
Advances in personalized medicine: translating genomic insights into targeted therapies for cancer treatment
Highlight box
Key findings
• Advances in genomic profiling and next-generation sequencing (NGS) have led to significant improvements in targeted cancer therapies, enhancing patient outcomes.
• Emerging technologies like clustered regularly interspaced short palindromic repeats (CRISPR) and artificial intelligence (AI) are refining personalized medicine by facilitating the identification of actionable mutations and optimizing treatment strategies.
What is known and what is new?
• Personalized medicine uses genomic insights to tailor therapies to individual genetic profiles, addressing cancer heterogeneity. Targeted therapies for known genetic drivers have improved survival rates. Integrating AI and machine learning into bioinformatics enhances data analysis and treatment selection, while CRISPR-based gene editing offers potential to correct specific mutations and develop innovative treatments.
• We propose a practical model integrating advanced genomics, CRISPR, and AI for personalized oncology. Emphasizing a multidisciplinary approach, we focus on transitioning new discoveries into routine care, overcoming data interpretation, access, and ethical barriers, and presenting actionable recommendations for broader adoption of personalized therapies.
What is the implication, and what should change now?
• The continued evolution of personalized medicine has the potential to transform cancer treatment paradigms, leading to more effective and individualized therapeutic options.
• Improved understanding of tumor genomics can guide clinical decision-making, ultimately enhancing patient care.
• Efforts should be made to increase accessibility to genomic testing and targeted therapies across diverse populations.
• Enhanced training for healthcare professionals in interpreting genomic data is essential to maximize the benefits of personalized medicine in clinical practice.
Introduction
Background
Personalized medicine has emerged as a revolutionary approach in modern healthcare, particularly in cancer treatment (1). Unlike traditional methods that rely on generalized treatment plans, personalized medicine leverages genomic insights to tailor therapies to an individual’s genetic profile (1). This paradigm shift has been made possible by advances in genomic technologies, enabling more precise diagnoses and targeted therapeutic strategies that improve patient outcomes while minimizing adverse effects (2).
Cancer is a highly heterogeneous disease characterized by complex genetic and molecular variations among patients (3). The advent of next-generation sequencing (NGS) and other genomic profiling techniques has provided invaluable insights into the genetic mutations and alterations that drive tumor growth and progression (4). These advancements have paved the way for targeted therapies, which selectively inhibit specific molecular pathways involved in cancer development. By identifying actionable mutations, clinicians can prescribe treatments that are more effective for individual patients, thereby enhancing therapeutic efficacy and reducing unnecessary side effects (2).
Rationale and knowledge gap
The integration of genomic technologies into oncology has led to significant improvements in patient care (5). Targeted therapies, such as tyrosine kinase inhibitors (TKIs) and monoclonal antibodies, have demonstrated superior efficacy in treating cancers with known genetic drivers (6,7). For example, epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer (NSCLC) can be effectively targeted using EGFR inhibitors (8), while HER2-positive breast cancers respond well to HER2-targeted therapies (9). Similarly, the identification of BRAF mutations in melanoma and KRAS mutations in colorectal cancer has enabled the development of highly specific therapeutic agents that significantly improve patient survival rates (10,11).
Beyond targeted therapies, emerging technologies such as clustered regularly interspaced short palindromic repeats (CRISPR) gene editing and AI are further refining the landscape of personalized medicine (12,13). CRISPR technology offers the potential to correct genetic mutations at the DNA level, opening new avenues for precision cancer treatment (12). AI and machine learning (ML) algorithms, on the other hand, are enhancing the interpretation of complex genomic data, facilitating the identification of novel biomarkers and optimizing treatment selection (13). These technological innovations are driving the transition from a one-size-fits-all approach to a more individualized treatment paradigm.
Objective
In this review, we discuss the role of genomic profiling in cancer treatment, highlighting its impact on therapy selection and patient outcomes. We also examine the challenges associated with accessibility, costs, and the need for robust bioinformatics infrastructure. Additionally, we explore emerging solutions such as AI and gene-editing technologies and their role in shaping the evolving landscape of precision medicine. Finally, we outline the future directions of genomic-guided cancer therapy, emphasizing the ongoing advancements and collaborative efforts required to fully realize the potential of personalized medicine in oncology. We present this article in accordance with the PRISMA-ScR reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-25-34/rc).
Methods
We conducted a literature search in PubMed on February 9, 2025, using a combination of MeSH terms and free-text keywords to identify original studies investigating genomic profiling with clinical relevance in cancer patients. The full search strategy is detailed in Table 1, and Figure 1 illustrates the study screening and selection process. The search covered the period from January 1, 1950 to February 9, 2025, with no language restrictions. Our PubMed search identified 238 studies. After screening titles and abstracts, 155 studies were excluded, leaving 83 studies for full-text review. Among these, 65 studies did not meet the inclusion criteria, and ultimately, 18 studies with clinical relevance were included in the review. The main findings of the 18 eligible studies are summarized in Table 2. Additionally, the risk of bias for the included studies was assessed using the Newcastle-Ottawa Scale (NOS).
Table 1
| Items | Specification |
|---|---|
| Date of search | 9 February, 2025 |
| Database searched | PubMed |
| Search term used | (“Genomics”[Mesh] OR “Genomic Profiling”[tiab] OR “Genomic Sequencing”[tiab] OR “NGS”[tiab] OR “Whole Exome Sequencing”[tiab] OR “Whole Genome Sequencing”[tiab] OR “Next-Generation Sequencing”[tiab]) AND (“Targeted Therap*”[tiab] OR “Targeted Treatment*”[tiab]) AND (“medical Oncology”[Mesh] OR “Cancer Therap*”[tiab] OR “Tumor Therap*”[tiab] OR “Cancer Treatment”[tiab] OR “Cancer Therap*”[tiab]) |
| Timeframe | 1950/01/01–2025/02/09 |
| Inclusion and exclusion criteria | Inclusion criteria: (I) original research articles involving cancer patients undergoing genomic profiling and targeted therapies; (II) studies must examine genomic technologies, targeted therapies, tumor heterogeneity, resistance mechanisms, or emerging technologies such as CRISPR and AI; (III) studies must report patient outcomes, including survival or mortality |
| Exclusion criteria: (I) non-original research articles, including reviews, commentaries, editorials, case reports, or opinion pieces; (II) studies conducted on non-cancer patient populations; (III) preclinical research lacking direct clinical relevance or studies that did not report patient outcomes; (IV) studies that did not assess genomic technologies in conjunction with targeted therapy | |
| Selection process | Both authors were involved in reviewing and selecting the relevant literature |
AI, artificial intelligence; CRISPR, clustered regularly interspaced short palindromic repeats.
Table 2
| Study | Design | Risk of bias | Cancer type | Genomic profiling method | Key findings | Clinical significance |
|---|---|---|---|---|---|---|
| Tsimberidou et al., 2017 (14) | Retrospective | Moderate | Advanced cancer (n=1,436) | Comprehensive genomic profiling (CGP) | 637 patients had actionable aberrations; those receiving molecularly targeted therapy (MTT, n=390) had improved response rates (11% vs. 5%; P=0.0099), longer failure-free survival (3.4 vs. 2.9 months; P=0.0015), and longer overall survival (8.4 vs. 7.3 months; P=0.041) compared to unmatched patients. However, targeting only the PI3K pathway did not improve outcomes | Highlights the clinical benefit of CGP-driven MTT but underscores the need for multi-pathway targeting in PI3K-altered tumors |
| Leroy et al., 2023 (15) | Retrospective | Low | Various cancers (n=416) | NGS-based CGP | 75% had actionable mutations; treatment modification occurred in 17.3%, more frequently in metastatic disease (OR =2.73, 95% CI: 1.31–5.71, P=0.007). Genomic-directed treatment changes were most common within 6 months but extended up to 24 months post-testing | Supports CGP utility in guiding treatment decisions, particularly in metastatic settings |
| Hughes et al., 2022 (16) | Retrospective | Low | NSCLC (n=248) | NGS and biomarker testing | 76% of metastatic NSCLC patients received first-line therapy. Median OS: 9.0 months (95% CI: 6.5–11.6). Targeted therapy significantly improved OS (28.7 vs. 6.6 months; P<0.001) | Highlights the need for comprehensive genomic profiling and early diagnosis in young NSCLC patients |
| Kim et al., 2023 (17) | Prospective | Moderate | NSCLC (n=152) | ctDNA vs. tissue sequencing | High cfDNA concentration at diagnosis was associated with poorer overall survival. The sensitivity and precision of ctDNA analysis compared to tissue sequencing were 58.4% and 61.5%, respectively | ctDNA shows prognostic value in NSCLC, but its lower sensitivity and precision limit its standalone use |
| Okamura et al., 2021 (18) | Retrospective | Low | Biliary tract cancer (n=121) | ctDNA-based profiling | Concordance between ctDNA and metastatic site tissue-DNA was higher than with primary tumor DNA. Molecularly matched therapy led to significantly longer progression-free survival (HR: 0.60, 95% CI: 0.37–0.99, P=0.047) and a higher disease control rate (61% vs. 35%, P=0.04) compared to unmatched regimens | ctDNA profiling aids treatment selection and improves outcomes in biliary tract cancer |
| Harttrampf et al., 2017 (19) | Prospective | Moderate | Pediatric refractory tumors (n=75) | Comparative genomic hybridization array, NGS, whole-exome and RNA sequencing | Molecular analysis identified actionable alterations in 60.9% of patients, leading to treatment modifications in 14 cases. Diagnosis was revised in three patients | Comprehensive genomic profiling is feasible in pediatric refractory tumors and reveals clinically relevant alterations, but access to targeted therapies remains a challenge |
| Riedl et al., 2021 (20) | Prospective | High | Advanced carcinoma (n=24) | ctDNA vs. tissue profiling | Matched treatments were identified in 46% of patients, but the median PFS ratio was <1.2 in all cases, leading to study termination. Combination therapy options were suggested in most cases | The study underscores the challenges of implementing ctDNA-driven precision oncology, highlighting the need for improved patient selection and optimized treatment matching strategies |
| Pleasance et al., 2022 (21) | Retrospective | Moderate | Advanced cancer (n=570) | WGTA | Clinically actionable targets were identified in 83% of patients, with 37% receiving WGTA-informed treatments. RNA expression data contributed to 67% of treatment decisions, with 25% based solely on RNA data. Clinical benefit was observed in 46% of WGTA-informed treatments | WGTA enhances treatment selection by integrating RNA expression and genomic data, expanding therapeutic options beyond standard sequencing |
