatic contraction and correlating work of breathing varies between people and raises the question, if US can be used to quantify the work of breathing. Furthermore, most studies evaluating aforementioned parameters were conducted in healthy volunteers, small or highly selected patient groups and/or during manoeuvres such as sniffing or forceful in- and exhalation. These settings are hardly comparable to ICU patients. Therefore, we conclude that today ultrasound has not been sufficiently validated to quantify diaphragm effort.
Consistency
Generating ultrasonographic images of the diaphragm requires a so called acoustic window, which is mainly provided by the liver and the spleen. However, large parts of the diaphragm are not accessible to US due to the lack of an acoustic window. Accordingly, we have to assume that the parts of the diaphragm that can be visualized are representative for the entire diaphragm.
Future
Study design
Clinical studies should be conducted to establish diaphragm ultrasound as a useful diagnostic tool, which improves patient outcome, such as prediction of extubation success. In addition, there is a need to validate ultrasound derived variables to quantify or even calculate indices for diaphragm effort such as PTP or work of breathing.
3D ultrasound
3D US is an innovative application to study diaphragm geometry (12). Quaranta reported changes in functional anatomy of the diaphragm in different postures and consequently, respiratory mechanics. This is an interesting future technique, although not feasible in ICU patients today. In addition, the clinical applications in our patients need to be established.
Speckle tracking
Speckle tracking is an ultrasound imaging method that uses naturally occurring speckle patterns to provide information on tissue’s deformation and motion. It is already established for the assessment of cardiac muscle and is now also slowly becoming available for the diaphragm muscle. In two different studies Ye and colleagues (13) and Hatam and colleagues (14) used this technique in 21 and 13 healthy volunteers respectively, to evaluate diaphragm strain, which is a value that describes active shortening of a given segment related to the length at a previous time point, as a new parameter of diaphragm function.
Ye and colleagues analysed three segments of the diaphragm and found that while the crura and the segment in the zone of apposition displayed changes in strain, the domes of the diaphragm do not. One of the explanations could be that certain parts of the muscle have different functional qualities than others and perhaps means that the force generation during inspiration is not uniform throughout the muscle.
Hatam and colleagues showed that diaphragm strain in healthy individuals increases with greater levels of pressure support ventilation, due to the fact that in healthy individuals the diaphragm resists the driving pressures and thus delivers more work. This further proves the utility of strain measurements to evaluate work delivered by the diaphragm.
The conclusion that can be drawn from both studies is that strain-measurements are a valuable new tool and could possibly allow us to evaluate the diaphragm during controlled modes of ventilation, during which parameters such as thickness and motion are not well correlated to diaphragm activity.
Contrast enhanced ultrasound
Ultrasound contrast agents are also an emerging field of research. For instance, microbubbles are gas-liquid emulsions, surrounded by a shell that prevents leakage and aggregation, with a size of 1 to 4 microns. The gaseous part creates a strong echogenic response which results in a high contrast-tissue ratio. This allows to highlight and better visualize the tissue of interest. To guarantee that that tissue is highlighted, the contrast agents can be made tissue-specific by directing them to bind specific molecular targets such as specific proteins or DNA. Other possibilities are quantitative assessment of perfusion by way of studying replenishment of microbubbles after purposefully destroying (15) them or of inflammation by targeting adhesion molecules (16). Currently there is no data available that these techniques are applicable for the diaphragm. In theory it should be possible however and research is needed to answer this question.
Conclusions
US of the diaphragm is a promising technique that offers information about diaphragm morphology and function. In ICU patients, it may already be of clinical value for instance to demonstrate severe diaphragm weakness (17,18) and patient-ventilator asynchrony (19). However, it should be kept in mind that clinicians should not be over enthusiastic regarding the interpretation of results obtained by diaphragm ultrasound. As described above, there still are many pitfalls, as validity of ultrasound derived data to assess breathing effort have not been very well validated and the impact of ultrasound as a diagnostic tool on clinical outcomes, has never been demonstrated. Probably, ultrasound can play a role as part of a diagnostic strategy, where other techniques to evaluate diaphragm function are used as well, or perhaps in the context of a more holistic approach by looking at multiple organs and tissues at the same time. Lungs and diaphragm for example, are functionally and anatomically closely related and combined evaluation could potentially have more value than each on its own. Mayo and colleagues (20) already proved such a holistic approach to be useful by detecting risk factors for weaning failure, which lie in heart-, lung- and diaphragm function. Future studies could look if a combination of other organs and/or tissues is viable as well.