| Ding et al., 2021 (22) | Retrospective | High | Lung cancer (n=12) | WES and RNA sequencing | Neoantigen vaccine therapy showed a 25% objective response rate and 75% disease control rate, with a median PFS of 5.5 months and OS of 7.9 months. All adverse events were grade 1–2 | Neoantigen-based vaccines demonstrate feasibility and safety in lung cancer, warranting further investigation for broader clinical application |
| Yuan et al., 2017 (23) | Retrospective | High | Metastatic breast cancer (n=44) | NGS | Actionable mutations were identified in 95% of patients, and 55% of those with actionable mutations received targeted therapy, with 70% showing assessable responses | Early NGS in MBC can uncover actionable targets and guide treatment, supporting its integration into clinical decision-making |
| Prager et al., 2019 (24) | Prospective | Moderate | Various cancers (n=55) | NGS | Molecular profiling-guided therapy led to longer PFS in 62% of patients, with a median PFS improvement from 61 to 112 days (P=0.002). Disease control was achieved in 56% of patients, and median OS was 348 days | Real-time molecular profiling enhances treatment personalization, leading to improved clinical outcomes and prolonged survival in selected patients |
| Dang et al., 2024 (25) | Retrospective | Moderate | Various cancers (n=94) | NGS | Actionable mutations were identified in 53.6% of patients. Among those, 11 received molecularly guided therapy. The PFS2/PFS1 ratio was ≥1.3 in 62.5% of evaluable patients, and median OS was 7.3 months | Molecular profiling informs targeted therapy decisions, but limited uptake of recommended treatments highlights challenges in clinical implementation |
| Dalens et al., 2023 (26) | Prospective | Moderate | Lung cancer (n=135) | WES | Certain germline SNPs in immunity-related genes negatively impacted survival on EGFR TKI treatment. SNPs in HLA-DRB5, KIR3DL1, and KIR3DL2 were linked to reduced PFS and OS | Highlights the role of germline variations in treatment response, suggesting potential biomarkers for refining targeted therapies |
| Martínez-Herrera et al., 2024 (27) | Retrospective | Low | NSCLC (n=127) | NGS | 32.3% of EGFR-negative patients had other actionable mutations (e.g., ALK, BRAF, ROS1, RET), supporting early targeted therapy. ALK and EGFR mutations were significantly associated with OS, while ALK, TMB, and brain MRI findings were linked to PFS | Emphasizes the necessity of comprehensive NGS in NSCLC, particularly in resource-limited settings, to optimize treatment strategies and improve patient outcomes |
| Gouton et al., 2022 (28) | Retrospective | Moderate | Solid tumors (n=191) | cfDNA genomic profiling | AMAs found in 52% of patients; most common alterations: TP53 (52%), KRAS (14%), DNMT3 (11%). Most common AMAs: CHEK2 (10%), PIK3CA (9%), ATM (7%). No significant difference in PFS (2.66 vs. 3.81 months, P=0.17), OS (5.3 vs. 7.1 months, P=0.64), or PFS2/PFS1 ratio ≥ 1.3 (20% vs. 24%, P=0.72) between MMT and non-MMT groups. MMT group: ORR 19%, disease control rate 32% | Feasibility of cfDNA profiling confirmed, but no clear survival benefit in unselected pan-cancer patients |
| Kim et al., 2022 (29) | Prospective | Low | Metastatic colorectal cancer (n=272) | Longitudinal ctDNA monitoring | Detectable ctDNA mutations in 90.3% of patients before treatment. ctDNA clearance post-chemotherapy correlated with longer PFS (adjusted HR: 0.22, 95% CI: 0.10–0.46). ctDNA progression preceded radiological PD in 58.1% (median 3.3 months). Resistant mutations (34.9%) and gene amplification (7.0%) detected at PD. Newly identified mutations in 16.3% of PD patients were potential candidates for targeted therapy | Supports ctDNA monitoring as a tool for early progression detection and guiding personalized treatment in metastatic CRC |
| Lin et al., 2020 (30) | Retrospective | Low | NSCLC (EGFR/ALK wild-type) (n=198) | TMB analysis by NGS | In EGFR-mutant patients treated with first-generation EGFR-TKIs, TMB-L group had significantly longer PFS (16.4 vs. 9.0 vs. 7.4 months; log-rank P=0.006). In EGFR/ALK wild-type patients receiving pemetrexed/platinum, the TMB-L group had a superior ORR (53.8% vs. 23% vs. 8.3%; log-rank P=0.037) and numerically longer PFS (6.9 vs. 4.3 vs. 4.6 months; P=0.22) | Highlights TMB as a potential biomarker influencing response to EGFR-TKIs and chemotherapy in NSCLC, though larger studies are needed for validation |
| Canino et al., 2024 (31) | Retrospective | Moderate | Metastatic breast cancer (n=101) | NGS | 75% had at least one actionable mutation. Nine received targeted therapy: eight with PIK3CA mutations received alpelisib/fulvestrant, and one with FGFR1/2 amplifications received TAS120. Median PFS for treated patients was 3.8 months. NGS results influenced therapy in 9% of cases | Highlights the potential of NGS in guiding treatment decisions for metastatic breast cancer, though limited sample size and follow-up suggest the need for further validation |
ALK, anaplastic lymphoma kinase; AMA, actionable molecular alteration; CI, confidence interval; CGP, comprehensive genomic profiling; cfDNA, cell-free DNA; CRC, colorectal cancer; ctDNA, circulating tumor DNA; EGFR, epidermal growth factor receptor; EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor; FGFR, fibroblast growth factor receptor; HR, hazard ratio; MTT, molecularly targeted therapy; NGS, next-generation sequencing; NSCLC, non-small cell lung cancer; OR, odds ratio; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PI3K, phosphatidylinositol 3-kinase; RET, rearranged during transfection; ROS1, ROS proto-oncogene 1; SNP, single nucleotide polymorphism; TKI, tyrosine kinase inhibitor; TMB, tumor mutational burden; WES, whole-exome sequencing; WGTA, whole-genome and transcriptome analysis.
Studies were included if they met the following criteria: (I) original research articles involving cancer patients undergoing genomic profiling and targeted therapies; (II) studies must examine genomic technologies, targeted therapies, tumor heterogeneity, resistance mechanisms, or emerging technologies such as CRISPR and AI; (III) studies must report patient outcomes, including survival or mortality.
Studies were excluded if they met any of the following criteria: (I) non-original research articles, including reviews, commentaries, editorials, case reports, or opinion pieces; (II) studies conducted on non-cancer patient populations; (III) preclinical research lacking direct clinical relevance or studies that did not report patient outcomes; (IV) studies that did not assess genomic technologies in conjunction with targeted therapy. Both authors reviewed the retrieved studies to assess their relevance. Discrepancies were resolved through discussion.
Results
Genomic technologies and the role of bioinformatics in data analysis
Epigenomics and phenomics data along with genomic technologies have become cornerstone tools in advancing personalized medicine. They have revolutionized our understanding and treatment of diseases, especially cancer (1,32). By enabling detailed analysis of an individual’s genetic makeup, these technologies provide critical insights into the molecular underpinnings of health and disease. Through high-throughput sequencing methods such as NGS, including targeted sequencing panels (TSP), whole exome sequencing (WES), and whole genome sequencing (WGS), researchers can identify specific genetic mutations and alterations that drive tumor development and progression (Figure 2) (4).
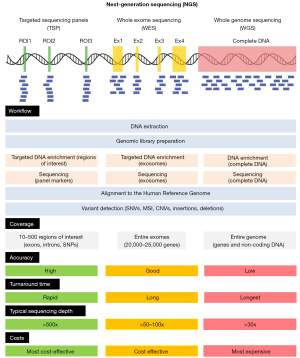
This wealth of genomic data empowers clinicians to tailor treatment strategies to each patient’s unique genetic profile, enhancing therapeutic efficacy while minimizing adverse effects. As a result, genomic technologies not only facilitate more precise diagnoses but also pave the way for innovative targeted therapies that hold great promise in transforming patient care within the realm of personalized medicine. In the following, we will provide a brief overview of these different sequencing techniques, highlighting their principles, applications, and significance in advancing personalized medicine. Additionally, we will mention some significant examples that illustrate how these technologies have been successfully applied in clinical settings to improve patient outcomes.
NGS has revolutionized genomic research by enabling rapid and cost-effective sequencing of DNA. Unlike traditional Sanger sequencing, which processes one sequence at a time, NGS can analyze millions of DNA fragments simultaneously (33). This parallel processing capability significantly enhances throughput and reduces costs, making it an invaluable tool for cancer genomics. The applications of NGS in oncology are vast. It facilitates comprehensive tumor profiling, enabling researchers to identify mutations, copy number variations, and other genomic alterations that drive cancer progression and clinical judgment in patients (34). Different platforms exist within the NGS landscape, such as Illumina’s sequencing technology and Ion Torrent’s semiconductor-based approach (35). These platforms have been instrumental in deciphering complex cancer genomes and guiding personalized treatment strategies. In particular, standardization in NGS workflow including template preparation (library preparation and amplification), sequencing, imaging, and data analysis has helped to increase the accuracy in data assembly and quality. However, the development of robust state-of-the-art bioinformatics pipelines to extract meaningful biological insights is still a significant topic (35).
TS panels are specialized tools used in genomics to analyze specific regions of DNA, allowing researchers to focus on a selected number of genes or genomic areas of interest for diagnosis, prognosis, and treatment monitoring (36). These panels contain a curated set of probes designed to capture and enrich targeted sequences (regions of interest), making it easier to identify variations such as single nucleotide polymorphisms (SNPs), insertions, deletions, and other genetic alterations associated with diseases. By concentrating on particular targets, these panels enhance the efficiency and accuracy of sequencing workflows while reducing costs and data complexity compared to whole-genome sequencing (37-39). They are widely used in clinical diagnostics, cancer research, and personalized medicine.
WES focuses specifically on the exons, which make up approximately 1.5% of the human genome but contain around 85% of known monogenic, disease-related variants (40). WES is particularly advantageous for identifying unbiased clinically relevant mutations and copy number alterations associated with various cancers (41). One notable example is its application in hereditary breast and ovarian cancer syndrome research, where WES has successfully identified pathogenic variants in genes such as BRCA1 and BRCA2. By concentrating on exonic regions, WES provides a cost-effective means to uncover significant genetic alterations that can inform therapeutic decisions (42).
WGS offers a more comprehensive analysis by sequencing the entire genome, including both coding and non-coding regions. The unique capabilities of WGS allow for the detection of structural variants, copy number variations, and regulatory elements that may play critical roles in tumor behavior. While WGS generates a vast amount of data compared to WES or TSP, its sensitivity enables researchers to uncover hidden complexities within cancer genomes. For instance, studies have demonstrated how WGS combined with specialized bioinformatics pipelines can detect novel fusion genes or previously unrecognized mutations that contribute to drug resistance or tumor recurrence (43,44). The breadth of information obtained from WGS positions it as a powerful tool in understanding cancer biology at an unprecedented level. Furthermore, combining the comprehensive genomic data of specific tumor types with detailed clinical information will create a groundbreaking research platform. This will enable a deeper understanding of the mechanisms behind treatment response, resistance development, and treatment failure. Ultimately, this approach will help identify unique characteristics of individual cancer genomes and facilitate the development of targeted cancer therapies (45,46).
It is evident that bioinformatics plays a pivotal role in the realm of personalized medicine, serving as the bridge between complex genomic data and actionable clinical insights. As advancements in sequencing technologies continue to evolve and produce vast amounts of ‘big data’, bioinformatics provides the necessary tools and methodologies to analyze, interpret, and integrate this information effectively (47). The primary objective is to translate raw genetic information into meaningful knowledge that can guide clinical personalized decision-making and improve patient outcomes (48). An exemplar Bioinformatics Multidrug Combination Protocol for Personalized Precision Medicine was developed for breast cancer patients. This protocol enables personalized drug combinations extracted from hundreds of drugs and thousands of drug combinations, offering more effective treatment options (49). The protocol integrates an individual pattern of perturbed gene expression for each patient, similar to a fingerprint, containing gene expression profiles different from healthy individuals. The algorithm can then extract an accurate personalized drug treatment, producing a more beneficial therapeutic effect in the respective patient (49).
At the heart of bioinformatics lies the data analysis pipeline, which includes several critical steps from sequencing to interpretation (Figure 3). Initially, raw sequencing data undergo quality control measures to filter out low-quality reads that could introduce errors into subsequent analyses (46,50). Following this step, the cleaned data is aligned against reference genomes using sophisticated pipelines such as the Burrows-Wheeler Aligner (BWA), Bowtie2, HISAT, or LongStrain (51-53). This alignment process is crucial for identifying genetic variants like SNPs, insertions, or deletions that may play a significant role in disease development. Variant calling is then performed using software like the Genome Analytic Tool Kit (GATK) or SAMtools to accurately identify these genetic alterations (54,55).
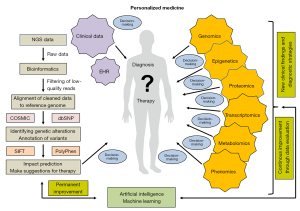
Once variants are identified, bioinformatics tools facilitate their annotation and interpretation through cross-referencing with established databases like the Catalogue of Somatic Mutations in Cancer (56) for cancer-related mutations or dbSNP for known polymorphisms. These resources provide context regarding whether specific mutations have been previously associated with clinical outcomes or biological functions related to cancer pathogenesis. Functional impact prediction tools such as Sorting Intolerant From Tolerant (57) or Polymorphism Phenotyping (58) further aid in assessing whether specific mutations are likely to affect protein function, thereby guiding therapeutic decisions.
Moreover, bioinformatics enables integrative genomics, by combing genomic data with other omics layers, such as transcriptomics and proteomics, to provide a comprehensive view of tumor biology. This approach allows researchers to uncover intricate interactions between genetic alterations and gene expression profiles influenced by environmental factors or treatment modalities. For example, understanding how specific mutations correlate with differential gene expression patterns can lead to more targeted therapeutic strategies customized for individual patients (45,46).
ML and AI are making significant progress in bioinformatics, providing innovative solutions for analyzing high-dimensional datasets produced by modern sequencing technologies and influencing clinical decision-making (13,59). These advanced computational techniques can detect patterns within genomic data that traditional analytical methods may overlook. By utilizing ML algorithms, researchers can forecast patient responses using their individual genomic profiles and uncover new biomarkers linked to treatment effectiveness or resistance. However, despite its tremendous potential benefits, bioinformatics faces several challenges that must be addressed to fully realize its promise in personalized medicine. Issues surrounding data storage capacity requirements arise due to the massive volumes of genomic data generated daily, thus necessitating robust infrastructure capable of supporting efficient access and processing capabilities. Additionally, ensuring reproducibility and accuracy throughout analyses conducted across varying contexts remains paramount for establishing trust in bioinformatic findings. Moreover, although AI has rapidly revolutionized diagnosis, prognosis, treatment, and prevention in oncology and related fields, there are also concerns about biases inherent in algorithms, reflecting the unconscious biases of their creators (60). Nevertheless, bioinformatics serves as an essential pillar supporting personalized medicine by transforming complex genomic data into actionable insights that inform clinical practice and pave new pathways toward improved patient care experiences overall.
Looking ahead, future directions in bioinformatics will focus on developing user-friendly interfaces and automating routine tasks to minimize barriers for clinicians seeking expertise in genomic analysis. Efforts aimed at enhancing collaboration between computational scientists and healthcare professionals will foster an environment conducive to translating genomic insights into real-world applications ultimately benefiting patients navigating complex diseases like cancer.
Key genomic alterations and targeted therapies in cancer
Advances in NGS and biomarker-driven approaches have led to improved clinical outcomes in multiple malignancies. Table 3 summarizes key genomic alterations identified through genomic profiling, their associated cancer types, Food and Drug Administration (FDA)-approved or investigational targeted therapies, and their clinical significance.
Table 3
| Gene/mutation | Cancer type | Targeted therapy | Clinical significance |
|---|---|---|---|
| EGFR mutations (L858R, Exon 19 deletions, T790M) (17,30,61) | NSCLC | Osimertinib, erlotinib, gefitinib | FDA-approved targeted therapies. Osimertinib is the standard of care for T790M-positive cases |
| ALK rearrangements (16,62,63) | NSCLC | Crizotinib, alectinib, lorlatinib | ALK inhibitors significantly prolong survival in ALK-positive NSCLC, with alectinib showing superior efficacy over crizotinib as a first-line therapy |
| KRAS mutations (G12C, G12D, G13D) (64,65) | NSCLC (G12C), colorectal cancer (investigational), pancreatic cancer (investigational) | Approved: sotorasib, adagrasib (for KRAS G12C-mutant NSCLC). Investigational: MEK inhibitors, pan-KRAS inhibitors for other KRAS mutations | KRAS G12C inhibitors are the first FDA-approved therapies for NSCLC. Other KRAS mutations remain under study |
| BRAF V600E (66-68) | Melanoma, colorectal cancer, thyroid cancer | Dabrafenib + trametinib, encorafenib + cetuximab | FDA-approved combination therapies, with dual BRAF/MEK inhibition being the preferred approach |
| PIK3CA mutations (31,48,64,69,70) | Breast cancer, colorectal cancer | Approved: alpelisib + fulvestrant (for HR+/HER2− breast cancer). Investigational: mTOR inhibitors in PIK3CA-mutant tumors | Alpelisib is FDA-approved for HR+/HER2− breast cancer. mTOR inhibitors are used in broader settings but not directly for PIK3CA mutations |
| HER2 (ERBB2) amplification/mutation (71-74) | Breast cancer, gastric cancer, NSCLC (HER2-mutant) | Trastuzumab, lapatinib, trastuzumab-deruxtecan (T-DXd, for NSCLC HER2 mutations) | Standard therapy for HER2+ tumors. T-DXd is FDA-approved for HER2-mutant NSCLC |
| TP53 mutations (72,75-79) | Various cancers | No direct targeted therapy | Associated with poor prognosis, resistance to therapy |
| MSI-H/dMMR (67,72,80) | Colorectal, endometrial, gastric cancer | Pembrolizumab, nivolumab | First FDA-approved biomarker-based therapy. Approved for MSI-H tumors regardless of origin |
| JAK2 p.V617F, JAK3 mutations (81,82) | Myeloproliferative neoplasms, hematologic malignancies | JAK inhibitors (ruxolitinib, fedratinib for myeloproliferative neoplasms); potential role in immunotherapy response for NSCLC (research phase) | JAK2 inhibitors are FDA-approved for MPNs. JAK3 mutations are under investigation for immunotherapy sensitivity |
| MET amplification, HER2 overexpression (73,83) | Lung cancer | Capmatinib, tepotinib, crizotinib (for MET amplification); HER2-targeted therapies for HER2 overexpression | MET exon 14 skipping and HER2-mutant NSCLC are FDA-approved targets for therapy. MET amplification is still under investigation |
| NTRK fusions (84,85) | Solid tumors | Larotrectinib, entrectinib | High response rates to TRK inhibitors. Approved for any NTRK+ tumor |
ALK, anaplastic lymphoma kinase; BRAF, v-Raf murine sarcoma viral oncogene homolog B; dMMR, deficient mismatch repair; EGFR, epidermal growth factor receptor; FDA, Food and Drug Administration; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; JAK, Janus kinase; KRAS, Kirsten rat sarcoma viral oncogene homolog; MEK, mitogen-activated protein kinase kinase; MET, mesenchymalepithelial transition; MPN, myeloproliferative neoplasm; MSI-H, microsatellite instability-high; mTOR, mechanistic target of rapamycin; NSCLC, nonsmall cell lung cancer; NTRK, neurotrophic receptor tyrosine kinase; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; T-DXd, trastuzumab-deruxtecan; TP53, tumor protein p53; TRK, tropomycin receptor kinase.
Certain genomic alterations serve as crucial therapeutic targets across multiple malignancies. In NSCLC, EGFR mutations (L858R, exon 19 deletions, T790M) drive tumor growth through aberrant activation of the EGFR signaling pathway, making them prime candidates for EGFR TKIs (17,30). Similarly, ALK rearrangements and ROS1 fusions in NSCLC (62) define subsets of patients who benefit from ALK and ROS1 inhibitors, respectively (16,63).
In colorectal, melanoma, and thyroid cancers, BRAF V600E mutations lead to constitutive activation of the MAPK pathway, rendering tumors susceptible to BRAF/MEK inhibition (66,86). KRAS G12C mutations, once deemed undruggable, have become actionable with recent advancements in KRAS inhibitors, although other KRAS variants remain under investigation (64,65).
In breast and gastric cancers, HER2 (ERBB2) amplification is a well-characterized oncogenic driver, guiding the use of HER2-targeted therapies (71,72). PIK3CA mutations, particularly in hormone receptor-positive (HR+)/HER2-negative breast cancer, are associated with PI3K/AKT pathway activation and response to PI3K inhibitors (31,64,69,87,88).
Another emerging class of actionable alterations includes tumors with high microsatellite instability (MSI-H) and deficient mismatch repair (dMMR) (67,72). These tumors, found in colorectal, endometrial, and other malignancies, exhibit high mutation burdens and respond exceptionally well to immune checkpoint inhibitors, leading to tumor-agnostic FDA approvals (72,80). In hematologic cancers, mutations in JAK2 (p.V617F) and JAK3 drive myeloproliferative neoplasms, with JAK inhibitors demonstrating significant clinical benefit (89,90). Similarly, MET amplification and exon 14 skipping mutations in NSCLC have emerged as critical therapeutic targets (91). Rare but clinically significant alterations, such as NTRK gene fusions, occur across various solid tumors and have led to the development of tissue-agnostic therapies with durable response rates (84).
While genomic profiling has significantly improved treatment stratification, not all mutations translate into therapeutic success. TP53 mutations, prevalent in multiple cancers, remain challenging due to the lack of direct targeting strategies. However, they provide valuable prognostic information and guide treatment selection (72,75-79).
Clinical significance of genomic profiling in oncology
Genomic profiling has revolutionized patient outcomes by enabling personalized therapy selection and identifying prognostic biomarkers. Tables 2,3 summarize the clinical significance of various genomic profiling methods, including NGS, comprehensive genomic profiling (CGP), WES, and circulating tumor DNA (ctDNA) analysis.