Regardless of the strategy of use, the role of US will change as new exciting techniques are underway.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zambon M, Greco M, Bocchino S, et al. Assessment of diaphragmatic dysfunction in the critically ill patient with ultrasound: a systematic review. Intensive Care Med 2017;43:29-38. [Crossref] [PubMed]
- Carrillo-Esper R, Pérez-Calatayud ÁA, Arch-Tirado E, et al. Standardization of Sonographic Diaphragm Thickness Evaluations on Healthy Volunteers. Respir Care 2016;61:920-4. [Crossref] [PubMed]
- Ferrari G, De Filippi G, Elia F, et al. Diaphragm ultrasound as a new index of discontinuation from mechanical ventilation. Crit Ultrasound J 2014;6:8. [Crossref] [PubMed]
- Farghaly S, Hasan AA. Diaphragm ultrasound as a new method to predict extubation outcome in mechanically ventilated patients. Aust Crit Care 2017;30:37-43. [Crossref] [PubMed]
- DiNino E, Gartman EJ, Sethi JM, et al. Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax 2014;69:423-7. [Crossref] [PubMed]
- Thimmaiah VT. MJ G, Jain KP. Evaluation of Thickness of Normal Diaphragm by B Mode Ultrasound. International Journal of Contemporary Medical Research 2016;3:2658-60.
- Santana PV, Prina E, Albuquerque AL, et al. Identifying decreased diaphragmatic mobility and diaphragm thickening in interstitial lung disease: the utility of ultrasound imaging. J Bras Pneumol 2016;42:88-94. [Crossref] [PubMed]
- Goligher EC, Fan E, Herridge MS, et al. Evolution of Diaphragm Thickness During Mechanical Ventilation: Impact Of Inspiratory Effort. Am J Respir Crit Care Med 2015;192:1080-8. [Crossref] [PubMed]
- Goligher EC, Laghi F, Detsky ME, et al. Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: feasibility, reproducibility and validity. Intensive Care Med 2015;41:642-9. [Crossref] [PubMed]
- Vivier E, Mekontso Dessap A, Dimassi S, et al. Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Med 2012;38:796-803. [Crossref] [PubMed]
- Umbrello M, Formenti P, Longhi D, et al. Diaphragm ultrasound as indicator of respiratory effort in critically ill patients undergoing assisted mechanical ventilation: a pilot clinical study. Crit Care 2015;19:161. [Crossref] [PubMed]
- Quaranta M, Salito C, Magalotti E, et al. Non-invasive three-dimensional imaging of human diaphragm in-vivo. Conf Proc IEEE Eng Med Biol Soc 2008;2008:5278-81.
- Ye X, Xiao H, Bai W, et al. Two-dimensional strain ultrasound speckle tracking as a novel approach for the evaluation of right hemidiaphragmatic longitudinal deformation. Exp Ther Med 2013;6:368-72. [PubMed]
- Hatam N, Goetzenich A, Rossaint R, et al. A novel application for assessing diaphragmatic function by ultrasonic deformation analysis in noninvasively ventilated healthy young adults. Ultraschall Med 2014;35:540-6. [Crossref] [PubMed]
- Wei K, Jayaweera AR, Firoozan S, et al. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 1998;97:473-83. [Crossref] [PubMed]
- Lindner JR, Song J, Christiansen J, et al. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation 2001;104:2107-12. [Crossref] [PubMed]
- Lerolle N, Guérot E, Dimassi S, et al. Ultrasonographic diagnostic criterion for severe diaphragmatic dysfunction after cardiac surgery. Chest 2009;135:401-7. [Crossref] [PubMed]
- Mariani LF, Bedel J, Gros A, et al. Ultrasonography for Screening and Follow-Up of Diaphragmatic Dysfunction in the ICU: A Pilot Study. J Intensive Care Med 2016;31:338-43. [Crossref] [PubMed]
- Matamis D, Soilemezi E, Tsagourias M, et al. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med 2013;39:801-10. [Crossref] [PubMed]
- Mayo P, Volpicelli G, Lerolle N, et al. Ultrasonography evaluation during the weaning process: the heart, the diaphragm, the pleura and the lung. Intensive Care Med 2016;42:1107-17. [Crossref] [PubMed]