Multiple studies confirm that genomic profiling facilitates the identification of actionable mutations, leading to improved treatment responses. For instance, Tsimberidou et al. (14) demonstrated that molecularly targeted therapy (MTT) based on CGP led to significantly longer failure-free survival (3.4 vs. 2.9 months; P=0.002) and overall survival (OS) (8.4 vs. 7.3 months; P=0.04). Similarly, Prager et al. (24) showed that NGS-based molecular profiling extended progression-free survival (PFS) from 61 to 112 days (P=0.002), reinforcing the role of personalized treatment selection.
In NSCLC, Hughes et al. (16) reported that patients who received targeted therapy had a dramatically improved median OS of 28.7 months compared to 6.6 months for those without targeted options (P<0.001). These findings highlight the necessity of early genomic testing to ensure that eligible patients benefit from precision medicine.
On these grounds, ctDNA analysis offers a minimally invasive alternative for tumor profiling, particularly for patients with insufficient tissue samples. Kim et al. (17) demonstrated that ctDNA had prognostic value in NSCLC, with high cell-free DNA (cfDNA) concentrations at diagnosis correlating with poorer survival. Similarly, Okamura et al. (18) showed that ctDNA profiling in biliary tract cancer led to significantly longer PFS (HR: 0.60, P=0.047) and a higher disease control rate (61% vs. 35%, P=0.04) when using molecularly matched therapy. However, despite its promise, liquid biopsy faces challenges. Riedl et al. (20) reported that matched treatment based on ctDNA findings did not result in meaningful PFS improvement, with a median PFS ratio of <1.2 in all cases. Similarly, Gouton et al. (28) found no significant survival difference between patients who received molecularly matched therapy and those who did not (PFS: 2.66 vs. 3.81 months, P=0.17). These findings suggest that while ctDNA profiling is valuable, its predictive accuracy and clinical application need refinement.
Beyond DNA mutation analysis, incorporating RNA expression data has been shown to enhance treatment stratification. Pleasance et al. (21) found that whole-genome and transcriptome analysis (WGTA) identified clinically actionable targets in 83% of advanced cancer patients, with 37% receiving WGTA-informed treatments. RNA expression data contributed to 67% of treatment decisions, highlighting its added value over DNA-based methods alone.
Neoantigen-based therapies also benefit from genomic profiling. Ding et al. (22) investigated whole-exome sequencing and RNA sequencing to guide neoantigen vaccine development in lung cancer, reporting a 25% objective response rate, 75% disease control rate, and a median OS of 7.9 months. These findings suggest that integrating genomic profiling into immunotherapy selection could further personalize treatment approaches. TMB has emerged as a potential biomarker for therapy response. Lin et al. (30) demonstrated that in EGFR-mutant NSCLC, patients with low TMB had significantly better PFS (16.4 vs. 9.0 vs. 7.4 months, P=0.006) on first-generation EGFR-TKIs. Similarly, in EGFR/ALK wild-type NSCLC patients treated with chemotherapy, those with low TMB had a superior objective response rate (53.8% vs. 23% vs. 8.3%, P=0.037), though PFS improvement was not statistically significant. These findings suggest that TMB could serve as a biomarker to refine treatment selection in lung cancer.
Challenges in real-world implementation
Despite the transformative role of genomic profiling in oncology, several real-world challenges hinder its widespread implementation. In particular, access to genomic testing remains highly variable across regions and healthcare systems (19,76,92). In low- and middle-income countries (LMICs), limited infrastructure, high costs, and absent reimbursement policies significantly restrict the availability of molecular diagnostics. Even in high-income countries, disparities exist between well-equipped academic centers and community hospitals with fewer resources. Martínez-Herrera et al. (27) emphasized this inequity by showing that 32.3% of EGFR-negative NSCLC patients in underserved areas harbored other actionable mutations that would have gone undetected without access to comprehensive NGS.
Germline profiling, though potentially valuable in predicting treatment response, remains underutilized. Dalens et al. (26) reported that certain germline SNPs in immunity-related genes negatively impacted survival in patients receiving EGFR TKI therapy. However, ethical concerns, unclear regulatory frameworks, and minimal integration into routine clinical workflows have limited its application. Standardized guidelines and further research are necessary to incorporate germline data effectively into precision oncology.
Interpreting complex genomic data is another major barrier for both oncologists and patients. Thavaneswaran et al. (93) found that while most oncologists referred patients for CGP, their confidence in interpreting and communicating these results was moderate, and significantly lower for germline findings. Similarly, Bylstra et al. (94) noted that patients often struggled to understand genomic information, while clinicians cited time constraints as a barrier to effective communication. These findings underscore the need for enhanced clinical training, robust decision-support tools, and accessible patient education materials to bridge the genomic literacy gap.
Furthermore, not all genomic findings translate into improved clinical outcomes. Dang et al. (25) observed that although 53.6% of patients had actionable alterations, only 11 received molecularly guided therapy, highlighting a significant gap between molecular insights and treatment implementation. Similarly, in pediatric oncology, Harttrampf et al. (19) found that while 60.9% of refractory pediatric tumors had targetable alterations, only a small number of cases were able to access appropriate therapies due to availability and access issues.
High drug prices, lack of insurance coverage, and systemic inefficiencies further limit the practical application of precision oncology. In many LMICs, targeted therapies remain prohibitively expensive, and healthcare systems lack the capacity to support advanced diagnostics. Even where therapies exist, regulatory restrictions and fragmented care models often delay or prevent their use. Addressing these barriers will require comprehensive policy reforms, tiered pricing strategies, and global health initiatives aimed at expanding equitable access.
To improve the clinical utility of genomic profiling, more integrated approaches are needed. The use of molecular tumor boards (MTBs) has shown promise in supporting personalized treatment decisions. For instance, Canino et al. (31) reported that NGS influenced therapy decisions in 9% of metastatic breast cancer cases. Expanding real-time genomic profiling in clinical trials can also help validate emerging biomarkers and inform treatment pathways.
Moreover, multi-omic approaches that integrate genomic, transcriptomic, and proteomic data are poised to refine precision oncology further. Pleasance et al. (21) demonstrated that combining RNA and DNA data enhances treatment selection, supporting a shift toward broader molecular characterization. However, realizing the full benefits of these advances will require coordinated efforts to address systemic barriers through targeted clinical training, patient education, standardized workflows, and supportive policy frameworks ensuring equitable implementation of precision oncology.
Proposed conceptual model
To address these real-world limitations, we propose a conceptual model (Figure 4) that integrates advanced genomic technologies, collaborative expertise, and adaptive clinical workflows into a patient-centered framework for personalized oncology. This model emphasizes the seamless incorporation of CGP, sophisticated bioinformatics interpretation, and multidisciplinary decision-making to guide individualized treatment strategies.
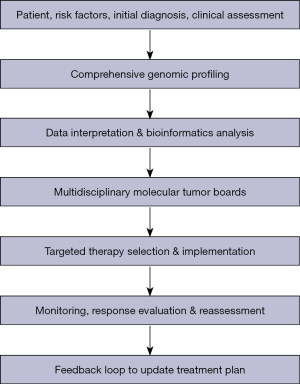
The patient journey begins with a standard clinical workup, including imaging, risk assessment, and histopathological evaluation, followed by in-depth genomic testing—ranging from targeted panels to exome or whole-genome sequencing—to detect actionable molecular alterations. These data are processed through validated bioinformatics pipelines that prioritize clinically relevant mutations and potential therapeutic options.
A multidisciplinary MTB—including oncologists, pathologists, geneticists, bioinformaticians, and other relevant specialists—reviews the molecular findings in conjunction with the patient’s clinical context to determine a personalized treatment plan. This plan is not static; it evolves through ongoing monitoring using imaging, biomarkers, and repeat genomic testing such as ctDNA, allowing real-time adaptation of therapy in response to treatment response or disease progression.
Based on this model, the proposed clinical guidelines highlight key implementation steps: clearly defined eligibility criteria for genomic testing, robust informed consent procedures, and selection of appropriate sequencing platforms tailored to clinical need. Standardized bioinformatics workflows are essential to ensure reproducibility, accurate variant calling, and functional annotation (Table 4).
Table 4
| Steps | Descriptions |
|---|---|
| Patient evaluation and genomic testing | |
| Eligibility criteria | Consider comprehensive genomic testing for patients diagnosed with advanced, metastatic, or refractory cancers, or those with early-stage cancers where targeted therapies may improve outcomes. For certain tumor types (e.g., NSCLC, breast cancer, colorectal cancer, melanoma), strongly recommend genomic testing to identify driver mutations (e.g., EGFR, HER2, KRAS, BRAF). Include germline testing if there is a strong family history or suspicion of hereditary cancer syndromes (e.g., BRCA1/BRCA2) |
| Informed consent | Clearly explain the benefits and limitations (e.g., false negatives, uncertain variants, cost) of genomic testing. Discuss potential incidental germline findings and how they may affect family members |
| Choice of test | Targeted gene panels allow rapid, cost-effective method when specific mutations are strongly suspected. Whole exome or whole genome sequencing offers broad coverage, particularly useful for complex cases or when partial testing is inconclusive. Liquid biopsy (ctDNA) should be considered when tissue biopsy is not feasible or to monitor minimal residual disease and emerging resistance |
| Data analysis and bioinformatics | |
| Standardized workflows | Adopt validated pipelines for read alignment, variant calling, and annotation (e.g., GATK, COSMIC, ClinVar). Integrate RNA expression profiling when feasible to refine interpretation for novel target discovery |
| Multidisciplinary interpretation | Ensure that bioinformaticians, molecular biologists, and clinicians work together to validate mutations of VUS, integrate functional predictions, and produce comprehensive reports |
| AI and machine learning deployment | Use AI/ML algorithms to prioritize variants, predict drug response, or classify tumor subtypes. Validate predictions with established clinical and laboratory tests before making final treatment decisions |
| MTB review | |
| Regular case discussions | Convene an MTB to discuss comprehensive genomic profiles in light of each patient’s clinical presentation. Collaborate with medical oncologists, pathologists, radiologists, palliative care experts, and genetic counselors as needed |
| Ethical considerations | Be transparent about how decisions are made, including how to deal with incidental findings or lack of data on certain variants. Discuss data privacy, especially if sharing genomic data across research networks |
| Decision support | Incorporate clinical decision support tools (e.g., OncoKB, CIViC, or local validated databases) to link detected mutations with potential therapeutic targets, investigational drugs, or clinical trials |
| Targeted therapy and combination treatments | |
| Drug selection | Prioritize FDA-approved therapies that match to the patient’s actionable mutations, taking into account safety, patient preference, and comorbidities. If no standard targeted agent is available, consider investigational options through clinical trials |
| Combination therapy | Evaluate synergies using AI-driven or evidence-based prediction models to overcome eventual resistance (e.g., EGFR inhibitors plus anti-angiogenic drugs). Avoid overlapping toxicities by careful dosing and close monitoring of adverse events |
| CRISPR gene-editing approaches (investigational) | If available, consider for refractory cases and ensure robust informed consent detailing potential risks and benefits of a research protocol |
| Monitoring, outcome evaluation, and follow-up | |
| Assessment frequency | Base imaging, biomarker testing, and genomic re-analysis on tumor type and treatment regimen (e.g., every 6–12 weeks). Use ctDNA to detect early molecular progression or the emergence of resistance |
| Adaptive treatment strategies | At signs of progression or unacceptable toxicity, re-biopsy or perform repeat genomic/concomitant transcriptomic testing to understand new resistance mechanisms. Escalate treatment to novel agents, combination regimens, or metabolic therapies |
| Data sharing and reanalysis | Encourage data sharing (in accordance with privacy and national regulations) to improve variant classification over time. Regularly re-examine previously inconclusive or “unknown significance” variants against updated databases |
| Infrastructure, training, and policy considerations | |
| Clinical infrastructure | Establish specialized centers with access to high-throughput sequencing technologies and in-house or collaborative expert bioinformatics support |
| Healthcare professional education | Provide ongoing training for oncologists, pathologists, genetic counselors, nurses, and allied staff in the interpreting genomic data and communication of results to patients |
| Equity and accessibility | Advocate for insurance coverage or national programs to subsidize genomic diagnostics, particularly in underserved or low-income areas. Facilitate telemedicine or remote consultation service to widen patient access to specialized expertise. |
| Regulation and ethics | Develop clear guidelines for the use of AI in patient care, focusing on transparency, bias mitigation, and safety. Promote the responsible use of patient genomic data, respecting privacy regulations (e.g., GDPR in Europe, HIPAA in the US) and the ethical principles of beneficence, autonomy, and justice |
| Anticipated benefits and future directions | |
| Enhanced treatment efficacy | Personalized selection of targeted agents can improve response rates and overall survival in various solid and hematologic malignancies |
| Reduced toxicity | By targeting treatments to specific molecular alterations, potential off-target side effects can be minimized |
| Rapid innovation | The integration of emerging technologies (e.g., CRISPR, advanced AI/ML) is opening new avenues for real-time re-stratification and next-generation drug development |
| Increased collaboration | The continued expansion of public/private databases and multi-institutional studies promotes a global approach to refining guidelines, especially for rare mutations |
| Iterative improvement | As more data accumulates, guidelines and best practices will evolve, continuously improving precision cancer care through an adaptive learning healthcare system |
AI, artificial intelligence; BRAF, v-Raf murine sarcoma viral oncogene homolog B; CRISPR, clustered regularly interspaced short palindromic repeats; ctDNA, circulating tumor DNA; EGFR, epidermal growth factor receptor; FDA, Food and Drug Administration; GDPR, General Data Protection Regulation; HER2, human epidermal growth factor receptor 2; HIPAA, Health Insurance Portability and Accountability Act; KRAS, Kirsten rat sarcoma viral oncogene homolog; ML, machine learning; MTB, molecular tumor board; NSCLC, non-small cell lung cancer; VUS, variants of uncertain significance.
MTBs play a central role in interpreting complex genomic data and addressing ethical and legal issues such as incidental germline findings and patient data privacy. Therapeutic decisions prioritize FDA-approved targeted agents matched to the tumor’s molecular profile, which may include combination therapies or investigational approaches, like CRISPR-based gene-editing strategies, especially in cases of acquired resistance.
Regular outcome assessment through imaging, molecular biomarkers, and adaptive treatment adjustments ensures that care remains responsive to each patient’s evolving disease trajectory. The model also emphasizes the importance of healthcare infrastructure, ongoing training for clinical staff, and policy development to support equitable access to genomic testing and the appropriate use of AI-driven decision-support systems.
In summary, this conceptual framework offers a practical, iterative approach to precision oncology by connecting cutting-edge genomic science with real-world clinical application. By placing the patient at the center of an integrated, data-driven ecosystem, it aims to maximize therapeutic efficacy, support dynamic care planning, and promote equitable implementation of personalized cancer treatment worldwide.
Discussion
Key findings
The conceptual model proposed in this review underscores the significance of integrating genomic insights, real-time data analytics, and multidisciplinary care into a cohesive framework for personalized oncology. Two transformative technologies, CRISPR gene editing and AI, are leading the way in this innovation, each making unique contributions to the advancement of cancer care. These technologies not only support but also enhance the fundamental pillars of the personalized oncology model, providing new opportunities to enhance diagnostic accuracy, therapeutic precision, and clinical outcomes.
CRISPR technology and gene editing in personalized cancer therapy
CRISPR technology and gene editing have revolutionized personalized medicine, especially in cancer treatment (95). By allowing precise editing of specific genetic sequences involved in tumorigenesis, CRISPR enables researchers and clinicians to create targeted therapies tailored to an individual’s unique mutational profile. This capability represents a significant departure from traditional “one-size-fits-all” treatments, which are often associated with limited efficacy and adverse effects, and towards more refined, mutation-specific interventions (96,97).
CRISPR-Cas9, a widely used gene-editing system, uses RNA-guided endonucleases to introduce double-strand breaks at specific genomic locations (Figure 5) (95). These breaks activate the cell’s natural repair mechanisms, allowing for either gene disruption or the insertion of desired genetic material. This precision editing opens the door for innovative therapeutic approaches, such as silencing oncogenes or reactivating tumor suppressor genes, with a level of specificity that surpasses pervious gene-editing tools.

A significant application of CRISPR technology lies in its potential for screening and correcting mutations that drive cancer development (98). Many cancers are fueled by specific genetic aberrations that confer growth advantages. Preclinical studies have demonstrated the feasibility of correcting such mutations in genes like TP53, PTEN, KRAS, and ARID1A using CRISPR-Cas9 systems, restoring their tumor-suppressive functions or altering oncogenic signaling pathways (99,100).
CRISPR also enables combinatorial targeting of multiple genes within a signaling network, addressing the complexity of cancer biology and drug resistance (98). By designing multiplexed CRISPR strategies, researchers can disrupt several genes simultaneously, which may help overcome tumor heterogeneity and reduce the likelihood of therapeutic escape mechanisms.
A pivotal moment in the evolution of genome editing was recently marked by regulatory approval. In 2023, the FDA approved CASGEVYTM, the first CRISPR/Cas9-based gene therapy. This marks a major milestone in clinical gene editing. CASGEVY is a one-time, cell-based therapy for patients aged 12 years and older with sickle cell disease and recurrent vaso-occlusive crises (101). The treatment involves editing the patient’s hematopoietic stem cells to reduce the expression of the BCL11A gene, a repressor of γ-globin. This results in increased fetal hemoglobin production, effectively mitigating disease symptoms (102). The success of CASGEVYTM demonstrates the therapeutic viability of CRISPR-Cas9 and is likely to inspire future gene-editing-based treatments for other genetic diseases, including many forms of cancer.
In addition to correcting mutations, CRISPR technology is actively being explored to enhance immunotherapy, a transformative field in cancer treatment. While immune checkpoint inhibitors have shown remarkable efficacy against certain malignancies (103), responses can be inconsistent due to variability within tumor microenvironments. Researchers are investigating how they can use CRISPR tools not only to enhance anti-tumor immunity but also engineer immune cells (e.g., T-cells) so they better recognize and eliminate malignant cells based on their unique genetic signatures (104).
One example is chimeric antigen receptor (CAR)-T cell therapy, where a patient’s T-cells are modified ex vivo using viral vectors to target tumor-specific antigens like CD19, found in B-cell malignancies such as acute lymphoblastic leukemia (ALL) (105,106). However, the use of viral vectors carries risks of off-target effects and presents logistical challenges in clinical production (107). CRISPR/Cas9 offers a more precise and scalable alternative, potentially increasing safety and expanding applicability across a wider range of solid and hematologic tumors.
Despite the remarkable progress, challenges remain. Ethical concerns surrounding human genome manipulating must be addressed within robust regulatory frameworks before clinical implementation (108). Equitable access to CRISPR-based therapies and diverse representation in clinical trials are also critical to ensure that innovations benefit all populations.
Another area requiring continued focus is the safe and efficient delivery of CRISPR components to target tissues or cells without affecting healthy adjacent tissue. Current delivery methods, such as liposomes, nanoparticles, and plasmid DNA constructs, rely heavily on traditional transfection techniques, which may be inadequate given biological complexities (95). Future advancements may stem from integrating emerging technologies like nanotechnology, biomaterials, and principles from synthetic biology, aimed at optimizing targeting efficiency while minimizing off-target effects and unintended consequences (95,109).
In conclusion, the convergence of genome editing, biotechnology, and personalized medicine principles is reshaping the future of cancer care. Tools like CRISPR not only provide unmatched precision in modifying disease-driving genes but also support broader strategies involving immune modulation and combination therapies. The approval of CASGEVYTM, the first CRISPR therapy, illustrates that we are entering a new era where gene editing can revolutionize treatment approaches. Despite ongoing challenges related to ethics, access, safety, and clinical translation, continued interdisciplinary collaboration will be crucial in realizing the full potential of CRISPR-based personalized therapies and enhancing outcomes for cancer patients globally.
Artificial intelligence (AI) and ML in personalized cancer therapy
The integration of AI and ML into personalized medicine is rapidly transforming treatment selection in cancer care (110). As the volume of genomic and clinical data continues to grow, AI algorithms offer a powerful means of extracting meaningful patterns and insights that may be difficult or impossible for human researchers to discern (47). By leveraging predictive analytics, AI can assist clinicians in identifying the most effective, individualized treatment options based on a patient’s unique genetic profile, tumor characteristics, and clinical history.
One of the most significant advantages of AI and ML lies in their capacity to process and analyze vast, heterogeneous datasets at unprecedented speed and scale (47). Cancer research generates diverse data types, including genomic and transcriptomic sequences, proteomic signatures, clinical trial outcomes, imaging data, and demographic information. Advanced AI techniques are capable of integrating and analyzing this multimodal data to provide a more holistic understanding of each patient’s condition.
Moreover, AI-driven platforms are revolutionizing biomarker discovery, a critical step in developing targeted therapies (111). ML algorithms can sift through large-scale datasets, derived from clinical trials, biobanks, or real-world evidence, to identify novel biomarkers associated with specific cancer subtypes or therapeutic responses. These insights enable clinicians to stratify patients more precisely and select therapies with higher probabilities of success based on individual molecular features.
Another exciting application of AL in oncology is in the optimizing combination therapies (49), a strategy commonly employed to overcome tumor resistance. AI models trained on historical treatment outcomes can predict synergistic interactions between therapeutic agents, allowing the development of personalized drug regimens that maximize efficacy while minimizing toxicity. In addition, AI-powered survival analysis models are being used to predict patient outcomes by incorporating factors such as genetic mutations, treatment history, comorbidities, and demographic variables (112). These tools support risk stratification and aid in tailoring treatment intensity, surveillance strategies, and follow-up care to patient-specific prognostic profiles.
Despite the clear potential benefits of AI and ML, several challenges must be addressed to facilitate seamless integration into existing workflows. Issues related to data interoperability, standardization, and system compatibility must be overcome to ensure the reliability and scalability of AI applications in healthcare settings (113,114). Equally important are concerns surrounding data privacy and cybersecurity, given the sensitive nature of genomic and health-related information. Adherence to stringent regulatory frameworks is essential to protect patient confidentiality while enabling responsible data sharing for innovation.
As discussed, ethical implications of AI applications in clinical decision-making require ongoing scrutiny. Transparency and accountability in algorithmic predictions are crucial for building trust among patients and providers. Ensuring fairness and mitigating biases within AI models are necessary to prevent disparities in care and to promote equitable access to advanced technologies.
As AI continues to evolve, interdisciplinary collaboration is vital for ensuring its responsible and effective application (115,116). Engaging experts from diverse fields, including bioinformatics, oncology, computational biology, ethics, law, and patient advocacy, can guide the development of AI solutions that are both scientifically sound and socially responsible. Incorporating stakeholder feedback throughout the design and implementation process also fosters the creation of user-friendly, accessible interfaces that promote adoption across varied clinical settings.
In conclusion, the convergence of AI and ML with advances in genomics and biotechnology heralds a new era of personalized cancer therapy. Intelligent systems are poised to enhance decision-making, improve treatment precision, and significantly extend survival rates by aligning therapeutic strategies with individual patient needs. As these technologies mature, ensuring ethical, equitable, and interdisciplinary integration will be key to realizing their full potential in transforming cancer care worldwide.
Strengths and limitations
This review highlights significant advancements in personalized medicine, particularly in the context of cancer treatment, by synthesizing current knowledge on genomic profiling and targeted therapies. Its strengths lie in the comprehensive overview of emerging technologies such as CRISPR and AI, which are reshaping treatment paradigms and enhancing the precision of therapeutic strategies. However, limitations include the potential for selection bias in included studies and the challenge of generalizing findings across diverse patient populations due to variations in access to genomic testing and targeted therapies. Additionally, while the review discusses technological innovations, it may not fully address the ethical implications and practical challenges associated with implementing these advancements in clinical practice. Finally, economic aspects are not discussed which might be a limitation, specifically in low-income countries.
Comparison with similar research
Several recent reviews have documented significant advancements in personalized medicine within oncology (117-120). Building upon this growing body of literature, our scoping review offers a comprehensive and up-to-date synthesis of recent innovations, with a particular emphasis on advances in genomic profiling and targeted therapies for cancer treatment.
What distinguishes our review from previous works is its holistic approach, which not only synthesizes established practices but also integrates insights from emerging technologies that are shaping the future of precision oncology. Specifically, we highlight the transformative roles of CRISPR gene editing and AI, exploring how these innovations are being incorporated into clinical decision-making to support more accurate, individualized treatment strategies.
Our analysis further addresses critical implementation challenges, including issues related to data interpretation, accessibility, and integration into routine clinical workflows, areas that are often underrepresented in prior reviews. By contextualizing these barriers within the broader framework of precision oncology, we provide practical insights that may inform future research, policy development, and clinical application.
Moreover, our review introduces an up-to-date conceptual model and practice-oriented recommendations for integrating personalized medicine into oncology care. This framework is designed to guide clinicians, researchers, and healthcare systems in navigating the complexities of translating genomic insights into actionable, patient-centered therapies.
Explanations of findings
The findings of this review emphasize the transformative impact of genomic profiling on personalized medicine, particularly in cancer treatment. By identifying specific genetic alterations that drive tumor growth, clinicians can tailor targeted therapies for individual patients, enhancing treatment effectiveness and overall outcomes. For example, identifying actionable mutations like EGFR or BRAF alterations allows the selection of treatments that target the molecular pathways involved in cancer progression. Additionally, advancements in technologies such as NGS have significantly improved our ability to conduct comprehensive genomic analyses, providing deeper insights into tumor heterogeneity and resistance mechanisms. The integration of AI and ML into bioinformatics further enhances this process by enabling more accurate interpretation of complex genomic data and facilitating optimal treatment selection based on patient-specific profiles. Together, these points illustrate how leveraging genomic insights not only guides clinical decision-making but also paves the way for innovative therapeutic strategies that show promise for enhancing patient care in oncology moving forward.
Implications and actions needed
The implications of this review are profound, as they highlight the necessity for a paradigm shift in cancer treatment towards a more personalized approach rooted in genomic insights. To fully realize the potential of personalized medicine, several actions are needed. First, there must be an increased emphasis on expanding access to genomic testing and targeted therapies across diverse populations to ensure equitable healthcare delivery. This includes addressing barriers such as cost, availability of testing facilities, and training for healthcare professionals in interpreting genomic data effectively. Additionally, fostering interdisciplinary collaboration among oncologists, geneticists, bioinformaticians, and policymakers is crucial to develop comprehensive frameworks that integrate genomic profiling into routine clinical practice. Furthermore, ongoing research should focus on refining bioinformatics tools and developing robust guidelines for the ethical application of emerging technologies like CRISPR and AI in patient care. By taking these steps, the medical community can enhance treatment personalization and improve outcomes for cancer patients globally.
Conclusions
Personalized medicine is transforming the field of oncology, with genomic profiling emerging as a key factor in customizing cancer treatments for individual patients. The integration of advanced technologies like NGS, CRISPR-based gene editing, and AI has sped up the progress of identifying actionable mutations, predicting treatment outcomes, and creating more precise therapeutic regimens. However, despite these advancements, the path to fully integrated precision oncology remains intricate. Looking forward, a practical vision for personalized cancer care involves tackling various crucial challenges. These challenges include ensuring fair access to genomic testing, improving healthcare infrastructure, enhancing clinician education, and establishing solid ethical and regulatory frameworks to govern the use of genomic data. Moreover, the transition of research breakthroughs into everyday clinical practice requires collaboration across disciplines, consistent funding, and strategies that prioritize the need of patients. To truly redefine oncology care, future initiatives must prioritize not only innovation but also inclusivity, scalability, and sustainability. Personalized medicine will not be a one-size-fits-all solution; it will require flexible models that can adapt alongside scientific advancements and healthcare system capabilities. By aligning technological progress with practical and ethical considerations, we can move closer to a future where cancer treatment is not only more effective, but also more equitable, accessible, and responsive to the diverse needs of patients worldwide.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the PRISMA-ScR reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-25-34/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-25-34/prf
Funding: This work was supported by grants from
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-25-34/coif). R.W. serves as an unpaid editorial board member of Annals of Translational Medicine from August 2024 to July 2026. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Molla G, Bitew M. Revolutionizing Personalized Medicine: Synergy with Multi-Omics Data Generation, Main Hurdles, and Future Perspectives. Biomedicines 2024;12:2750. [Crossref] [PubMed]
- Su J, Yang L, Sun Z, et al. Personalized Drug Therapy: Innovative Concept Guided With Proteoformics. Mol Cell Proteomics 2024;23:100737. [Crossref] [PubMed]
- Zhang J, Späth SS, Marjani SL, et al. Characterization of cancer genomic heterogeneity by next-generation sequencing advances precision medicine in cancer treatment. Precis Clin Med 2018;1:29-48. [Crossref] [PubMed]
- Satam H, Joshi K, Mangrolia U, et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology (Basel) 2023;12:997. [Crossref] [PubMed]
- Avci CB, Bagca BG, Shademan B, et al. Precision oncology: Using cancer genomics for targeted therapy advancements. Biochim Biophys Acta Rev Cancer 2025;1880:189250. [Crossref] [PubMed]
- Li J, Gong C, Zhou H, et al. Kinase Inhibitors and Kinase-Targeted Cancer Therapies: Recent Advances and Future Perspectives. Int J Mol Sci 2024;25:5489. [Crossref] [PubMed]
- Jin S, Sun Y, Liang X, et al. Emerging new therapeutic antibody derivatives for cancer treatment. Signal Transduct Target Ther 2022;7:39. [Crossref] [PubMed]
- Fu K, Xie F, Wang F, et al. Therapeutic strategies for EGFR-mutated non-small cell lung cancer patients with osimertinib resistance. J Hematol Oncol 2022;15:173. [Crossref] [PubMed]
- Swain SM, Shastry M, Hamilton E. Targeting HER2-positive breast cancer: advances and future directions. Nat Rev Drug Discov 2023;22:101-26. [Crossref] [PubMed]
- Zhu G, Pei L, Xia H, et al. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol Cancer 2021;20:143. [Crossref] [PubMed]
- Castellani G, Buccarelli M, Arasi MB, et al. BRAF Mutations in Melanoma: Biological Aspects, Therapeutic Implications, and Circulating Biomarkers. Cancers (Basel) 2023;15:4026. [Crossref] [PubMed]
- Selvakumar SC, Preethi KA, Ross K, et al. CRISPR/Cas9 and next generation sequencing in the personalized treatment of Cancer. Mol Cancer 2022;21:83. [Crossref] [PubMed]
- Taherdoost H, Ghofrani A A. I's role in revolutionizing personalized medicine by reshaping pharmacogenomics and drug therapy. Intelligent Pharmacy. 2024;2:643-50. [Crossref]
- Tsimberidou AM, Hong DS, Ye Y, et al. Initiative for Molecular Profiling and Advanced Cancer Therapy (IMPACT): An MD Anderson Precision Medicine Study. JCO Precis Oncol 2017;2017:PO.17.00002.
- Leroy K, Audigier Valette C, Alexandre J, et al. Retrospective analysis of real-world data to evaluate actionability of a comprehensive molecular profiling panel in solid tumor tissue samples (REALM study). PLoS One 2023;18:e0291495. [Crossref] [PubMed]
- Hughes DJ, Kapiris M, Podvez Nevajda A, et al. Non-Small Cell Lung Cancer (NSCLC) in Young Adults, Age < 50, Is Associated with Late Stage at Presentation and a Very Poor Prognosis in Patients That Do Not Have a Targeted Therapy Option: A Real-World Study. Cancers (Basel) 2022;14:6056. [Crossref] [PubMed]
- Kim S, Kim S, Kim SH, et al. Clinical validity of oncogenic driver genes detected from circulating tumor DNA in the blood of lung cancer patients. Transl Lung Cancer Res 2023;12:1185-96. [Crossref] [PubMed]
- Okamura R, Kurzrock R, Mallory RJ, et al. Comprehensive genomic landscape and precision therapeutic approach in biliary tract cancers. Int J Cancer 2021;148:702-12. [Crossref] [PubMed]
- Harttrampf AC, Lacroix L, Deloger M, et al. Molecular Screening for Cancer Treatment Optimization (MOSCATO-01) in Pediatric Patients: A Single-Institutional Prospective Molecular Stratification Trial. Clin Cancer Res 2017;23:6101-12. [Crossref] [PubMed]
- Riedl JM, Hasenleithner SO, Pregartner G, et al. Profiling of circulating tumor DNA and tumor tissue for treatment selection in patients with advanced and refractory carcinoma: a prospective, two-stage phase II Individualized Cancer Treatment trial. Ther Adv Med Oncol 2021;13:1758835920987658. [Crossref] [PubMed]
- Pleasance E, Bohm A, Williamson LM, et al. Whole-genome and transcriptome analysis enhances precision cancer treatment options. Ann Oncol 2022;33:939-49. [Crossref] [PubMed]
- Ding Z, Li Q, Zhang R, et al. Personalized neoantigen pulsed dendritic cell vaccine for advanced lung cancer. Signal Transduct Target Ther 2021;6:26. [Crossref] [PubMed]
- Yuan Y, Yost SE, Yim J, et al. Genomic mutation-driven metastatic breast cancer therapy: a single center experience. Oncotarget 2017;8:26414-23. [Crossref] [PubMed]
- Prager GW, Unseld M, Waneck F, et al. Results of the extended analysis for cancer treatment (EXACT) trial: a prospective translational study evaluating individualized treatment regimens in oncology. Oncotarget 2019;10:942-52. [Crossref] [PubMed]
- Dang M, Schritz A, Goncharenko N, et al. Impact of molecular diagnostics and targeted cancer therapy on patient outcomes (MODIFY): a retrospective study of the implementation of precision oncology. Mol Oncol 2024; Epub ahead of print. [Crossref] [PubMed]
- Dalens L, Niogret J, Richard C, et al. Durable response of lung carcinoma patients to EGFR tyrosine kinase inhibitors is determined by germline polymorphisms in some immune-related genes. Mol Cancer 2023;22:120. [Crossref] [PubMed]
- Martínez-Herrera JF, Sánchez Domínguez G, Juárez-Vignon Whaley JJ, et al. Mutation profile in liquid biopsy tested by next generation sequencing in Mexican patients with non-small cell lung carcinoma and its impact on survival. J Thorac Dis 2024;16:161-74. [Crossref] [PubMed]
- Gouton E, Malissen N, André N, et al. Clinical Impact of High Throughput Sequencing on Liquid Biopsy in Advanced Solid Cancer. Curr Oncol 2022;29:1902-18. [Crossref] [PubMed]
- Kim S, Lim Y, Kang JK, et al. Dynamic changes in longitudinal circulating tumour DNA profile during metastatic colorectal cancer treatment. Br J Cancer 2022;127:898-907. [Crossref] [PubMed]
- Lin C, Shi X, Zhao J, et al. Tumor Mutation Burden Correlates With Efficacy of Chemotherapy/Targeted Therapy in Advanced Non-Small Cell Lung Cancer. Front Oncol 2020;10:480. [Crossref] [PubMed]
- Canino F, Tornincasa A, Bettelli S, et al. Real-World Data and Clinical Implications of Next-Generation Sequencing (NGS)-Based Analysis in Metastatic Breast Cancer Patients. Int J Mol Sci 2024;25:2490. [Crossref] [PubMed]
- Yurkovich JT, Evans SJ, Rappaport N, et al. The transition from genomics to phenomics in personalized population health. Nat Rev Genet 2024;25:286-302. [Crossref] [PubMed]
- Katara A, Chand S, Chaudhary H, et al. Evolution and applications of Next Generation Sequencing and its intricate relations with chromatographic and spectrometric techniques in modern day sciences. Journal of Chromatography Open 2024;5:100121. [Crossref]
- Colomer R, Miranda J, Romero-Laorden N, et al. Usefulness and real-world outcomes of next generation sequencing testing in patients with cancer: an observational study on the impact of selection based on clinical judgement. EClinicalMedicine 2023;60:102029. [Crossref] [PubMed]
- Mandlik JS, Patil AS, Singh S. Next-Generation Sequencing (NGS): Platforms and Applications. J Pharm Bioallied Sci 2024;16:S41-5. [Crossref] [PubMed]
- Pei XM, Yeung MHY, Wong ANN, et al. Targeted Sequencing Approach and Its Clinical Applications for the Molecular Diagnosis of Human Diseases. Cells 2023;12:493. [Crossref] [PubMed]
- Shin H, Sa JK, Bae JS, et al. Clinical Targeted Next-Generation sequencing Panels for Detection of Somatic Variants in Gliomas. Cancer Res Treat 2020;52:41-50. [Crossref] [PubMed]
- Kopetz S, Mills Shaw KR, Lee JJ, et al. Use of a Targeted Exome Next-Generation Sequencing Panel Offers Therapeutic Opportunity and Clinical Benefit in a Subset of Patients With Advanced Cancers. JCO Precis Oncol 2019;3:PO.18.00213.
- Krebs E, Weymann D, Ho C, et al. Real-world cost-effectiveness of multi-gene panel sequencing to inform therapeutic decisions for advanced non-small cell lung cancer: a population-based study. Lancet Reg Health Am 2024;40:100936. [Crossref] [PubMed]
- Lee Gregory M, Burton VJ, Shapiro BK. Chapter 3 - Developmental Disabilities and Metabolic Disorders. In: Zigmond MJ, Rowland LP, Coyle JT. editors. Neurobiology of Brain Disorders. San Diego: Academic Press; 2015:18-41.
- Menzel M, Ossowski S, Kral S, et al. Multicentric pilot study to standardize clinical whole exome sequencing (WES) for cancer patients. NPJ Precis Oncol 2023;7:106. [Crossref] [PubMed]
- Doddato G, Valentino F, Giliberti A, et al. Exome sequencing in BRCA1-2 candidate familias: the contribution of other cancer susceptibility genes. Front Oncol 2021;11:649435. [Crossref] [PubMed]
- Hafstað V, Häkkinen J, Persson H. Fast and sensitive validation of fusion transcripts in whole-genome sequencing data. BMC Bioinformatics 2023;24:359. [Crossref] [PubMed]
- Pan X, Tu H, Mohamed N, et al. FindDNAFusion: An Analytical Pipeline with Multiple Software Tools Improves Detection of Cancer-Associated Gene Fusions from Genomic DNA. J Mol Diagn 2024;26:140-9. [Crossref] [PubMed]
- Pan-cancer analysis of whole genomes. Nature 2020;578:82-93. [Crossref] [PubMed]
- Zhao EY, Jones M, Jones SJM. Whole-Genome Sequencing in Cancer. Cold Spring Harb Perspect Med 2019;9:a034579. [Crossref] [PubMed]
- Kumar P, Paul RK, Roy HS, et al. Big Data Analysis in Computational Biology and Bioinformatics. Methods Mol Biol 2024;2719:181-97. [Crossref] [PubMed]
- Yang X, Huang K, Yang D, et al. Biomedical Big Data Technologies, Applications, and Challenges for Precision Medicine: A Review. Glob Chall 2024;8:2300163. [Crossref] [PubMed]
- Mokhtari M, Khoshbakht S, Akbari ME, et al. BMC3PM: bioinformatics multidrug combination protocol for personalized precision medicine and its application in cancer treatment. BMC Med Genomics 2023;16:328. [Crossref] [PubMed]
- Cabello-Aguilar S, Vendrell JA, Solassol J. A Bioinformatics Toolkit for Next-Generation Sequencing in Clinical Oncology. Curr Issues Mol Biol 2023;45:9737-52. [Crossref] [PubMed]
- Langmead B, Wilks C, Antonescu V, et al. Scaling read aligners to hundreds of threads on general-purpose processors. Bioinformatics 2019;35:421-32. [Crossref] [PubMed]
- Zhou B, Wang C, Putzel G, et al. An integrated strain-level analytic pipeline utilizing longitudinal metagenomic data. Microbiol Spectr 2024;12:e0143124. [Crossref] [PubMed]
- Saada B, Zhang T, Siga E, et al. Whole-Genome Alignment: Methods, Challenges, and Future Directions. Applied Sciences 2024;14:4837. [Crossref]
- McKenna A, Hanna M, Banks E, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 2010;20:1297-303. [Crossref] [PubMed]
- Li H, Handsaker B, Wysoker A, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009;25:2078-9. [Crossref] [PubMed]
Catalogue of Somatic Mutations in Cancer (COSMIC) Sorting Intolerant From Tolerant (SIFT) - Polymorphism Phenotyping (PolyPhen-2). Prediction of functional effects of human nsSNPs. Available online: http://genetics.bwh.harvard.edu/pph2/
- Parekh AE, Shaikh OA. Artificial intelligence (AI) in personalized medicine: AI-generated personalized therapy regimens based on genetic and medical history: short communication. Ann Med Surg (Lond) 2023;85:5831-3. [Crossref] [PubMed]
- Corti C, Cobanaj M, Dee EC, et al. Artificial intelligence in cancer research and precision medicine: Applications, limitations and priorities to drive transformation in the delivery of equitable and unbiased care. Cancer Treat Rev 2023;112:102498. [Crossref] [PubMed]
- Sha C, Lee PC. EGFR-Targeted Therapies: A Literature Review. J Clin Med 2024;13:6391. [Crossref] [PubMed]
- Kang HN, Choi JW, Shim HS, et al. Establishment of a platform of non-small-cell lung cancer patient-derived xenografts with clinical and genomic annotation. Lung Cancer 2018;124:168-78. [Crossref] [PubMed]
- Peng L, Zhu L, Sun Y, et al. Targeting ALK Rearrangements in NSCLC: Current State of the Art. Front Oncol 2022;12:863461. [Crossref] [PubMed]
- Myint KZY, Shimabuku M, Horio R, et al. Identification of circulating tumour DNA (ctDNA) from the liquid biopsy results: Findings from an observational cohort study. Cancer Treat Res Commun 2023;35:100701. [Crossref] [PubMed]
- Santarpia M, Ciappina G, Spagnolo CC, et al. Targeted therapies for KRAS-mutant non-small cell lung cancer: from preclinical studies to clinical development-a narrative review. Transl Lung Cancer Res 2023;12:346-68. [Crossref] [PubMed]
- Baygin RC, Yilmaz KC, Acar A. Characterization of dabrafenib-induced drug insensitivity via cellular barcoding and collateral sensitivity to second-line therapeutics. Sci Rep 2024;14:286. [Crossref] [PubMed]
- Fujii S, Kotani D, Hattori M, et al. Rapid Screening Using Pathomorphologic Interpretation to Detect BRAFV600E Mutation and Microsatellite Instability in Colorectal Cancer. Clin Cancer Res 2022;28:2623-32. [Crossref] [PubMed]
- Gouda MA, Subbiah V. Expanding the Benefit: Dabrafenib/Trametinib as Tissue-Agnostic Therapy for BRAF V600E-Positive Adult and Pediatric Solid Tumors. Am Soc Clin Oncol Educ Book 2023;43:e404770. [Crossref] [PubMed]
- Yu P, Wang Y, Yu Y, et al. Deep Targeted Sequencing and Its Potential Implication for Cancer Therapy in Chinese Patients with Gastric Adenocarcinoma. Oncologist 2021;26:e756-68. [Crossref] [PubMed]
- Wang H, Tang R, Jiang L, et al. The role of PIK3CA gene mutations in colorectal cancer and the selection of treatment strategies. Front Pharmacol 2024;15:1494802. [Crossref] [PubMed]
- Toss A, Piacentini F, Cortesi L, et al. Genomic alterations at the basis of treatment resistance in metastatic breast cancer: clinical applications. Oncotarget 2018;9:31606-19. [Crossref] [PubMed]
- Zeng HH, Yang Z, Qiu YB, et al. Detection of a novel panel of 24 genes with high frequencies of mutation in gastric cancer based on next-generation sequencing. World J Clin Cases 2022;10:4761-75. [Crossref] [PubMed]
- Oh DY, Jung K, Song JY, et al. Precision medicine approaches to lung adenocarcinoma with concomitant MET and HER2 amplification. BMC Cancer 2017;17:535. [Crossref] [PubMed]
- Zhu K, Yang X, Tai H, et al. HER2-targeted therapies in cancer: a systematic review. Biomark Res 2024;12:16. [Crossref] [PubMed]
- Ohnami S, Ohshima K, Nagashima T, et al. Comprehensive characterization of genes associated with the TP53 signal transduction pathway in various tumors. Mol Cell Biochem 2017;431:75-85. [Crossref] [PubMed]
- Popa AM, Stejeroiu MA, Iaciu C, et al. Role of Tumor Molecular Profiling With FoundationOne®CDx in Advanced Solid Tumors: A Single-Centre Experience From Romania. Cureus 2023;15:e50709. [Crossref] [PubMed]
- Liao R, Yi G, Shen L, et al. Genomic features and its potential implication in bone oligometastatic NSCLC. BMC Pulm Med 2023;23:59. [Crossref] [PubMed]
- Groisberg R, Hong DS, Holla V, et al. Clinical genomic profiling to identify actionable alterations for investigational therapies in patients with diverse sarcomas. Oncotarget 2017;8:39254-67. [Crossref] [PubMed]
- Roy-Chowdhuri S, de Melo Gagliato D, Routbort MJ, et al. Multigene clinical mutational profiling of breast carcinoma using next-generation sequencing. Am J Clin Pathol 2015;144:713-21. [Crossref] [PubMed]
- Sahin IH, Akce M, Alese O, et al. Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br J Cancer 2019;121:809-18. [Crossref] [PubMed]
- Li SD, Ma M, Li H, et al. Cancer gene profiling in non-small cell lung cancers reveals activating mutations in JAK2 and JAK3 with therapeutic implications. Genome Med 2017;9:89. [Crossref] [PubMed]
- Nair PC, Piehler J, Tvorogov D, et al. Next-Generation JAK2 Inhibitors for the Treatment of Myeloproliferative Neoplasms: Lessons from Structure-Based Drug Discovery Approaches. Blood Cancer Discov 2023;4:352-64. [Crossref] [PubMed]
- Dong Y, Xu J, Sun B, et al. MET-Targeted Therapies and Clinical Outcomes: A Systematic Literature Review. Mol Diagn Ther 2022;26:203-27. [Crossref] [PubMed]
- Hondelink LM, Schrader AMR, Asri Aghmuni G, et al. The sensitivity of pan-TRK immunohistochemistry in solid tumours: A meta-analysis. Eur J Cancer 2022;173:229-37. [Crossref] [PubMed]
- Dunn DB. Larotrectinib and Entrectinib: TRK Inhibitors for the Treatment of Pediatric and Adult Patients With NTRK Gene Fusion. J Adv Pract Oncol 2020;11:418-23. [PubMed]
- Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2018;19:603-15. [Crossref] [PubMed]
- He Y, Sun MM, Zhang GG, et al. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther 2021;6:425. [Crossref] [PubMed]
- Yang H, Xiao X, Zeng L, et al. Integrating cfDNA liquid biopsy and organoid-based drug screening reveals PI3K signaling as a promising therapeutic target in colorectal cancer. J Transl Med 2024;22:132. [Crossref] [PubMed]
- Gou P, Zhang W, Giraudier S. Insights into the Potential Mechanisms of JAK2V617F Somatic Mutation Contributing Distinct Phenotypes in Myeloproliferative Neoplasms. Int J Mol Sci 2022;23:1013. [Crossref] [PubMed]
- Bader MS, Meyer SC. JAK2 in Myeloproliferative Neoplasms: Still a Protagonist. Pharmaceuticals (Basel) 2022;15:160. [Crossref] [PubMed]
- Wolf J, Hochmair M, Han JY, et al. Capmatinib in MET exon 14-mutated non-small-cell lung cancer: final results from the open-label, phase 2 GEOMETRY mono-1 trial. Lancet Oncol 2024;25:1357-70. Erratum in: Lancet Oncol 2024;25:e542. [Crossref] [PubMed]
- Ishimaru S, Shimoi T, Sunami K, et al. Platform trial for off-label oncology drugs using comprehensive genomic profiling under the universal public healthcare system: the BELIEVE trial. Int J Clin Oncol 2024;29:89-95. [Crossref] [PubMed]
- Thavaneswaran S, Ballinger M, Butow P, et al. The experiences and needs of Australian medical oncologists in integrating comprehensive genomic profiling into clinical care: a nation-wide survey. Oncotarget 2021;12:2169-76. [Crossref] [PubMed]
- Bylstra Y, Lysaght T, Thrivikraman J, et al. Ethical frameworks for obtaining informed consent in tumour profiling: an evidence-based case for Singapore. Hum Genomics 2017;11:31. [Crossref] [PubMed]
- Sayed N, Allawadhi P, Khurana A, et al. Gene therapy: Comprehensive overview and therapeutic applications. Life Sci 2022;294:120375. [Crossref] [PubMed]
- Abdelnour SA, Xie L, Hassanin AA, et al. The Potential of CRISPR/Cas9 Gene Editing as a Treatment Strategy for Inherited Diseases. Front Cell Dev Biol 2021;9:699597. [Crossref] [PubMed]
- Chehelgerdi M, Chehelgerdi M, Khorramian-Ghahfarokhi M, et al. Comprehensive review of CRISPR-based gene editing: mechanisms, challenges, and applications in cancer therapy. Mol Cancer 2024;23:9. [Crossref] [PubMed]
- Heitink L, Whittle JR, Vaillant F, et al. In vivo genome-editing screen identifies tumor suppressor genes that cooperate with Trp53 loss during mammary tumorigenesis. Mol Oncol 2022;16:1119-31. [Crossref] [PubMed]
- Lew TE, Minson A, Dickinson M, et al. Treatment approaches for patients with TP53-mutated mantle cell lymphoma. Lancet Haematol 2023;10:e142-54. [Crossref] [PubMed]
- Mehrabadi S, Salmani Izadi F, Pasha S, et al. The Potential Therapeutic Applications of CRISPR/Cas9 in the Treatment of Gastrointestinal Cancers. Curr Mol Med 2024; Epub ahead of print. [Crossref] [PubMed]
- Adashi EY, Gruppuso PA, Cohen IG. CRISPR Therapy of Sickle Cell Disease: The Dawning of the Gene Editing Era. Am J Med 2024;137:390-2. [Crossref] [PubMed]
- Singh A, Irfan H, Fatima E, et al. Revolutionary breakthrough: FDA approves CASGEVY, the first CRISPR/Cas9 gene therapy for sickle cell disease. Ann Med Surg (Lond) 2024;86:4555-9. [Crossref] [PubMed]
- Shiravand Y, Khodadadi F, Kashani SMA, et al. Immune Checkpoint Inhibitors in Cancer Therapy. Curr Oncol 2022;29:3044-60. [Crossref] [PubMed]
- Chen X, Zhong S, Zhan Y, et al. CRISPR-Cas9 applications in T cells and adoptive T cell therapies. Cell Mol Biol Lett 2024;29:52. [Crossref] [PubMed]
- Sun D, Shi X, Li S, et al. CAR-T cell therapy: A breakthrough in traditional cancer treatment strategies Mol Med Rep 2024;29:47. (Review). [Crossref] [PubMed]
- Saleh K, Pasquier F, Bigenwald C, et al. CAR T-Cells for the Treatment of B-Cell Acute Lymphoblastic Leukemia. J Clin Med 2023;12:6883. [Crossref] [PubMed]
- Ottaviano G, Georgiadis C, Gkazi SA, et al. Phase 1 clinical trial of CRISPR-engineered CAR19 universal T cells for treatment of children with refractory B cell leukemia. Sci Transl Med 2022;14:eabq3010. [Crossref] [PubMed]
- Gonzalez-Avila LU, Vega-López JM, Pelcastre-Rodríguez LI, et al. The Challenge of CRISPR-Cas Toward Bioethics. Front Microbiol 2021;12:657981. [Crossref] [PubMed]
- Hunt JMT, Samson CA, Rand AD, et al. Unintended CRISPR-Cas9 editing outcomes: a review of the detection and prevalence of structural variants generated by gene-editing in human cells. Hum Genet 2023;142:705-20. [Crossref] [PubMed]
- Belbase P, Bhusal R, Ghimire SS, et al. Assuring assistance to healthcare and medicine: Internet of Things, Artificial Intelligence, and Artificial Intelligence of Things. Front Artif Intell 2024;7:1442254. [Crossref] [PubMed]
- Prelaj A, Miskovic V, Zanitti M, et al. Artificial intelligence for predictive biomarker discovery in immuno-oncology: a systematic review. Ann Oncol 2024;35:29-65. [Crossref] [PubMed]
- Huang Y, Li J, Li M, et al. Application of machine learning in predicting survival outcomes involving real-world data: a scoping review. BMC Med Res Methodol 2023;23:268. [Crossref] [PubMed]
- Mahmood U, Shukla-Dave A, Chan HP, et al. Artificial intelligence in medicine: mitigating risks and maximizing benefits via quality assurance, quality control, and acceptance testing. BJR Artif Intell 2024;1:ubae003.
- Kuziemsky CE, Chrimes D, Minshall S, et al. AI Quality Standards in Health Care: Rapid Umbrella Review. J Med Internet Res 2024;26:e54705. [Crossref] [PubMed]
- Schmutz JB, Outland N, Kerstan S, et al. AI-teaming: Redefining collaboration in the digital era. Curr Opin Psychol 2024;58:101837. [Crossref] [PubMed]
- Abbonato D, Bianchini S, Gargiulo F, et al. Interdisciplinary research in artificial intelligence: Lessons from COVID-19. Quantitative Science Studies 2024;922-35. [Crossref]
- Krzyszczyk P, Acevedo A, Davidoff EJ, et al. The growing role of precision and personalized medicine for cancer treatment. Technology 2018;6:79-100. (Singap World Sci). [Crossref] [PubMed]
- Saeed RF, Awan UA, Saeed S, et al. Targeted Therapy and Personalized Medicine. Cancer Treat Res 2023;185:177-205. [Crossref] [PubMed]
- Dumbrava EI, Meric-Bernstam F. Personalized cancer therapy-leveraging a knowledge base for clinical decision-making. Cold Spring Harb Mol Case Stud 2018;4:a001578. [Crossref] [PubMed]
- Riedl JM, Moik F, Esterl T, et al. Molecular diagnostics tailoring personalized cancer therapy-an oncologist's view. Virchows Arch 2024;484:169-79. [Crossref] [PubMed]



